Decreased Serum Levels of the Insulin Resistance-Related microRNA miR-320a in Patients with Polycystic Ovary Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort and Preanalytics
2.2. RNA Isolation
2.3. cDNA Synthesis and qPCR
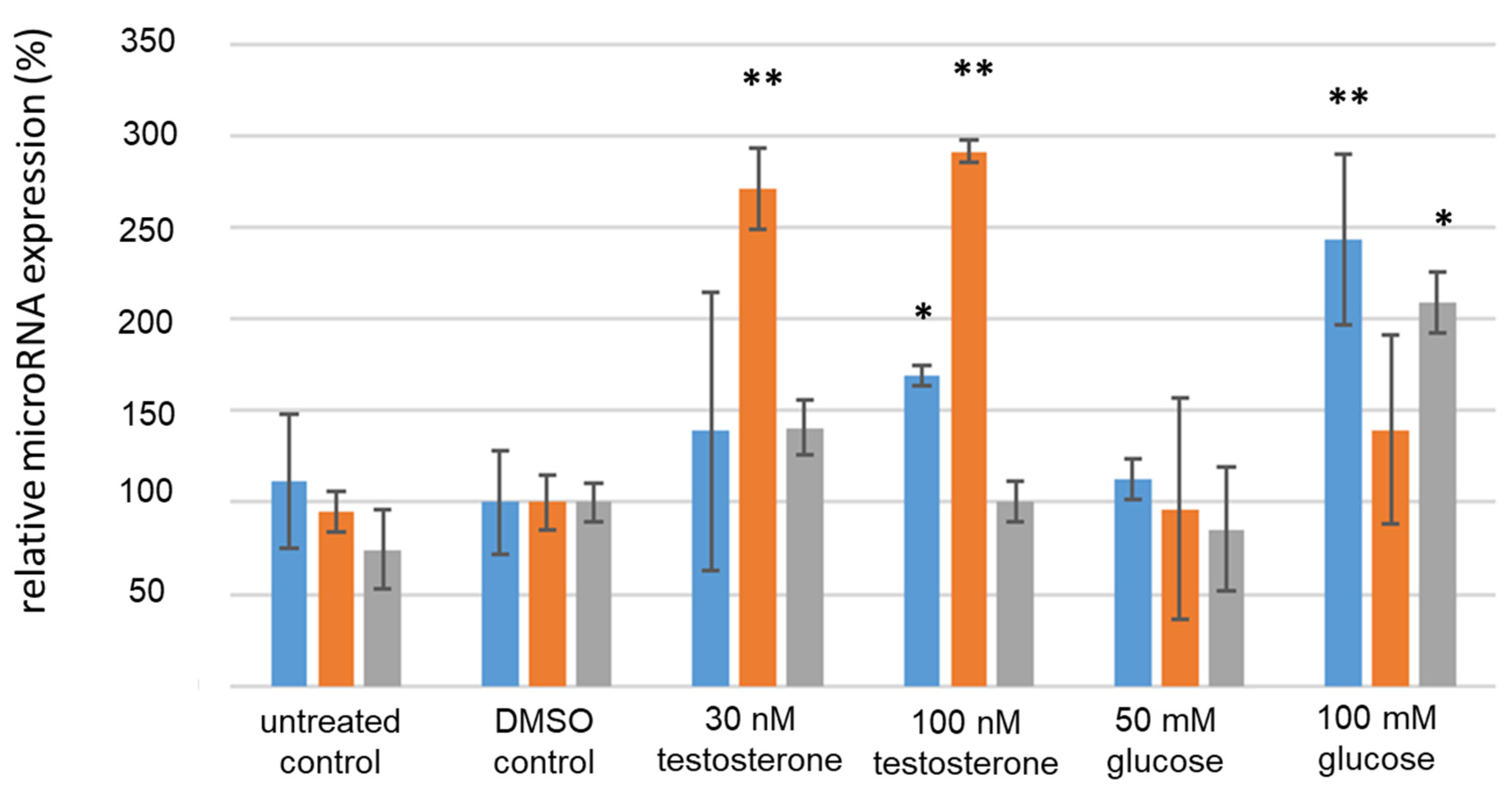
2.4. Cell Culture
2.5. Statistics
3. Results
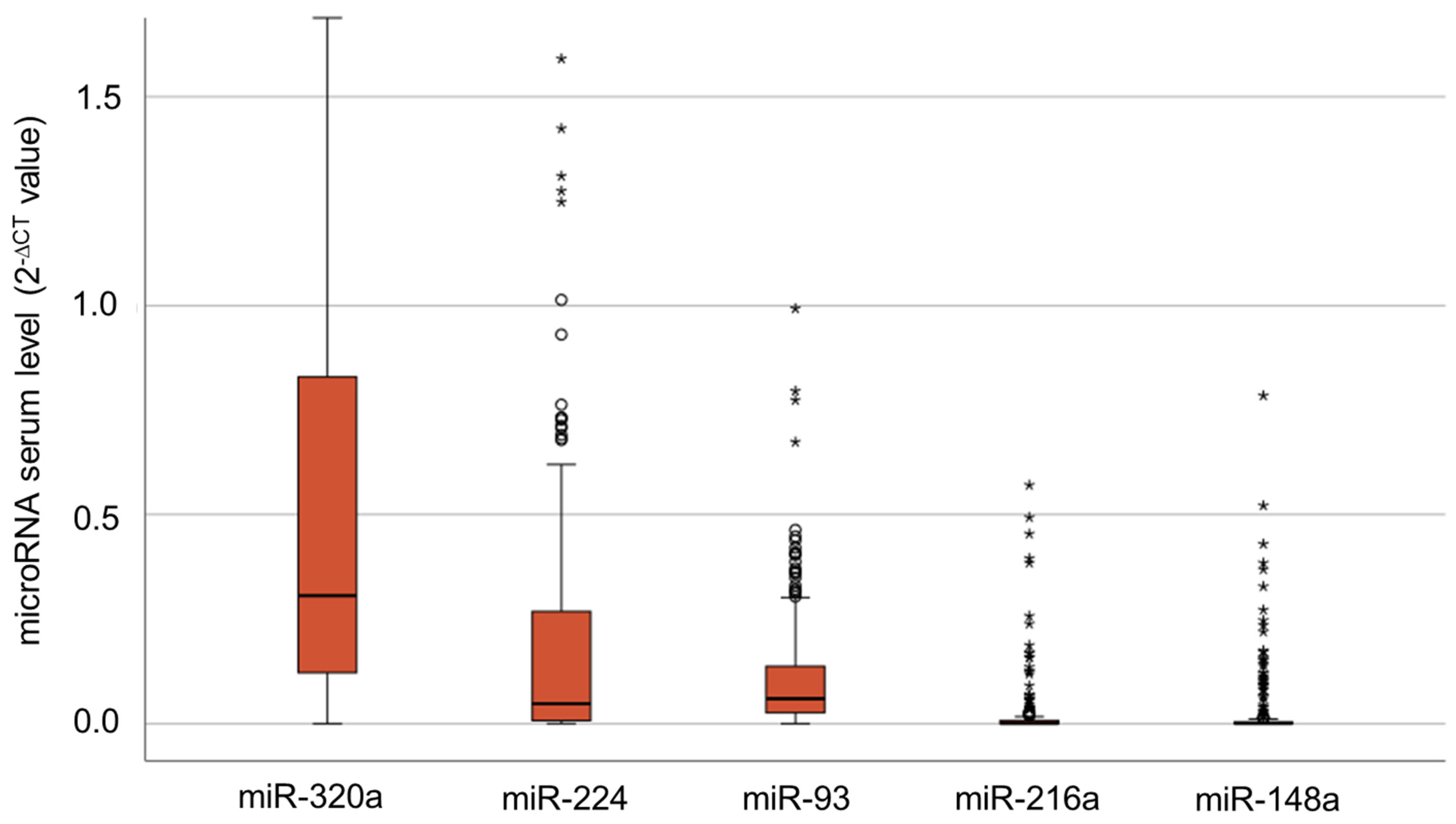
3.1. Serum Levels of IR-Associated microRNAs
