Abstract
Gynecological cancers (GC) represent some of the most frequently diagnosed malignancies in women worldwide. Long-non-coding RNAs (lncRNAs) are regulatory RNAs increasingly being recognized for their role in tumor progression and metastasis in various cancers. Urothelial cancer-associated 1 (UCA1) is a lncRNA, first found deregulated in bladder cancer, and many studies have exposed its oncogenic effects in more tumors since. However, the role of UCA1 in gynecological malignancies is still unclear. This review aims to analyze and define the role of UCA1 in GC, in order to identify its potential use as a diagnostic, prognostic, or therapeutic biomarker of GC. By employing the search terms “UCA1”, “breast cancer”, “endometrial cancer”, “ovarian cancer”, “cervical cancer”, “vaginal cancer”, and “vulvar cancer” in the PubMed database for the literature review, we identified a total of sixty-three relevant research articles published between 2014 and 2024. Although there were some opposing results, UCA1 was predominantly found to be upregulated in most of the breast, endometrial, ovarian, cervical, and vulvar cancer cells, tissue samples, and mouse xenograft models. UCA1 overexpression mainly accounts for enhanced tumor proliferation and increased drug resistance, while also being associated with some clinicopathological features, such as a high histological grade or poor prognosis. Nonetheless, no reviews were identified about the involvement of UCA1 in vaginal carcinogenesis. Therefore, further clinical trials are required to explore the role of UCA1 in these malignancies and, additionally, examine its possible application as a target for upcoming treatments, or as a novel biomarker for GC diagnosis and prognosis.
1. Introduction
Gynecological cancers (GC) can be generally divided into two categories: breast cancer (BC) and tumors of the female genital tract, such as endometrial cancer (EC), ovarian cancer (OC), cervical cancer (CC), vaginal cancer (VGC), and vulvar cancer (VC). BC is the most frequently diagnosed malignancy in women across the globe, with approximately 298,000 new cases reported in 2023 [1]. Mammography and breast examination represent the two main screening methods for BC. Histopathological examination of tumor biopsies represents the gold standard for diagnosis [2]. The latest research proves that about 70% of all cases are estrogen receptor-positive (ER+), whereas triple-negative BC (TNBC), lacking estrogen receptors, progesterone receptors (PR), and the human epidermal growth factor receptor 2 (Her2), constitute 10–20% of BC cases [3,4]. The therapeutic approach for BC patients generally consists of chemotherapy, hormone therapy, and Her2-targeted drugs, such as trastuzumab and pertuzumab, combined with surgery and radiotherapy, depending on the staging [5].
EC is among the most prevalent tumors found in women globally, as it represents approximately 7% of all gynecological malignancies [6,7]. The median age of diagnosis is estimated at 63, considering that it mainly affects women of ages between 55 and 64, during the postmenopausal phase [7]. Based on the histopathology observed, there are two main categories of endometrial cancer: type I and type II. The first one derives from atypical endometrial hyperplasia and is directly linked to prolonged exposure to high levels of ERs. On the other hand, type II is characterized as ER-independent and it predominantly impacts postmenopausal women, as it develops from atrophic endometrium [7]. The main operative therapeutic strategy for EC is total hysterectomy with bilateral salpingo-oophorectomy in postmenopausal patients [8]. However, surgery is often combined with adjuvant chemotherapy when it comes to women with high–intermediate and high-risk ECs, as well as recurrent disease [7]. Despite current therapies, the prognosis rate for patients of the latter category remains poor. More specifically, five-year survival rates range from 23% to 72%, due to a low understanding of the molecular mechanisms behind the progression of the disease [6,7].
OC is the seventh most common gynecological malignancy, with 140,000 deaths per year reported [9,10]. In 2021, 13,770 deaths from OC were documented according to the American Cancer Society [11]. The vast majority of ovarian tumors are epithelial, which present with vague persistent gastrointestinal and urologic symptoms, such as excessive bloating and discomfort [12]. Due to the lack of screening tests for OC, patients are mostly diagnosed in the advanced stages, as determined from the tumor node metastasis classification of malignant tumors (TNM), where treatment options are severely limited [13]. More precisely, the primary approach entails surgical tumor removal combined with chemotherapy [13]. Despite all the well-developed therapies available for OC patients, OC remains the main cause of death among GC [13].
CC is the second most commonly diagnosed gynecological tumor worldwide, and thus it undoubtedly poses a major threat to women’s health [14]. Each year, about 570,000 new incidents are reported, despite the massive evolution of prevention strategies during the last decades [14,15]. The main cause of CC is high-risk Human Papillomavirus (HPV) infection, which is transmitted sexually. Due to the lack of evident symptoms at early stages, many patients are often diagnosed as having advanced CC [14]. Depending on the clinical staging, radical hysterectomy with pelvic lymph node dissection and radiotherapy with or without the combination of platinum-based chemotherapy remain the standard treatments for patients with CC [14].
VGC is one of the most uncommon GCs, mostly found in postmenopausal women. Nevertheless, the growing prevalence of high-risk HPV exposure has led to an increasing incidence of VGC diagnoses [16]. Radiation therapy, particularly brachytherapy, is the preferred approach for early-stage VGC, aiming to preserve vaginal anatomy and function. However, treatment should be tailored to each patient’s unique circumstances [17]. For patients with metastatic VGC, a more systematic strategy with the use of immunotherapy is being reviewed [18].
VC is one of the rarest tumors of the female genital tract, primarily impacting postmenopausal women [19]. According to the latest findings, there are two types of VC. Type I mainly afflicts younger patients and is caused by the Human Papillomavirus, resulting in vulvar intraepithelial neoplasia. Type II, conversely, predominantly manifests in elderly patients and may arise from vulvar non-neoplastic epithelial disorders, secondary to chronic inflammation [20]. Even though VC mainly presents asymptomatic, some cases are characterized by vulvar pruritus or pain, or are presented in the forms of lumps and ulcers [19]. The most common subtype of VC is the vulvar squamous cell carcinoma (VSCC), representing approximately 5% of all gynecological malignancies [21]. The principal treatment approach involves surgical excision of the tumor, particularly for VSCC, while chemoradiation is predominantly reserved for advanced stages [19].
Although major advancements regarding the diagnosis, prognosis, and therapeutic efficacy of treatment strategies for GC have been accomplished over the past years, novel biomarkers and potential therapeutic targets are urgently needed to facilitate a more effective management of such carcinomas.
Over recent decades, a huge advancement in RNA profiling technologies has been made, indicating that there is a large amount of DNA that is transcribed but does not code for any protein. The majority of the human genome is being transcribed into non-coding RNAs, with long non-coding RNAs (lncRNAs) representing an important subclass [5]. LncRNAs are usually over 200 nucleotides long and modulate a variety of physiological processes, such as gene transcription, cell differentiation, and chromosome inactivation [15,22]. Moreover, recent evidence suggests that dysregulated lncRNAs contribute to tumorigenesis and chemoresistance [15,22]. Therefore, lncRNAs offer promising avenues as diagnostic and prognostic biomarkers, as well as targets for therapeutic intervention in diverse malignancies.
Urothelial carcinoma-associated 1 (UCA1) is a long non-coding RNA (lncRNA) with three exons that encode a 1.4 kb isoform, which acts as a tumor-enhancing gene, and a 2.2 kb isoform, characterized as the cancer upregulated drug-resistant gene in doxorubicin (DOX)-resistant epidermoid carcinoma A431 cells [22]. In recent times, a multitude of studies have been conducted to closely investigate the involvement of UCA1 in various malignancies. It was initially identified as an oncogenic lncRNA in bladder cancer, and increasing evidence indicates its pivotal role in melanoma, colorectal, gastric, and hepatocellular cancer as well [5,23]. UCA1 facilitates the reproduction of cancer cells through interacting with tumor-suppressing microRNAs (miR) and proteins, as well as signaling pathways that can regulate the post-transcriptional expression of genes involved in fundamental cell processes, such as proliferation, differentiation, and invasion [24].
Despite the accumulating evidence suggesting that overexpression of UCA1 in certain cancer entities can modulate tumorigenesis, the underlying molecular mechanisms regarding the regulatory roles of UCA1 in GC are still unknown. Consequently, it is imperative to invest efforts in understanding the purpose of UCA1 in this group of malignancies. This review aims to thoroughly examine the potential utility of UCA1 as a valuable tool in the diagnosis, prognosis, and treatment of GC.
2. Methods
The MEDLINE (PubMed) database was utilized for the literature review, and the data analysis consisted entirely of original research articles written in English, which focus on the role of UCA1 in GC, whereas the publications focusing on the correlation between UCA1 and other malignancies were ruled out. A total of 100 articles were identified after employing the search terms “UCA1”, “breast cancer”, “endometrial cancer”, “ovarian cancer”, “cervical cancer”, “vaginal cancer”, and “vulvar cancer”. During the initial selection process, 25 articles were excluded, and among the 75 remaining studies, 63 relevant research articles that met the inclusion criteria were chosen for the final literature review. Figure 1 depicts the aforementioned selection process.
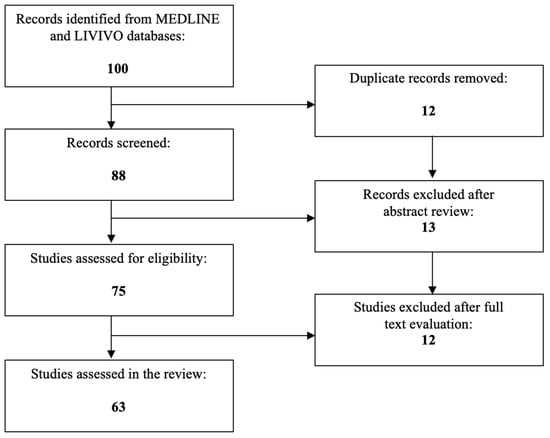
Figure 1.
PRISMA flow diagram visually summarizing the screening process.
3. The Role of UCA1 in BC Oncogenesis, Proliferation, and Invasion
Numerous research groups have explored the involvement of UCA1 in BC carcinogenesis and the mechanisms behind it.
Zhou et al. observed that the downregulation of the insulin-like growth factor 2 messenger RNA-binding protein (IMP1) in the ER + PR + Her2-negative (−) or luminal A subtype T47D and MCF7 cells resulted in enhanced UCA1 expression, increasing their invasiveness. In the T47D and triple-negative MDA-MB-231 cells, this was achieved through the sponging of miR-122-5p by UCA1, and was reversed when IMP1 bound to UCA1, leading to increased pyruvate kinase M2 (PKM2) and insulin-like growth factor 1 receptor (IGF-1R) levels [24].
Furthermore, Zhang et al. found that lncRNAs UCA1 and MACC1-AS1 are mutually coordinated in MCF7 and MDA-MB-231 cells, and through sponging different miRNAs, they are able to upregulate the expression of their target mRNAs associated with oncogenic characteristics, such as increased metastasis or poor prognosis. For instance, both lncRNAs simultaneously suppressed miR-384, miR-181d-5p, and miR-10b-5p, which increased the mRNA TBL1X expression, resulting in the enhanced proliferation of MDA-MB-231 cells [25].
Moreover, Xiao and Wu discovered that the overexpression of UCA1 mediated the activation of the Wingless-related integration site (Wnt)/β-catenin pathway in MDA-MB-231 cells, leading to enhanced Epithelial–Mesenchymal Transition (EMT), which is a key factor for metastasis. Additionally, the silencing of UCA1 reduced the cells’ invasion, upregulated the transmembrane protein E-cadherin, and downregulated N-cadherin, Vimentin, and the transcription factor Snail [22].
Additionally, Mota et al. found that UCA1 was downregulated in the triple-negative SUM159 cells that re-express the tumor suppressor protein Merlin but upregulated in the Merlin-deficient MCF7 and triple-negative MCF10AT cells. Its overexpression in the latter increased hexokinase 2 (HK2), inducing aerobic glycolysis, and promoted the phosphorylation of protein kinase B (AKT) and signal transducer and activator of transcription 3 (STAT3), enhancing the cells’ proliferation and decreasing their apoptosis. The transcription effectors Hippo and transforming growth factor-β (TGF-β) upregulated the UCA1 expression, further enhancing these effects [26].
Lee at al. discovered that the depletion of special AT-rich sequence-binding protein 1 (SATB1), which accounts for aggressive BC progression, increased UCA1 levels, resulting in the enhanced proliferation of MDA-MB-231 cells, while the simultaneous silencing of SATB1 and UCA1 suppressed their survival. UCA1 overexpression was also associated with increased H3K4 trimethylation (H3K4me3) and decreased H3K27 trimethylation (H3K27me3) levels, which are involved in the epigenetic modification of the lysins in Histone H3 protein and, thus, regulate gene expression [27].
In addition, Lee et al. studied the expression levels of various lncRNAs, including UCA1, in multiple BC cell lines. The results indicated that UCA1 was downregulated in the MDA-MB-231, MCF7, T47D, and Her2+ SKBR3 cells, but upregulated in the normal epithelial MCF10A cells [28].
The study of Hiemer et al. revealed that TGF-β and the transcriptional co-activator with PDZ-binding motif (TAZ) and Yes-associated protein (YAP) cooperatively upregulate UCA1 in MCF7 cells and the metastatic MDA-MB-231-derived LM2-4 cell line, increasing their oncogenic activity. UCA1 knockdown, on the other hand, decreased the migration of the TGF-β-treated LM2-4 cells [29].
Alkhathami et al. demonstrated the significant upregulation of UCA1 in the serum of untreated invasive ductal carcinoma (IDC) patients compared to healthy controls, with a remarkable increase in advanced and distant organ metastatic disease patients compared to those with early-stage disease [5].
After evaluating the expression of multiple lncRNAs in blood samples of untreated BC patients, Pourramezan et al. identified that UCA1 was significantly upregulated in BC patients compared to healthy women; however, the correlation between UCA1 levels and clinicopathological characteristics, such as race, histological grade, tumor size, TNM staging, and molecular subtypes, was not statistically significant [30].
The study of Liu et al. revealed that UCA1 levels were substantially more upregulated in the plasma of TNBC patients than the non-TNBC (NTNBC) patients, especially in those with lymph node metastasis at the time of diagnosis, which provides substantial evidence that UCA1 can be used as a TNBC-specific diagnostic biomarker [4].
Of note, El-Helkan et al. found the UCA1 expression to be markedly upregulated in the plasma of non-metastatic BC (NMBC) patients, while also discovering the considerable downregulation of UCA1 in left breast tumors of metastatic BC patients (MBC), indicating its significant association with laterality in MBC [2].
In 2017, Jiang et al. attempted to discover the specific genetic variants within multiple lncRNAs that can possibly affect the susceptibility to BC in Chinese women. UCA1 was identified as one of the significantly differentially expressed lncRNAs between the five paired tumor and normal tissues [31].
Guo et al. evaluated the UCA1 expression to be upregulated and enhance tumor development in the established transcriptional tumor suppressor ARID1A-depleted JIMT1 (Her2+), HCC1937, MDA-MB-468 (both triple-negative), and MCF7 cells, whereas the inhibition of UCA1 by ARID1A suppressed their proliferation and invasion abilities. Moreover, the ARID1A knockdown decreased the binding of CCAAT enhancer binding protein alpha gene (CEBPa) to UCA1, while ARID1A and CEBPa cooperatively downregulated UCA1 and limited the proliferation and migration of MCF7 and MDA-MB-468 cells. UCA1 overexpression abolished the tumor-suppressing effects of ARID1A in vivo, confirming the in vitro results. Lastly, although not statistically significant, the increased UCA1 levels were associated with poor prognosis in BC tissues [32].
Furthermore, Li et al. determined that UCA1 increases tumor growth in MDA-MB-231 cells by upregulating the protein tyrosine phosphatase 1B (PTP1B) expression, and the same effect was facilitated via the inhibition of miR-206 by UCA1 in MCF7 cells. All these results were evident in vivo as well, while the analyzed tumor specimens exhibited an increase of PTP1B expression and its positive correlation with UCA1 [33].
Moreover, Guo-Yin Li et al. discovered that through the upregulation of the Suppressor of Mothers against Decapentaplegic protein 3 (SMAD3) and extracellular signal-regulated kinase (ERK), TGF-β was able to increase the expression of lncRNAs UCA1 and AC026904.1 in MCF7 and MDA-MB-231 cells. These lncRNAs cooperated to upregulate the Snail family transcriptional repressor 2 gene (Slug) and promote EMT in the cells through the inhibition of miR-1 and miR-203. They were also found more overexpressed in the triple-negative MDA-MB-231, MDA-MB-436, and BT549 (IDC-type) cells than in the MCF7, T47D, and triple-positive BT474 cells. UCA1 was able to promote invasion and metastasis in vivo, as well. The analysis of BC tissues additionally showed the upregulation of Slug by both lncRNAs, as well as the overexpression of UCA1 in IDC and metastatic specimens, its association with poor prognosis, and its ability to promote metastasis by downregulating E-cahderin [34].
Zhao et al. found UCA1 to be significantly increased in MCF7, MDA-MB-231, and especially T47D cells. UCA1 knockdown in the latter triggered the expression of the Methyltransferase 14, N6-Adenosine-Methyltransferase Subunit (METTL14), leading to the upregulation of miR-375 and the downregulation of the transcription factor SRY (sex-determining region Y)-box 2 (SOX12), and finally suppressing the proliferation–migration of the T47D cells and increasing their apoptosis. The tumor-suppressing effects from the UCA1 inhibition and its association with the METTL14-miR-375-SOX12 axis were evident in vivo, as well. Lastly, the SOX12 and UCA1 expressions were measured higher in the BC tissues compared to the normal ones, while UCA1 overexpression was associated with short overall survival [35].
Additionally, Yin et al. found UCA1 to be more upregulated in the established Natural Killer (NK)-resistant MDA-MB-231 and MCF7 cells compared to the parental lines, suggesting UCA1’s ability to enhance NK resistance. UCA1 also upregulated the UL16 binding protein 2 (ULBP2), and by sponging miR-26b-5p, increased the disintegrin and metalloproteinase 17 (ADAM17) expression, which accelerated the shedding of ULBP2 from the surface of the cells and, therefore, promoted their resistance to NK. Lastly, the UCA1, ADAM17, and ULBP2 expressions were calculated higher in bone metastasis tissues of BC compared to the primary BC tissues [36].
Zhang et al. identified that the inhibition of miR-185-5p by UCA1 results in the increased proliferation and suppressed apoptosis of MCF7 cells, while the UCA1 knockdown induces the opposite outcomes. The negative correlation of UCA1 and miR-185-5p was also proven in the analysis of BC tissues, which also demonstrated upregulated UCA1 and downregulated miR-185-5p levels [37].
The study that Tuo et al. conducted revealed that UCA1 can increase the proliferation and reduce the apoptosis of MDA-MB-231 cells through the sponging of miR-143. The evaluation of BC specimens additionally showed the inverse expression between them [38].
Zhao et al. found UCA1 to be downregulated in the MCF7 cell line and to regulate the tumor necrosis factor (TNF) pathway, by controlling the expression of chemokine ligand 6 (CXCL6) and mitogen-activated protein kinase 8 (MAP3K8), that act as mediators of infection. UCA1 expression was estimated to be suppressed in the luminal BC tissues, as well, and low UCA1 levels were identified as a biomarker of poor overall survival [39].
In addition, Choudhry et al. exposed MCF7 cells to hypoxic conditions, and found that the hypoxia-inducible transcription factor 1α (HIF-1α) can upregulate the UCA1 expression and subsequently enhance the cells’ survival. UCA1 was also overexpressed in the hypoxia-grown SKBR3, MDA-MB-468, MDA-MB-231, and BT474 cells, while the T47D and triple-negative BT-20 cell lines exhibited a lower increase in UCA1. Finally, the tissue analysis demonstrated that BC specimens expressed UCA1 in higher levels compared to the healthy tissues [40].
Chen et al. studied the correlation between UCA1 and macrophage infiltration, by exposing MCF7 and T47D cells to conditioned medium (CM) containing cultured human leukemia monocytic (THP-1) macrophages. The results indicated that through the activation of AKT signaling, the macrophage CM was able to upregulate the UCA1 expression and, therefore, induce significantly increased invasion abilities in the cells. The THP-1 CM-infiltrated BT474 cells overexpressed UCA1, as well. Furthermore, UCA1 levels were remarkably upregulated in BC tissues, and UCA1 overexpression was associated with advanced BC stages [41].
Finally, Záveský et al. noticed that the metastatic MDA-MB-231-derived 231BoM-1833 and 231BrM-2a variants exhibited considerably upregulated UCA1 expression, while the same was observed for the no-special-type (NST) invasive BC tissues. Notably, significantly elevated UCA1 expression in the tissues was correlated with multifocality, whereas lymph node metastasis and high UCA1 levels were slightly associated [42].
Table 1 briefly summarizes the aforementioned findings.

Table 1.
The role of UCA1 in BB oncogenesis, proliferation, and invasion.