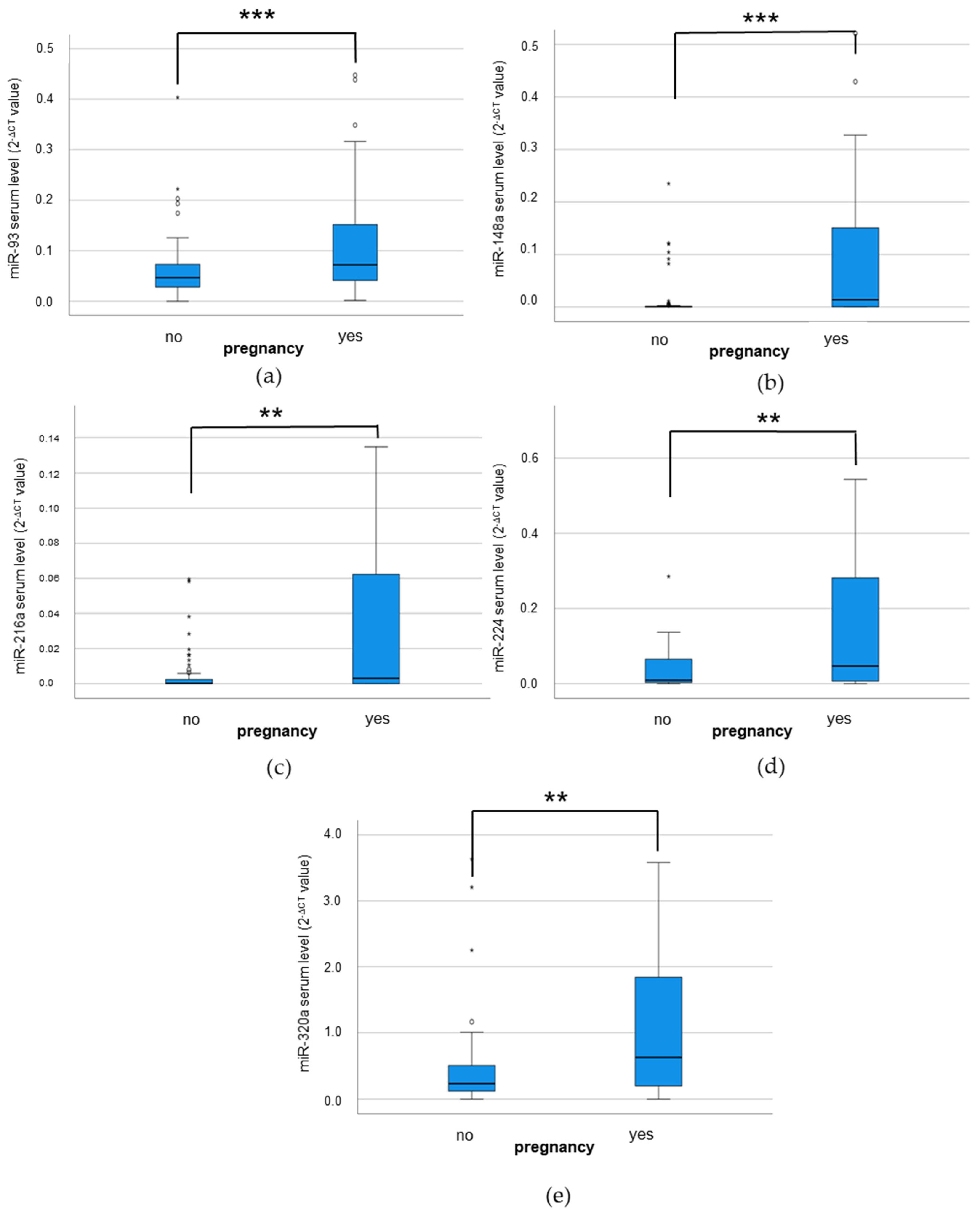
3.2. Correlation Analyses via Bivariate Correlation Analyses
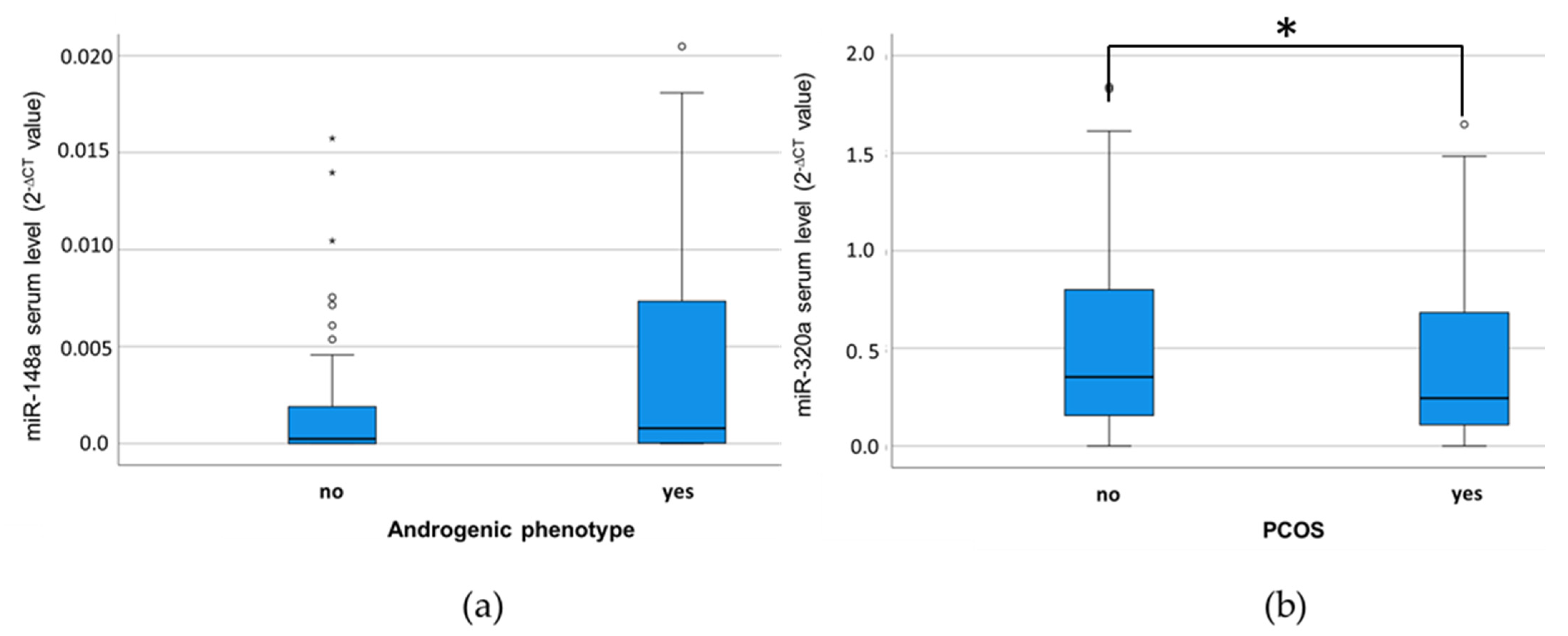
3.3. MiR-320a Serum Levels Are Associated with a PCOS Diagnosis