4. Therapeutic Implications of UCA1 in BC
Many studies have been conducted to investigate the role of UCA1 in BC chemoresistance, and the repercussions of its interactions with various drugs and natural elements in BC treatment.
Okcanoğlu et al. found that the Aurora kinase inhibitor CCT137690 is able to downregulate UCA1 in MDA-MB-231 cells, possibly through the direct inhibition of fibroblast growth factor receptor 1 (FGFR1); however, the latter was not confirmed [43].
Furthermore, Mokhtary et al. investigated the potential use of UCA1 in BC gene therapy. They synthesized a UCA1 short-hairpin RNA (shRNA) to cause UCA1 knockdown via RNA interference (RNAi), and then established a UCA1 shRNA complex formed by a vesicular nanocarrier consisting of polysorbate 80 or Tween 80 (T), squalene (S), and cationic lipid didodecyldimethylammonium bromide (DDAB), with a cationic polymer (PEI) ((T:S)1040 μM with PEI). The UCA1 shRNA-(T:S) with PEI complex was used to treat MCF7 cells, which exhibited increased apoptosis and G2/M cell cycle arrest. This evidence suggests that UCA1 downregulation suppresses tumor growth by inducing cell cycle arrest in MCF7 cells. Accordingly, the application of UCA1 RNAi through the vesicular nanocarrier (T:S)1040 μM with PEI constitutes a promising strategy for the future of BC gene treatment [44].
Moreover, Rezaie et al. detected that UCA1 can be successfully downregulated by quercetin, a flavonoid with known anti-cancer properties, leading to G2 cell cycle arrest in MCF7 cells, suppressed proliferation, and enhanced apoptosis [45].
Zhu et al. investigated the involvement of UCA1 in trastuzumab resistance and found that the established trastuzumab-resistant SKBR3 cells exhibited higher UCA1 levels than the parental cells. The UCA1 knockdown upregulated miR-18a, and downregulated its direct target YAP1, facilitating the trastuzumab-triggered apoptosis of the cells, and significantly limiting their invasiveness [46].
The study of Jiang et al. illustrated that UCA1 was significantly differentially expressed between Adriamycin (ADR)-sensitive MCF7 and ADR-resistant MCF7 cells, suggesting of UCA1 ability to mediate ADR resistance [47].
Additionally, Wu and Luo examined the role of UCA1 in tamoxifen resistance. They found that UCA1 levels were lower in the tamoxifen-sensitive MCF7 cells compared to the MCF7-derived tamoxifen-resistant LCC2 and LCC9 cells, which overexpressed AKT and the mammalian target of rapamycin (mTOR) but exhibited decreased viability and increased apoptosis following the UCA1 knockdown. The MCF7 cells demonstrated tamoxifen resistance after being infected with UCA1 particles; however, their exposure to rapamycin abolished the protective effect of UCA1 on them. These findings suggest that UCA1 induces tamoxifen resistance by activating the AKT/mTOR pathway [48].
Li et al. also found that the tamoxifen-resistant LCC2, LCC9, and BT474 cells exhibited markedly overexpressed UCA1 compared to MCF7 cells. After treating the latter with tamoxifen, they estimated a significant upregulation of UCA1 and HIF-1a, and a decrease in miR-18a, which promoted the cells’ viability. The silencing of UCA1 in LCC9 and BT474 cells had the opposite effects and enhanced their sensitivity to tamoxifen. Altogether, tamoxifen increases UCA1 in ER+ cells, leading to the inhibition of miR-18a and the upregulation of HIF-1a, all of which contribute to acquired tamoxifen resistance [49].
Besides, Xu et al. isolated the exosomes released from MCF7 and LCC2 cells and identified that LCC2 cells, and particularly their exosomes, overexpressed UCA1 compared to MCF7 cells. The latter were treated with LCC2/exosomes and exposed to tamoxifen, which increased their viability and decreased their apoptosis. On the contrary, the ability of the LCC2/exosomes with deficient UCA1 expression to promote tamoxifen resistance in MCF7 cells was significantly reduced [50].
Furthermore, Liu et al. noticed that the generated tamoxifen-resistant MCF7 and T47D cells (MCF7-R and T47D-R) exhibited higher UCA1 expression compared to their parental lines, resulting in increased proliferation and migration abilities. Their treatment with tamoxifen, however, caused the downregulation of UCA1, which decreased their survival and enhanced their sensitivity to tamoxifen through the inhibition of β-catenin. The in vivo experiment validated all the aforementioned findings. Lastly, the stage III and IV hormone receptor-positive (HR+) tissues expressed higher UCA1 and β-catenin levels than the specimens of stages I and II, while UCA1 overexpression was associated with poor prognosis [23].
In addition, Zhuo Li et al. found that LCC2 and LCC9 cells demonstrated higher UCA1 expression than the MCF7 and T47D cells. Furthermore, through inducing G2/M cycle arrest, UCA1 knockdown inhibited the phosphoinositide 3 kinase (PI3K)/AKT axis, which downregulated CAMP-responsive element binding protein (CREB), leading to increased apoptosis and tamoxifen sensitivity in LCC2 and LCC9 cells. The enforced UCA1 expression on MCF7 and T47D cells recruited the enhancer of zeste homolog 2 (EZH2), which downregulated the cyclin-dependent kinase inhibitor p21, and decreased the tamoxifen sensitivity of the cells. Finally, the overexpression of UCA1 promoted tumor progression in HR+ BC tissues [3].
Liu et al. observed that the established paclitaxel (PTX)-resistant MCF7 cells (MCF7/PTX) expressed higher UCA1 levels than the MCF7 and MCF10A cells, and UCA1 was able to promote PTX resistance by upregulating cyclin-dependent kinase 12 (CDK12) through the sponging of miR-613 in MCF7/PTX cells. The in vivo experiment showed that UCA1 increased the tumor volume and induced PTX resistance via the miR-613/CDK12 axis. Lastly, PTX-resistant tissues exhibited more upregulated UCA1 expression that the PTX-sensitive specimens [51].
Moreover, Huang et al. discovered that hnRNP I, a member of the heterogeneous nuclear ribonucleoproteins family (hnRNP), increases UCA1 stability in MCF7 and MDA-MB-231 cells, making them resistant to doxorubicin (DOX). Additionally, by competing with the cyclin-dependent kinase inhibitor protein p27, UCA1 promotes tumor development in MCF7 cells. In the in vivo experiment, UCA1 enhanced cancer proliferation, while the tissue specimens analysis showed that UCA1 promotes tumor growth by suppressing p27 [52].
Wo et al. demonstrated that the upregulation of UCA1 by TFG-β resulted in enhanced EMT and DOX resistance in MCF7, MDA-MB-231, and MDA-MB-468 cells. Moreover, BC tissues exhibited more elevated UCA1 levels compared to normal specimens [53].
Table 2 briefly summarizes the aforementioned findings.

Table 2.
Therapeutic implications of UCA1 in BC.
5. The Role of UCA1 in EC Oncogenesis, Proliferation, and Invasion
Two study groups have explored the contribution of UCA1 in EC development.
Liu et al. generated three primary EC cell lines derived from endometrioid adenocarcinoma patients and found that UCA1 enhances their proliferation by sponging miR-143-3p and upregulating Kruppel-like factor 5 (KLF5) and promotes EMT through downregulating miR-1-3p and increasing the relaxin-like family peptide receptor 1 (RXFP1), respectively. The in vivo experiment revealed that UCA1 silencing suppressed cancer development, while UCA1 was overexpressed in the endometrioid EC tissues and closely correlated with tumor growth, metastasis, and poor overall survival [8].
Lu et al. demonstrated that UCA1 silencing decreased the migration and invasion of type II adenosquamous carcinoma HTB-111 and type I endometrioid adenocarcinoma Ishikawa cells. Furthermore, the adenocarcinoma and non-adenocarcinoma tissue types I and II, predominantly the lymph node metastasis specimens, exhibited considerably elevated UCA1 expression. Upregulated UCA1 levels were associated with distant metastasis, advanced stage, high histological grade, and poor prognosis [6].
Table 3 briefly summarizes the aforementioned findings.

Table 3.
The role of UCA1 in EC oncogenesis, proliferation, and invasion.
6. Therapeutic Implications of UCA1 in EC
The study that Dong et al. conducted revealed that the invasive, sphere-forming, and PTX-resistant derivatives of the poorly differentiated endometrioid type II HEC-50 cells exhibited significantly more overexpressed UCA1 levels compared to their parental cell line [54].
Table 4 briefly summarizes the aforementioned findings.

Table 4.
Therapeutic implications of UCA1 in EC.
7. The Role of UCA1 in OC Oncogenesis, Proliferation, and Invasion
Several studies have investigated the contribution of UCA1 to OC pathogenesis.
Liu et al. demonstrated the considerable upregulation of UCA1 in the metastatic SKOV3.ip1 cells compared to their parental serous cystadenocarcinoma SKOV3 line, which indicates the heightened potential of UCA1 to promote metastasis [55].
Furthermore, Qiu et al. additionally showed the correlation of UCA1 with metastasis in OC. They observed that OC tissues exhibited more elevated UCA1 expression than the benign and normal ovarian specimens, while this overexpression was significantly associated with some clinicopathological characteristics, including staging, grade, peritoneal effusion, and lymph node metastasis [56].
Moreover, Lin et al. found that UCA1 increased YAP expression after binding to its regulator, Angiomotin (AMOT), enhancing the interaction between YAP and AMOT, and therefore mediating the YAP dephosphorylation and nuclear translocation. This resulted in the increased survival and proliferation of high-grade serous adenocarcinoma CaOV3 and hereditary BC-OC syndrome (BRCA1)-associated UWB1.289 cells, serous cystadenocarcinoma OVCA429 cells, and ovarian surface epithelial OSEC4C2 cells. Moreover, silencing of UCA1 led to significant tumor suppression in vivo. UCA1 was overexpressed in high-grade serous adenocarcinoma tissues and significantly associated with prognosis, while its locus was marked by a tumor-specific super-enhancer, regulating its expression. Treatment with the inhibitor of the bromodomain and extra-terminal domain (BET) family of proteins (+)-JQ1, however, resulted in the downregulation of UCA1 in CaOV3 and UWB1.289 cells, confirming the research suggesting that super-enhancer-associated genes are sensitive to (+)-JQ1 [57].
The study of Xu et al. indicated that through sponging miR-99b-3p, UCA1 was able to regulate the expression of serine/arginine-rich splicing factor protein kinase-1 (SRPK1) and increase the viability of OC cells, which overexpressed UCA1. The examined OC tissues exhibited upregulation of UCA1, as well [58].
Additionally, Yang et al. showed the UCA1 overexpression in SKOV3, and particularly in mucinous cystadenocarcinoma OMC685 and endometrioid adenocarcinoma A2780 cells, in comparison to the normal ovarian IOSE386 cells. UCA1 was able to promote the migration and invasion of the last two OC cells by inhibiting miR-485-5p and subsequently upregulating matrix metallopeptidase 14 (MMP14). The positive correlation of UCA1 and MMP14 was additionally demonstrated in the epithelial OC tissues (EOC), where UCA1 levels were increased and associated with the International Federation of Gynecology and Obstetrics (FIGO) stage, lymph node metastasis, and poor prognosis [59].
Table 5 briefly summarizes the aforementioned findings.

Table 5.
The role of UCA1 in OC oncogenesis, proliferation, and invasion.
8. Therapeutic Implications of UCA1 in OC
The role of UCA1 in OC chemoresistance was the subject of interest for multiple researchers.
The study of Wang et al. revealed the significant upregulation of UCA1 in the generated PTX-resistant SKOV3 (SKOV3/PTX) and high-grade serous adenocarcinoma HeyA8 (HeyA8/PTX) cells compared to their parental lines. Inhibition of UCA1 in the PTX-resistant cells, however, led to the upregulation of miR-129 and the following depletion of ATP binding cassette subfamily B member 1 (ABCB1), which promoted the PTX-induced apoptosis of the cells [60].
Horita et al. found that both UCA1 and Oncolytic Vaccinia Virus (OVV) expressions were upregulated in the serous cystadenocarcinoma PTX-resistant KFTX and KFTXlow cells compared to PTX-sensitive KFlow cells, indicative of UCA1’s ability to promote both PTX resistance and viral replication. Moreover, SKOV3 and clear cell adenocarcinoma RMG-1 cells exhibited higher UCA1 and enhanced green fluorescent protein (EGFP) levels, and more cytopathic effects and OVV replication than serous cystadenocarcinoma SHIN3 and high-grade serous adenocarcinoma ES-2 and OVCAR3 cells. Therefore, UCA1 can control the oncolytic properties of OVV, and potentially serve as a valuable biomarker for predicting its efficacy in OC. Additionally, by triggering the Cell Division Cycle 42 (Cdc42) expression, UCA1 enhanced the cell-to-cell spread of OVV in SKOV3 cells. The in vivo experiment also illustrated that OVV treatment should be preferred over PTX for tumors overexpressing UCA1, and vice versa [61].
In addition, Li et al. demonstrated the remarkable UCA1 overexpression in A2780, serous cystadenocarcinoma OAW42, high-grade serous adenocarcinoma OVCAR4, and especially SKOV3 and HeyA8 cells, compared to IOSE-386 cells. Notably, UCA1 was estimated as even more overexpressed in the established SKOV3/PTX and HeyA8/PTX cells, which exhibited a substantial decrease in their proliferation, migration, and invasion, as well as an increase in their apoptosis following the inhibition of UCA1. UCA1 was additionally able to promote PTX resistance in these cells via simultaneously downregulating miR-654-5p and upregulating Salt-Inducible Kinase 2 (SIK2). Lastly, OC tissues showed higher UCA1 and lower miR-654-5p levels than the adjacent normal specimens [62].
Zhang et al. found that UCA1 was overexpressed in OEC tissues and significantly correlated with advanced FIGO stage, lymph node metastasis, short survival rates, and resistance to chemotherapy in OEC tissues. Additionally, even though the cisplatin (DDP)-resistant patients exhibited higher UCA1 expression than the DDP-sensitive group, this disparity was not statistically significant [63].
The study of Li et al. indicated that the established DDP-resistant A2780 (A2780/DDP) and SKOV3 (SKOV3/DDP) cells overexpressed UCA1 in comparison to their parental lines and the normal epithelial IOSE-80 cells. Nonetheless, a remarkable decrease in their proliferation and an increase in their DDP-induced apoptosis was observed following UCA1 knockdown. Moreover, the inhibition of miR-143 and subsequent upregulation of FOS-like 2 AP-1 transcription factor subunit (FOSL2) by UCA1 facilitated its effects on DDP resistance in the cells. UCA1 enhanced cell growth and reduced the DDP sensitivity in vivo, as well. The DDP-sensitive serous OC tissues showed a higher UCA1 upregulation than the normal ones; however, the highest UCA1 levels were detected in the DDP-resistant specimens, which additionally exhibited suppressed miR-143 and heightened FOSL2 levels, respectively. Lastly, the DDP-resistant serum-derived exosomes demonstrated UCA1 overexpression and its negative association with miR-143 [64].
Besides, Wambecke et al. revealed that UCA1 was responsible for enhancing DDP resistance to both DDP-resistant OAW42 (OAW42/DDP) cells and their parental line, whereas UCA1 silencing induced a S-G2/M phase block in the former cells and increased apoptosis at G1 phase in DDP-sensitive OAW42 and high-grade serous adenocarcinoma OVCAR3 cells. Additionally, suppression of the short isoform of UCA1 significantly increased the DDP sensitivity to OAW42/DDP cells through the upregulation of miR-27a-5p and the following downregulation of ubiquitin-conjugating enzyme E2 N (UBE2N), which increased B-cell lymphoma 2 (Bcl2)-like protein 11 (BIM). Finally, OC tissues with high UCA1 levels presented a significantly shorter median progression-free survival (PFS) compared to the tissues with a lower UCA1 expression [65].
Wang et al. showcased the ability of UCA1 to promote the proliferation, migration, invasion, and resistance of SKOV3 cells to DDP, through the upregulation of SRPK1, and the subsequent overexpression of the antiapoptotic Bcl2 protein and the downregulation of the proapoptotic Bcl-2-Associated X-protein (Bax), caspase-3, and caspase-9 levels. Furthermore, the EOC tissues displayed markedly overexpressed UCA1 and SRPK1 levels compared to the normal specimens [66].
Table 6 briefly summarizes the aforementioned findings.

Table 6.
Therapeutic implications of UCA1 in OC.
9. The Role of UCA1 in CC Oncogenesis, Proliferation, and Invasion
Nine research groups have, to this day, uncovered the role of UCA1 in the tumorigenesis of CC.
Duan et al. discovered that UCA1 knockdown suppressed tumor progression and enhanced the apoptosis of HeLa cells (adenocarcinoma) through the downregulation of β-catenin and transcription factor 4 (TCF-4) [67].
Yan et al. found UCA1 to be upregulated in human CC cells, while its inhibition led to the overexpression of miR-206 and the suppression of the vascular endothelial growth factor (VEGF). This resulted in a significant decrease in the proliferation, migration, invasion, and viability of the cells [68].
Furthermore, Gao et al. demonstrated that the CaSki-derived exosomes exhibited overexpressed UCA1 and SRY-Box Transcription Factor 2 (SOX2) levels, but decreased miR-122-5p expression, whereas the silencing of UCA1 in the CD133+CaSki stem cells (squamous cell carcinoma or SCC) was able to upregulate miR-122-5p and downregulate SOX2, resulting in their suppressed proliferation, migration, and invasion. In vivo, UCA1 knockdown increased the apoptosis and reduced the tumor volume in the mice [69].
Moreover, He et al. found that UCA1 was overexpressed in SiHa, ME180, C33a (all SCC), CaSki, and HeLa cells, but downregulated in the epithelial non-cancerous Ect1/E6E7 cells. UCA1 promoted the proliferation and invasion of SiHa and CaSki cells through sponging miR-204 and upregulating Kinesin Family Member 20A (KIF20A). The in vivo experiment revealed significant tumor suppression following the UCA1 knockdown. Lastly, CC specimens exhibited upregulated UCA1 and low survival rates, while the inhibition of UCA1 decreased the KIF20A expression [70].
An et al. identified UCA1 to be upregulated in HeLa, SiHa, and particularly ME180 cells, leading to a significant increase in proliferation, migration, and invasion in the first two. UCA1 overexpression was shown to downregulate the SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily d, member 3 (SMARCD3) via ubiquitin-mediated proteolysis in HeLa and ME180 cells, resulting in the progression of tumor growth. The ME180 mouse xenograft models exhibited a decrease in cancer development following the silencing of UCA1, while demonstrating a negative correlation between UCA1 and SMARCD3. Finally, UCA1 was evaluated as overexpressed in both tumor tissues and CC plasma exosomes [71].
Additionally, An et al. showed that HeLa, CaSki, and ME180 cells exhibited significant UCA1 upregulation, which enhanced their proliferation and invasion abilities. This was also accomplished in the SiHa cell line through the inhibition of miR-299-3p. The downregulation of miR-299-3p and subsequent tumor development caused by UCA1 overexpression were obvious in the CC tissues, as well [72].
Besides, Wei et al. showed that UCA1 promoted the proliferation, migration, and invasion of HeLa cells by downregulating miR-145, which was additionally evident in the CC tissues, where UCA1 accelerated the cancer progression [14].
Wu et al. identified that N3CA (SCC), RL95-2, lshikawa3H12, HEC-1A (all adenocarcinoma), and especially HeLa and HEC-1B (adenocarcinoma) cells overtly express UCA1. UCA1 seemed to promote the proliferation and glycolysis of the last two through sponging miR-493-5p, which targeted HK2. UCA1 expression was additionally examined in 20 CC specimens, revealing upregulation in 13 of them [73].
Yang et al. found that UCA1 suppressed miR-155 in order to promote EMT and enhance the proliferation, migration, and invasion of HeLa cells. The CC tissues exhibited decreased miR-155 and enhanced UCA1 expression, with the latter being significantly associated with short overall survival [74].
Table 7 briefly summarizes the aforementioned findings.

Table 7.
The role of UCA1 in CC oncogenesis, proliferation, and invasion.
10. Therapeutic Implications of UCA1 in CC
The following experiments have been conducted to study the participation of UCA1 in CC chemoresistance and radioresistance, respectively.
In 2017, Wang et al. were the first to identify that the established DDP-resistant HeLa cells exhibited higher UCA1 levels than their parental cells. UCA1 enhanced DDP resistance to the former cells by limiting apoptosis via caspase 3 downregulation and CDK2 upregulation, while promoting proliferation through survivin upregulation and p21 suppression [15].
Fan et al. generated the irradiation-resistant SiHa and HeLa cells (IRR), and observed that they exhibited more upregulated UCA1 expression and glycolysis compared to their parental cells, which is indicative of UCA1’s capability to induce radioresistance in CC [75].
Table 8 briefly summarizes the aforementioned findings.

Table 8.
Therapeutic implications of UCA1 in CC.
11. Therapeutic Implications of UCA1 in VC
To date, Gao et al. are the sole research group to explore the role of UCA1 in VC, particularly in the resistance of VSCC to DDP. They found UCA1 to be upregulated in A431 and CAL-39 cells following their exposure to cancer-associated fibroblasts (CAF)-derived exosomes, and CAF-derived exosomal UCA1 mediated DDP resistance to both cell lines by sponging miR-103a and upregulating mitosis inhibitor protein kinase WEE1. In vivo, UCA1 enhanced both tumor development and DDP resistance in the mice. Finally, VSCC tissues exhibited decreased miR-103a and overexpressed UCA1 levels, with the latter being significantly correlated with advanced clinical stage and lymph node metastasis [21].
Table 9 briefly summarizes the aforementioned findings.

Table 9.
Therapeutic implications of UCA1 in VC.
12. Discussion
Gynecological malignancies affect a huge amount of the female population each year. It is consequently of utmost importance to identify novel molecular markers involved in their pathogenesis, in order to develop innovative prognostic tools and therapeutic approaches. Emerging evidence has indicated that UCA1 is frequently found dysregulated in several tumors, such as bladder, colorectal, and gastric cancers [5,23]. It plays a critical role in developing previous carcinomas by modulating the proliferation, invasion, and apoptosis of cancer cells. A great number of review articles have, to date, been published on the involvement of UCA1 in the tumorigenesis of GC and its contribution to chemoresistance. Nonetheless, no study review has been published on the concrete role of UCA1 in GC oncogenesis, prevention, and therapy. The current work, to our knowledge, constitutes the most inclusive, up-to-date review of the literature that comprehensively summarizes the numerous effects of UCA1 on GC.
The majority of the research has focused on the involvement of UCA1 in the tumorigenesis and chemoresistance of BC. The luminal-A subtype representative MCF7 and T47D cells have been included in a very large number of studies. Almost all the experiments revealed the UCA1 overexpression in both cell lines, which significantly promoted their proliferation, invasion, and migration abilities. These functions were accomplished by the sponging effect of UCA1 on several miRNAs, including miR-122-5p [24], miR-206 [33], and miR-185-5p [37], or the upregulation of UCA1 by factors such as TGF-β [29,34], HIF-1a [40], and macrophages [41]. Nevertheless, there were two studies demonstrating that MCF7 and T47D cells exhibited lower UCA1 levels than the normal BC cells [28,39]. In one of those studies, MDA-MB-231 cells, which are the most prominently featured triple-negative cells in the reviews, also manifested decreased UCA1 expression [28]. However, the rest of the reviews revealed a significant upregulation of UCA1 in the MDA-MB-231 line, leading to their enhanced invasiveness, EMT, and reduced apoptosis [22,24]. The carcinogenic impact of UCA1 on these cells was accomplished through the upregulation of other mRNAs and proteins, such as TBL1X and PTP1B, respectively, and inhibition of miRNAs, such as miRNA-143, miR-1, and miR-203 [33,34,37,38]. Notably, UCA1 was shown to be responsible for maintaining the enhanced migratory characteristics of the MDA-MB-231-derived metastatic LM2-4 cells, while the additional MDA-MB-231-derived metastatic 231BoM-1833 and 231BrM-2a variants showed a significant expression of UCA1, as well [29,42]. Several more triple-negative cell lines were included in the studies, including MCF-10AT and SUM159 cells, which exhibited considerably downregulated UCA1 expression following their exposure to the tumor suppressor Merlin. The MDA-MB-468 and HCC1937 cells also displayed decreased UCA1 levels, after their exposure to another tumor suppressor, ARID1A [26,32]. In one study, the triple-negative MDA-MB-436 and BT549 cells exhibited significantly more elevated UCA1 expression compared to the MCF7 and MDA-MB-231 cells, while another study showed the BT-20 and MDA-MB-468 cells to overexpress UCA1 after being exposed to hypoxic conditions [34,40]. The induction of apoptosis resulting from UCA1 downregulation by ARID1A was similarly observed in Her2+ JIMT1 cells, while the Her2+ SKBR3 cells demonstrated significantly increased UCA1 expression in response to hypoxic conditions, as well [32,40]. Conversely, the study reporting low UCA1 levels in MCF7, T47D, and MDA-MB-231 cells also noted downregulated UCA1 expression in SKBR3 cells [28]. Lastly, among the examined cells, only one triple-positive cell line, BT474, was studied, exhibiting upregulated UCA1 expression after its interaction with HIF-1a and macrophages, respectively [40,41].
While no experiments were conducted exclusively in vivo, the majority of the aforementioned studies utilized BC mouse xenograft models. These models served to confirm the in vitro findings by demonstrating the promotion of tumor growth and suppression of metastasis, resulting from UCA1 overexpression and its interactions with various molecular entities, such as PTP1B and SOX12, in triple-negative and Her2+ mouse xenografts [32,33,34,35].
Four research groups assessed the levels of UCA1 in the blood of BC patients. UCA1 was found to be overtly expressed in all serum and plasma samples of patients with IDC and TNBC, compared to normal and NTNBC samples, respectively. Furthermore, UCA1 concentrations were measured to be even more elevated in the blood samples of patients in advanced stages, and women with lymph node and distant organ metastatic disease [4,5]. Nonetheless, one study found that UCA1 levels were not significantly correlated with clinicopathological characteristics, whereas another study proposed that UCA1 expression was reduced in left-sided MBC tumors, suggesting a potential correlation between UCA1 levels and laterality in MBC patients [2,30]. Almost all studies identified overexpressed UCA1 levels in BC tissues compared to the healthy ones, while additionally revealing the remarkable association of high UCA1 levels with short overall survival and advanced clinical stage disease [31,32,34,35,37,38,40,41]. One study, however, demonstrated the downregulated UCA1 expression to be significantly associated with poor prognosis for the luminal subtype BC patients [39]. Moreover, the studies revealed the enhanced ability of UCA1 to regulate the expression of various biomolecules, such as PTP1B and SOX12, and different miRNAs, such as miR-185-5p and miR-143, to further promote its oncogenic influence on BC tissues [33,35,37,38]. Interestingly, there was one study group that identified the significant correlation between high UCA1 expression and multifocality in NST invasive BC specimens [42]. Finally, one study revealed higher UCA1 expression in patients with DCIS compared to those with IDC, while another study demonstrated that UCA1 promoted bone metastasis by upregulating ADAM17 and ULBP2 [34,36]. Both findings suggest the amplified capability of UCA1 to induce migration, invasion, and tumor development in BC.
Researchers have shown significant interest in exploring the role of UCA1 in future BC treatments and its contribution to chemotherapy resistance. One study showed that UCA1 expression can be successfully suppressed in triple-negative cell lines by the Aurora kinase inhibitor CCT137690, while another study suggested that quercetin, an important phytochemical compound with anti-tumor characteristics, is able to downregulate UCA1 in MCF7 cells and subsequently cause cell cycle arrest at the G2/M phase, reducing tumor growth and triggering apoptosis [43,45]. The induction of G2 cell cycle arrest by UCA1 inhibition was replicated when MCF7 cells were transfected with shRNA UCA1 using a non-viral vesicular nanocarrier. This indicates that UCA1 RNAi may serve as a viable approach for future BC gene therapies [44]. Many studies identified the crucial role of UCA1 in mediating BC chemoresistance. For instance, UCA1 was observed to be overexpressed in Her2+ cells, leading to a notable reduction in the cells’ responsiveness to trastuzumab. However, this effect was promptly reversed upon UCA1 knockdown [46]. The inhibition of UCA1 led to increased sensitivity to PTX in MCF7 cells, as well [51]. One study indicated a possible correlation between UCA1 and ADR resistance in MCF7 cells, since the ADR-resistant cells exhibited significantly more elevated UCA1 expression compared to the ADR-sensitive ones [47]. The luminal-A-type cells also exhibited significant resistance to DOX upon UCA1 overexpression, attributed to its interactions with hnRNP or TGF-β. In contrast, UCA1 knockdown enhanced the sensitivity to DOX-induced and p27-induced apoptosis, as observed in triple-negative cell lines [52,53]. Five studies analyzed the involvement of UCA1 in tamoxifen resistance, and found that the luminal-A-type LCC2 and LCC9—tamoxifen-resistant cells—as well as BT474 cells expressed significantly higher UCA1 levels compared to the MCF7 controls, whereas the UCA1 inhibition resulted in enhanced sensitivity to tamoxifen, decreased cell viability, and deceleration of tumor growth [3,23,48,49,50]. In the in vivo experiments, luminal-A-type BC mouse xenograft models, which were resistant to tamoxifen and PTX, exhibited a notable increase in their sensitivity to these drugs, following UCA1 silencing [23,51,52].
Finally, analysis of BC tissue specimens revealed UCA1 overexpression in all tumor samples compared to healthy samples, contributing to accelerated cancer progression [3,23,52,53]. Two of those studies found UCA1 to be upregulated in HR+ BC tissues, while one of them demonstrated that UCA1 was notably overexpressed in stage III and IV tissues, with elevated UCA1 levels identified as a prognostic biomarker for poor survival [3,23]. The same study additionally discovered that the upregulation of β-catenin by UCA1 constitutes one of the underlying mechanisms participating in tamoxifen resistance in HR+ BC tissues [23].
The influence of UCA1 on EC was studied by two research groups. UCA1 was found to be overexpressed in the patient-derived endometrioid EC cells, enhancing proliferation and EMT through the miR-143-3p/KLF and miR-1-3-p/RXFP1 pathways, respectively [8]. In addition, both type I endometrioid Ishikawa and type II adenosquamous HTB-111 cells exhibited a remarkable suppression of their migration and invasion abilities, following the inhibition of UCA1 [6].
The reduction in tumor growth and size attributed to UCA1 knockdown was also observed in mice injected with primary endometrioid EC cells transfected with the UCA1 vector, thereby corroborating the in vitro findings [8].
The analysis of tissue specimens from both studies revealed significant upregulation of UCA1 in adenocarcinoma, including endometrioid, and non-adenocarcinoma tissues compared to normal samples. This heightened expression was correlated with accelerated cancer progression and metastasis. Additionally, one study demonstrated elevated UCA1 levels in lymph node and distant metastatic tissues, with a significant association observed between high UCA1 expression and advanced-stage disease, as well as high histological grade. Both research groups identified elevated UCA1 expression as a prognostic biomarker for poor overall survival [6,8].
Ultimately, one study group revealed a substantial upregulation of UCA1 in the invasive and sphere-forming type II endometrioid HEC-50 cells exhibiting PTX resistance compared to the parental HEC-50 cells. This suggests the significant involvement of UCA1 in mediating PTX resistance [54].
A total of five research groups showed interest in the correlation of UCA1 with the pathogenesis of OC. All studied OC cells displayed a significant overexpression of UCA1, leading to their enhanced proliferation, migration, and invasion activities [55,57,58,59]. In some cases, the presence of UCA1 alone was able to promote the cells’ growth and viability, while in others, the interaction of UCA1 with various biomolecules was needed to achieve its carcinogenic influence. For example, the sole expression of UCA1 in the serous cystadenocarcinoma SKOV3 cells and their metastatic SKOV3.ip1 variants was proven capable of increasing their survival, whereas the sponging of miR-485-5p and the upregulation of MMP14 greatly facilitated the UCA1 impact in the mucinous cystadenocarcinoma OMC685 and endometrioid adenocarcinoma A2780 cells [55,59]. Those two cell lines in particular were shown to exhibit the highest UCA1 levels out of all the cells included in the experiment [59]. Interestingly, one study demonstrated UCA1 as a super-enhancer-associated gene, particularly sensitive to treatment with the BET inhibitor (+)-JQ1 in the high-grade serous adenocarcinoma CaOV3 and UWB1.289 cells [57].
The last study additionally featured an in vivo experiment, showcasing the suppression of cancer development following the inhibition of UCA1 in the serous cystadenocarcinoma OVCA249 mouse xenograft models [57].
All analyzed OC tissues manifested UCA1 upregulation, a finding that was notably associated with FIGO staging, histological grade, peritoneal effusion, prognosis, and lymph node metastasis in the majority of cases [56,57,58,59].
The role of UCA1 as a mediator of PTX and DDP resistance in OC was the focus of research for multiple study groups. Two studies illustrated the ability of UCA1 to induce PTX resistance in SKOV3 and high-grade serous adenocarcinoma HeyA8 cells by regulating the expression of two miRNAs and their target genes: miR-129/ABCB1 and miR-654-5p/SIK, respectively [60,62]. The enhanced capacity of UCA1 to promote PTX resistance was further confirmed by observing higher UCA1 levels in serous cystadenocarcinoma PTX-resistant KFTX and KFTXlow cells, compared to the PTX-sensitive KFlow cells [61]. Another important finding of this study was the ability of UCA1 to facilitate cell-to-cell OVV spread in the SKOV3 line overexpressing UCA1, in contrast to the high-grade serous adenocarcinoma OVCAR3 and ES-2 lines displaying a lower UCA1 expression, and being more susceptible to PTX treatment, instead [61]. The results of the last study were further validated by an in vivo experiment using KFTX and KFlow mouse xenograft models [61]. Lastly, OC tissue analysis additionally verified the previously mentioned negative correlation between UCA1 and miR-654-5p, highlighting its impact on the response to PTX chemotherapy [62].
Two studies showcased the UCA1-induced DDP resistance in SKOV3 cells, accomplished through the upregulation of SRPK1 and antiapoptotic protein Bcl2, and the respective modulation of miR-143 and its target gene FOSL2 [64,66]. The downregulation of miR-143 by UCA1 conferred DDP resistance in A2780 cells, as well [64]. Conversely, silencing the short isoform of UCA1 elevated miR-27a-5p and BIM expressions, leading to enhanced sensitization of established DDP-resistant serous cystadenocarcinoma OAW42 cells to DDP [65]. The SKOV3 mouse xenografts additionally exposed the reduced DDP sensitivity and increased tumor progression caused by UCA1 overexpression [64]. Furthermore, it became evident that all scrutinized tissues exhibiting DDP resistance expressed higher levels of UCA1 compared to both DDP-sensitive and normal EOC tissues. This upregulation was often accompanied by the different expression of various biomolecules, such as SRKP1 and FOSL2 upregulation, or miR-143 downregulation [64,66]. The latter was also depicted in the analyzed OC serum-derived exosomes [64]. Although another study evaluated UCA1 as more upregulated in the DDP-resistant tissues compared to the sensitive ones, the difference did not attain statistical significance [63]. However, the same study, in conjunction with another, revealed a crucial association between UCA1 and clinicopathological features, including poor prognosis, lymph node metastasis, advanced FIGO stage, and shorter PFS [63,65].
The regulatory purpose of UCA1 In CC was studied by several groups, the majority of which examined its effects in the adenocarcinoma representative HeLa cells. All experiments revealed a significant overexpression of UCA1, leading to enhanced EMT, proliferation, and migration of the cells through the upregulation of the β-catenin/TCF-4 axis, and inhibition of miR-145, miR-493-5p, and miR-155 [14,67,70,72,73,74]. All additional cervical adenocarcinoma cell lines studied, including HEC-1A, Ishikawa3H12, and HEC-1B, exhibited exceptionally high levels of UCA1 expression, particularly the latter. This overexpression enhanced their malignant behavior and restrained their apoptosis [73]. UCA1 demonstrated consistent upregulation and carcinogenic effects across all SCC cells examined in the reviews. CaSki cells, for example, displayed increased proliferation and decreased apoptosis due to the upregulation of SOX2 and downregulation of miR-204 by UCA1 [69,70]. In the SiHa, Me180, and C33a cells, which are also representative of SCC of the cervix, UCA1 was found to be overexpressed and implicated in promoting tumor development [70].
Two of the referenced studies additionally conducted in vivo experiments, both demonstrating that UCA1 silencing suppressed tumor development and enhanced apoptosis in mice [69,70].
In five of the previous studies, the analysis was also conducted on CC tissue specimens, revealing significant overexpression of UCA1 in all malignant tissues. UCA1 was once again shown accountable for driving aggressive tumor growth, through its interactions with proteins such as KIF20A, and by sponging different miRNAs, such as miR-299-3p, miR-145, and miR-155 [14,70,72,73,74]. Finally, two research groups identified UCA1 as a factor contributing to shortened overall survival in patients, highlighting its association with poor prognosis [70,74].
Two study groups investigated the implications of UCA1 in the treatment of CC. The first one exposed the ability of UCA1 to induce DDP resistance to HeLa cells through its correlations with CDK12 and caspase 3, and the other study revealed that the overexpression of UCA1 in HeLa and SiHa cells played a pivotal role in mediating radioresistance [15,75].
Only one research group has, to date, delved into the role of UCA1 in VC. They showed exosomal UCA1 derived from CAF to induce DDP resistance to VSCC cells by suppressing miR-103a and upregulating WEE1. The in vivo experiment validated the previous findings, while the overexpression of UCA1 in the VSCC tissues was associated with advanced clinical stage disease and lymph node metastasis [21].
Unfortunately, no original research article has, to date, been published on the role of UCA1 in VGC. The lack of reviews regarding the correlation between UCA1 and VGC underlines the urgent need for future trials, in order to improve the management of female patients suffering from VGC.
Considering all these results collectively, it appears that UCA1 exerts an oncogenic role in GC, as evidenced by the majority of studies illustrating its ability to promote cancer proliferation and metastasis, and inhibit apoptosis through interactions with multiple proteins, miRNAs, and genes. Additionally, significantly elevated UCA1 expression was associated with advanced-stage disease, lymph node or distant organ metastasis, and poor overall survival, indicating its crucial value as a prognostic biomarker. The results further indicate that the upregulation of UCA1 plays a pivotal role in inducing chemoresistance, at least partially, in all studied GC entities, implying that targeting UCA1 therapeutically holds promise for the improvement of GC management. The findings across all investigated gynecological tumors were consistent, although some results deviated from the majority. For example, while the luminal-type MCF7 cells repeatedly exhibited elevated UCA1 levels in the majority of experiments, a single study revealed downregulated UCA1 levels in both MCF7 cells and luminal subtype tissues, which additionally served as a biomarker for poor prognosis [39]. In contrast to the findings of other works, a separate study observed downregulated UCA1 expression in all analyzed BC cell types, including luminal A, triple-negative, and Her2+ cells [28]. These discrepancies probably arise from variations in methodological approaches and sample sizes, as ranging from small-scale experiments to large clinical studies impacts the reliability and applicability of the results. Differences in cell culture conditions and data analysis techniques also contribute to the variability of outcomes. Furthermore, most experiments were conducted on a pre-clinical level, and the number of studies focusing on EC, CC, and VC is insufficient compared to the research revolving around the association between UCA1 and BC. It should also be mentioned that no studies have, to date, been conducted on the impact of UCA1 in vaginal oncogenesis, and the possible involvement of UCA1 in VGC chemoresistance, despite the encouraging results displayed from the research emphasizing the rest of the GCs. All the above findings accentuate the importance of performing larger and more meticulously planned clinical studies, to more effectively evaluate the various functions of UCA1 in individuals diagnosed with all kinds of gynecological malignancies.
13. Conclusions
To conclude, UCA1 seems to fulfill a crucial role in the progression of most GCs, mainly by promoting the cancer cells’ proliferation and migration abilities, while inhibiting apoptosis, through interacting with multiple signaling pathways and regulating the expression levels of different proteins and genes. In addition, UCA1 functions as a mediator for inducing drug resistance, therefore limiting the therapeutic efficacy of some first-line treatment options, as well as the overall survival of female patients. All this evidence strongly suggests that UCA1 could be targeted by therapeutic agents, and possibly used as a biomarker for the early diagnosis and prognosis of GC. Regardless, future randomized-controlled clinical trials are needed to test the application of such practices in the everyday management of women dealing with BC and tumors of the genital tract.
Author Contributions
Literature analysis and conceptualization, E.N. and I.P.; original draft preparation and writing, E.N. and I.P.; review and supervision, K.V., C.D., N.G., A.G., P.T., N.N., K.N. and I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- El-Helkan, B.; Emam, M.; Mohanad, M.; Fathy, S.; Zekri, A.R.; Ahmed, O.S. Long non-coding RNAs as Novel Prognostic Biomarkers for Breast Cancer in Egyptian Women. Sci. Rep. 2022, 12, 19498. [Google Scholar] [CrossRef]
- Li, Z.; Yu, D.; Li, H.; Lv, Y.; Li, S. Long Non-coding RNA UCA1 Confers Tamoxifen Resistance in Breast Cancer Endocrinotherapy through Regulation of the EZH2/p21 Axis and the PI3K/AKT Signaling Pathway. Int. J. Oncol. 2019, 54, 1033–1042. [Google Scholar] [CrossRef]
- Liu, M.; Xing, L.Q.; Liu, Y.J. A three-long Noncoding RNA Signature as a Diagnostic Biomarker for Differentiating between triple-negative and non-triple-negative Breast Cancers. Medicine 2017, 96, e6222. [Google Scholar] [CrossRef]
- Alkhathami, A.G.; Hamid, A.; Alfaifi, M.; Alshahrani, M.Y.; Verma, A.; Beg, A. Serum-Based lncRNA ANRIL, TUG1, UCA1, and HIT Expressions in Breast Cancer Patients. Dis. Markers 2022, 2022, 9997212. [Google Scholar] [CrossRef]
- Lu, L.; Shen, Y.; Tseng, K.F.; Liu, W.; Duan, H.; Meng, W. Silencing of UCA1, a Poor Prognostic factor, Inhibited the Migration of Endometrial Cancer Cell. Cancer Biomark. 2016, 17, 171–177. [Google Scholar] [CrossRef]
- SEER. Cancer of the Endometrium—Cancer Stat Facts. 2018. Available online: https://seer.cancer.gov/statfacts/html/corp.html (accessed on 7 March 2024).
- Liu, T.; Wang, X.; Zhai, J.; Wang, Q.; Zhang, B. Long Noncoding RNA UCA1 Facilitates Endometrial Cancer Development by Regulating KLF5 and RXFP1 Gene Expressions. Cancer Biother. Radiopharm. 2021, 36, 521–533. [Google Scholar] [CrossRef]
- Webb, P.M.; Jordan, S.J. Epidemiology of Epithelial Ovarian Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef]
- Cancer.org. Ovarian Cancer Statistics|How Common is Ovarian Cancer. 2024. Available online: https://www.cancer.org/cancer/types/ovarian-cancer/about/key-statistics.html#:~:text=About%2019%2C680%20women%20will%20receive%20a%20new%20diagnosis,during%20her%20lifetime%20is%20about%201%20in%2087 (accessed on 7 March 2024).
- Psilopatis, I.; Pergaris, A.; Vrettou, K.; Tsourouflis, G.; Theocharis, S. The EPH/Ephrin System in Gynecological Cancers: Focusing on the Roots of Carcinogenesis for Better Patient Management. Int. J. Mol. Sci. 2022, 23, 3249. [Google Scholar] [CrossRef]
- Orr, B.; Edwards, R.P. Diagnosis and Treatment of Ovarian Cancer. Hematol./Oncol. Clin. N. Am. 2018, 32, 943–964. [Google Scholar] [CrossRef]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef]
- Wei, H.; Qiu, Y.Q.; Zeng, Q.S.; Wang, S.F.; Yi, C.J. LncRNA UCA1 regulates proliferation, migration and invasion of cervical cancer cells by targeting miR-145. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3555–3564. [Google Scholar] [CrossRef]
- Wang, B.; Huang, Z.; Gao, R.; Zeng, Z.; Yang, W.; Sun, Y.; Wei, W.; Wu, Z.; Yu, L.; Li, Q.; et al. Expression of Long Noncoding RNA Urothelial Cancer Associated 1 Promotes Cisplatin Resistance in Cervical Cancer. Cancer Biother. Radiopharm. 2017, 32, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.S.; Rogers, L.J.; Cuello, M.A. Cancer of the vagina: 2021 Update. Int. J. Gynecol. Obstet. 2021, 155, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Jhingran, A. Updates in the Treatment of Vaginal Cancer. Int. J. Gynecol. Cancer 2022, 32, 344–351. [Google Scholar] [CrossRef]
- Kulkarni, A.; Dogra, N.; Zigras, T. Innovations in the Management of Vaginal Cancer. Curr. Oncol. 2022, 29, 3082–3092. [Google Scholar] [CrossRef]
- Olawaiye, A.B.; Cuello, M.A.; Rogers, L.J. Cancer of the vulva: 2021 Update. Int. J. Gynecol. Obstet. 2021, 155, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Canavan, T.P.; Cohen, D. Vulvar Cancer. Am. Fam. Physician 2002, 66, 1269–1274. Available online: https://pubmed.ncbi.nlm.nih.gov/12387439/ (accessed on 28 January 2024).
- Gao, Q.; Fang, X.; Chen, Y.; Li, Z.; Wang, M. Exosomal lncRNA UCA1 from cancer-associated fibroblasts enhances chemoresistance in vulvar squamous cell carcinoma cells. J. Obstet. Gynaecol. Res. 2021, 47, 73–87. [Google Scholar] [CrossRef]
- Xiao, C.; Wu, C.H.; Hu, H.Z. LncRNA UCA1 Promotes epithelial-mesenchymal Transition (EMT) of Breast Cancer Cells via Enhancing Wnt/beta-catenin Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2819–2824. Available online: https://pubmed.ncbi.nlm.nih.gov/27424981/ (accessed on 28 January 2024).
- Liu, H.; Wang, G.; Yang, L.; Qu, J.; Yang, Z.; Zhou, X. Knockdown of Long Non-Coding RNA UCA1 Increases the Tamoxifen Sensitivity of Breast Cancer Cells through Inhibition of Wnt/β-Catenin Pathway. PLoS ONE 2016, 11, e0168406. [Google Scholar] [CrossRef]
- Zhou, Y.; Meng, X.; Chen, S.; Li, W.; Li, D.; Singer, R.; Gu, W. IMP1 regulates UCA1-mediated cell invasion through facilitating UCA1 decay and decreasing the sponge effect of UCA1 for miR-122-5p. Breast Cancer Res. BCR 2018, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, Y.; Wu, J.D.; Zhou, Y.; Chen, W.; Gu, W. Two lncRNAs, MACC1-AS1 and UCA1, co-mediate the expression of multiple mRNAs through interaction with individual miRNAs in breast cancer cells. Non-Coding RNA Res. 2022, 7, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.S.V.; Jackson, W.P.; Bailey, S.K.; Vayalil, P.; Landar, A.; Rostas, J.W.; Mulekar, M.S.; Samant, R.S.; Shevde, L.A. Deficiency of tumor suppressor Merlin facilitates metabolic adaptation by co-operative engagement of SMAD-Hippo signaling in breast cancer. Carcinogenesis 2018, 39, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Kim, M.; Kim, H.P. Epigenetic regulation of long noncoding RNA UCA1 by SATB1 in breast cancer. BMB Rep. 2016, 49, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, H.Y.; Kim, W.W.; Lee, S.J.; Jeong, J.-H.; Kang, S.H.; Jung, J.H.; Chae, Y.S. Biological function of long noncoding RNA snaR in HER2-positive breast cancer cells. Tumor Biol. 2017, 39, 101042831770737. [Google Scholar] [CrossRef]
- Hiemer, S.E.; Szymaniak, A.D.; Varelas, X. The Transcriptional Regulators TAZ and YAP Direct Transforming Growth Factor β-induced Tumorigenic Phenotypes in Breast Cancer Cells. J. Biol. Chem. 2014, 289, 13461–13474. [Google Scholar] [CrossRef] [PubMed]
- Pourramezan, Z.; Attar, F.A.; Yusefpour, M.; Azizi, M.; Oloomi, M. Circulating LncRNAs landscape as potential biomarkers in breast cancer. Cancer Rep. 2023, 6, e1722. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Du, F.; Chen, F.; Qin, N.; Jiang, Z.; Zhou, J.; Jiang, T.; Pu, Z.; Cheng, Y.; Chen, J.; et al. Potentially functional variants in lncRNAs are associated with breast cancer risk in a Chinese population. Mol. Carcinog. 2017, 56, 2048–2057. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Y.; Mayakonda, A.; Madan, V.; Ding, L.-W.; Lin, L.-H.; Zia, S.; Gery, S.; Tyner, J.W.; Zhou, W.; et al. ARID1A and CEBPα cooperatively inhibit UCA1 transcription in breast cancer. Oncogene 2018, 37, 5939–5951. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, Q.; Qiu, J.; Pang, T.; Xian, J.; Zhang, X. Long non-coding RNA UCA1 promotes breast cancer by upregulating PTP1B expression via inhibiting miR-206. Cancer Cell Int. 2019, 19, 275. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-Y.; Wang, W.; Sun, J.-Y.; Xin, B.; Zhang, X.; Wang, T.; Zhang, Q.-F.; Yao, L.-B.; Han, H.; Fan, D.-M.; et al. Long non-coding RNAs AC026904.1 and UCA1: A “one-two punch” for TGF-β-induced SNAI2 activation and epithelial-mesenchymal transition in breast cancer. Theranostics 2018, 8, 2846–2861. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ling, X.; Xia, Y.; Yan, B.; Guan, Q. LncRNA UCA1 promotes SOX12 expression in breast cancer by regulating m6A modification of miR-375 by METTL14 through DNA methylation. Cancer Gene Ther. 2022, 29, 1043–1055. [Google Scholar] [CrossRef]
- Yin, J.-Y.; Zhou, Y.; Ding, X.-M.; Gong, R.-Z.; Zhou, Y.; Hu, H.-Y.; Liu, Y.; Lv, X.-B.; Zhang, B. UCA1 inhibits NKG2D-mediated cytotoxicity of NK cells to breast cancer. Curr. Cancer Drug Targets 2024, 24, 204–219. [Google Scholar] [CrossRef]
- Zhang, C.; Hua, Y.; Zhong, X. LncRNA UCA1 promotes tumorigenesis of breast cancer via targeting MiR-185-5p. Minerva Medica 2020, 113, 889–890. [Google Scholar] [CrossRef] [PubMed]
- Tuo, Y.L.; Li, X.M.; Luo, J. Long noncoding RNA UCA1 modulates breast cancer cell growth and apoptosis through decreasing tumor suppressive miR-143. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3403–3411. [Google Scholar]
- Zhao, H.; Liu, X.; Yu, L.; Lin, S.; Zhang, C.; Xu, H.; Leng, Z.; Huang, W.; Lei, J.; Li, T.; et al. Comprehensive landscape of epigenetic-dysregulated lncRNAs reveals a profound role of enhancers in carcinogenesis in BC subtypes. Mol. Ther. Nucleic Acids 2021, 23, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, H. UCA1 Overexpression Promotes Hypoxic Breast Cancer Cell Proliferation and Inhibits Apoptosis via HIF-1α Activation. J. Oncol. 2021, 2021, 5512156. [Google Scholar] [CrossRef]
- Chen, S.; Shao, C.; Xu, M.; Ji, J.; Xie, Y.; Lei, Y.; Wang, X. Macrophage infiltration promotes invasiveness of breast cancer cells via activating long non-coding RNA UCA1. Int. J. Clin. Exp. Pathol. 2015, 8, 9052–9061. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4583879/ (accessed on 28 January 2024).
- Záveský, L.; Jandáková, E.; Weinberger, V.; Minář, L.; Kohoutová, M.; Slanař, O. Long non-coding RNAs PTENP1, GNG12-AS1, MAGI2-AS3 and MEG3 as tumor suppressors in breast cancer and their associations with clinicopathological parameters. Cancer Biomark. 2024, 1–18. [Google Scholar] [CrossRef]
- Okcanoğlu, B.T.; Kayabaşı, Ç.; Gündüz, C. Effect of CCT137690 on long non-coding RNA expression profiles in MCF-7 and MDA-MB-231 cell lines. Bosn. J. Basic Med. Sci. 2019, 20, 56. [Google Scholar] [CrossRef]
- Mokhtary, P.; Javan, B.; Sharbatkhari, M.; Soltani, A.; Erfani-Moghadam, V. Cationic vesicles for efficient shRNA transfection in the MCF-7 breast cancer cell line. Int. J. Nanomed. 2018, 13, 7107–7121. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, F.; Mokhtari, M.J.; Kalani, M. Quercetin Arrests in G2 phase, Upregulates INXS LncRNA and Downregulates UCA1 LncRNA in MCF-7 Cells. Int. J. Mol. Cell. Med. 2021, 10, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Bai, W.D.; Ye, X.; Yang, A.; Jia, L. Long non-coding RNA UCA1 desensitizes breast cancer cells to trastuzumab by impeding miR-18a repression of Yes-associated protein 1. Biochem. Biophys. Res. Commun. 2018, 496, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Huang, O.; Xie, Z.; Wu, S.; Zhang, X.; Shen, A.; Liu, H.; Chen, X.; Wu, J.; Lou, Y.; et al. A novel long non-coding RNA-ARA: Adriamycin Resistance Associated. Biochem. Pharmacol. 2014, 87, 254–283. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Luo, J. Long Non-Coding RNA (lncRNA) Urothelial Carcinoma-Associated 1 (UCA1) Enhances Tamoxifen Resistance in Breast Cancer Cells via Inhibiting mTOR Signaling Pathway. Med. Sci. Monit. 2016, 22, 3860–3867. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Y.; Liu, A.; Tang, X. Long non-coding RNA UCA1 enhances tamoxifen resistance in breast cancer cells through a miR-18a-HIF1α feedback regulatory loop. Tumor Biol. 2016, 37, 14733–14743. [Google Scholar] [CrossRef]
- Xu, C.G.; Yang, M.F.; Ren, Y.Q.; Wu, C.H.; Wang, L.Q. Exosomes mediated transfer of lncRNA UCA1 results in increased tamoxifen resistance in breast cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4362–4368. Available online: https://pubmed.ncbi.nlm.nih.gov/27831634/ (accessed on 28 January 2024). [PubMed]
- Liu, C.; Jiang, F.; Zhang, X.; Xu, X. Long Non-Coding RNA UCA1 Modulates Paclitaxel Resistance in Breast Cancer via miR-613/CDK12 Axis. Cancer Manag. Res. 2020, 12, 2777–2788. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, N.; Watabe, K.; Lu, Z.; Wu, F.; Xu, M.; Mo, Y.-Y. Long non-coding RNA UCA1 promotes breast tumor growth by suppression of p27 (Kip1). Cell Death Dis. 2014, 5, e1008. [Google Scholar] [CrossRef]
- Wo, L.; Zhang, B.; You, X.; Hu, Y.; Gu, Z.; Zhang, M.; Wang, Q.; Lv, Z.; Zhao, H. Up-regulation of LncRNA UCA1 by TGF-β promotes doxorubicin resistance in breast cancer cells. Immunopharmacol. Immunotoxicol. 2022, 44, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Xiong, Y.; Yue, J.; Xu, D.; Ihira, K.; Konno, Y.; Kobayashi, N.; Todo, Y.; Watari, H. Long noncoding RNA NEAT1 drives aggressive endometrial cancer progression via miR-361-regulated networks involving STAT3 and tumor microenvironment-related genes. J. Exp. Clin. Cancer Res. 2019, 38, 295. [Google Scholar] [CrossRef]
- Liu, S.P.; Yang, J.X.; Cao, D.Y.; Shen, K. Identification of differentially expressed long non-coding RNAs in human ovarian cancer cells with different metastatic potentials. DOAJ Dir. Open Access J. 2013, 10, 138–141. [Google Scholar] [CrossRef]
- Qiu, Y.R.; Zhao, M.Y.; Sun, L.; Yang, B.C.; Hei, K.W.; Du, X.; Li, Y.M. Expression of IncRNA UCA1 in ovarian cancer and its clinical significance. Eur. J. Gynaecol. Oncol. 2017, 38, 191–195. Available online: https://pubmed.ncbi.nlm.nih.gov/29953778/ (accessed on 12 February 2024).
- Lin, X.; Spindler, T.J.; Fonseca, M.A.d.S.; Corona, R.I.; Seo, J.-H.; Dezem, F.S.; Li, L.; Lee, J.M.; Long, H.W.; Sellers, T.A.; et al. Super-Enhancer-Associated LncRNA UCA1 Interacts Directly with AMOT to Activate YAP Target Genes in Epithelial Ovarian Cancer. iScience 2019, 17, 242–255. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, L.-H.; Hong, Y.-N.; Xuan, C.; Yan, S.-L.; Lv, G.-L.; Jiang, Z.-G.; Ding, X.-F. Long Non-coding RNA UCA1 Regulates SRPK1 Expression Through miR- 99b-3p in Ovarian Cancer. Protein Pept. Lett. 2022, 29, 829–838. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, Y.; Wan, Y.; Zhang, L.; Qiu, J.; Zhou, S.; Cheng, W. UCA1 functions as a competing endogenous RNA to suppress epithelial ovarian cancer metastasis. Tumor Biol. 2016, 37, 10633–10641. [Google Scholar] [CrossRef]
- Wang, J.; Ye, C.; Liu, J.; Hu, Y. UCA1 confers paclitaxel resistance to ovarian cancer through miR-129/ABCB1 axis. Biochem. Biophys. Res. Commun. 2018, 501, 1034–1040. [Google Scholar] [CrossRef]
- Horita, K.; Kurosaki, H.; Nakatake, M.; Kuwano, N.; Oishi, T.; Itamochi, H.; Sato, S.; Kono, H.; Ito, M.; Hasegawa, K.; et al. lncRNA UCA1-Mediated Cdc42 Signaling Promotes Oncolytic Vaccinia Virus Cell-to-Cell Spread in Ovarian Cancer. Mol. Ther.-Oncolytics 2019, 13, 35–48. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Wang, X.-L.; Dang, Y.; Zhu, X.-Z.; Zhang, Y.-H.; Cai, B.-X.; Zheng, L. Long non-coding RNA UCA1 promotes the progression of paclitaxel resistance in ovarian cancer by regulating the miR-654-5p/SIK2 axis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 591–603. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, X.; Zhang, L.; Zhang, X.; Sheng, H.; Tao, K. UCA1 overexpression predicts clinical outcome of patients with ovarian cancer receiving adjuvant chemotherapy. Cancer Chemother. Pharmacol. 2016, 77, 629–634. [Google Scholar] [CrossRef]
- Li, Z.; Niu, H.; Qin, Q.; Yang, S.; Wang, Q.; Yu, C.; Wei, Z.; Jin, Z.; Wang, X.; Yang, A.; et al. lncRNA UCA1 Mediates Resistance to Cisplatin by Regulating the miR-143/FOSL2-Signaling Pathway in Ovarian Cancer. Mol. Ther.-Nucleic Acids 2019, 17, 92–101. [Google Scholar] [CrossRef]
- Wambecke, A.; Ahmad, M.; Morice, P.; Lambert, B.; Weiswald, L.; Vernon, M.; Vigneron, N.; Abeilard, E.; Brotin, E.; Figeac, M.; et al. The lncRNA “UCA1” modulates the response to chemotherapy of ovarian cancer through direct binding to miR-27a-5p and control of UBE2N levels. Mol. Oncol. 2021, 15, 3659–3678. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, J.; Xie, X.; Hu, J.; Chen, L.; Hu, Q.; Guo, H.; Yu, C. Involvement of SRPK1 in cisplatin resistance related to long non-coding RNA UCA1 in human ovarian cancer cells. Neoplasma 2015, 62, 432–438. [Google Scholar] [CrossRef]
- Duan, D.M.; Zhang, L.; Hua, F. LncRNA UCA1 inhibits proliferation and promotes apoptosis of cervical cancer cells by regulating β-catenin/TCF-4. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5963–5969. [Google Scholar] [CrossRef]
- Yan, Q.; Tian, Y.; Hao, F. Downregulation of lncRNA UCA1 inhibits proliferation and invasion of cervical cancer cells through miR-206 expression. Oncol. Res. 2018. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, Q.; Ji, M.; Guo, X.; Li, L.; Su, X. Exosomal lncRNA UCA1 modulates cervical cancer stem cell self-renewal and differentiation through microRNA-122-5p/SOX2 axis. J. Transl. Med. 2021, 19, 229. [Google Scholar] [CrossRef]
- He, Q.; Meng, J.; Liu, S.; Zeng, Q.; Zhu, Q.; Wei, Z.; Shao, Y. Long non-coding RNA UCA1 upregulates KIF20A expression to promote cell proliferation and invasion via sponging miR-204 in cervical cancer. Cell Cycle 2020, 19, 2486–2495. [Google Scholar] [CrossRef]
- An, L.; Dong, K.; Chi, S.; Wei, S.; Zhang, J.; Yu, Z.; Zhang, Q.; Zhang, T.; Cheng, S.; Shi, R.; et al. lncRNA UCA1 promotes tumor progression by targeting SMARCD3 in cervical cancer. Mol. Carcinog. 2023, 63, 384–399. [Google Scholar] [CrossRef]
- An, M.; Xing, X.; Chen, T. Long non-coding RNA UCA1 enhances cervical cancer cell proliferation and invasion by regulating microRNA-299-3p expression. Oncol. Lett. 2021, 22, 772. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, D.; Cui, Y.; Shen, G.; Li, Y.; Wei, F. Long non-coding RNA UCA1 modulates the glycolysis of cervical cancer cells by miR-493-5p/HK2. Int. J. Clin. Exp. Pathol. 2018, 11, 3943–3951. [Google Scholar] [PubMed]
- Yang, T.J.; Wang, L.; Zhang, Y.; Zheng, J.D.; Liu, L. LncRNA UCA1 regulates cervical cancer survival and EMT occurrence by targeting miR-155. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9869–9879. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Huang, C.; Li, J.; Gao, T.; Lin, Z.; Yao, T. Long non-coding RNA urothelial cancer associated 1 regulates radioresistance via the hexokinase 2/glycolytic pathway in cervical cancer. Int. J. Mol. Med. 2018, 42, 2247–2259. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).