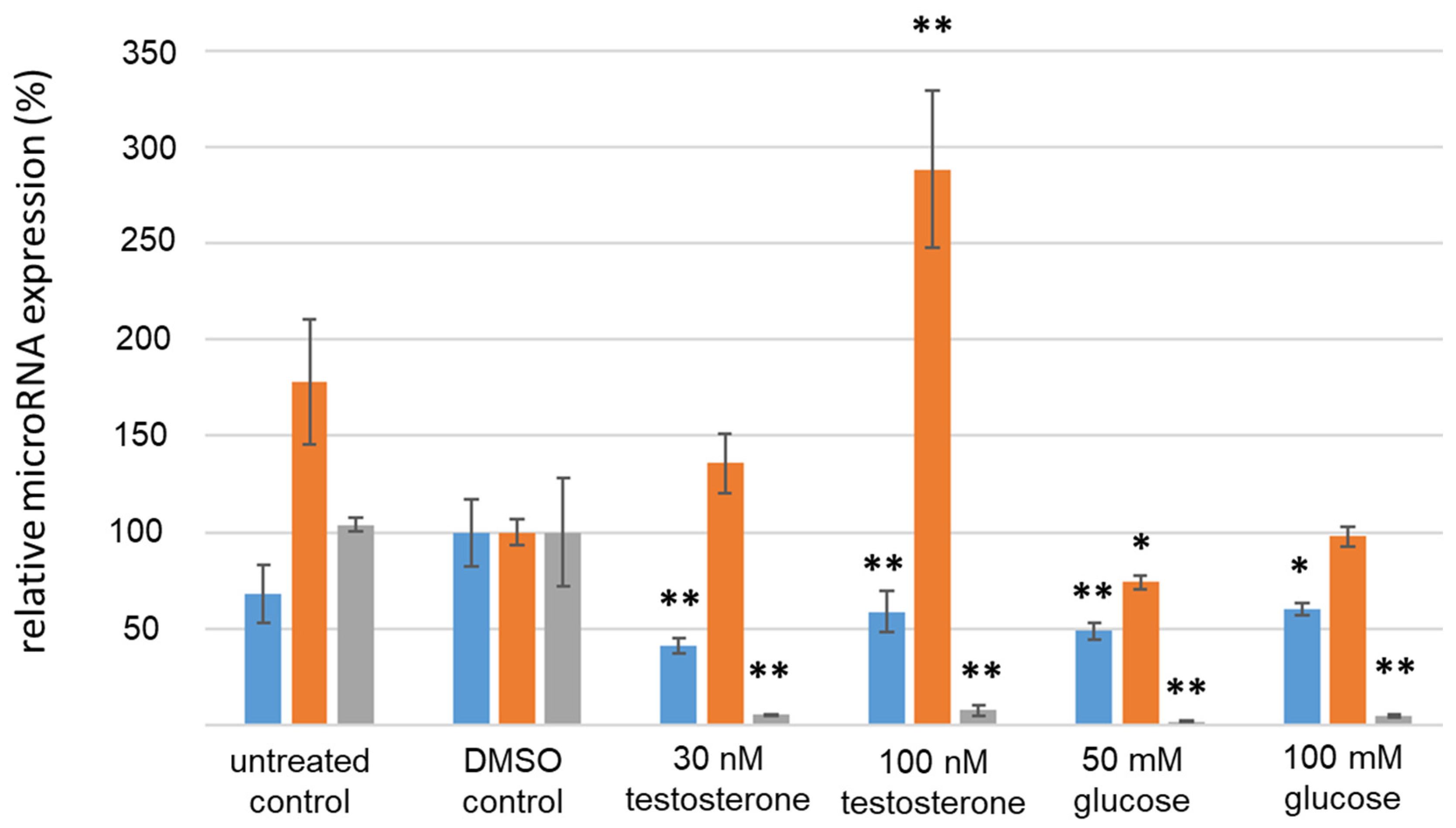
3.4. Analysis of IR-Associated microRNA Expression under Hyperglycemic/Hyperandrogenemic Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Hum. Reprod. 2017, 32, 1786–1801. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.J. Physiological Aspects of Female Fertility: Role of the Environment, Modern Lifestyle, and Genetics. Physiol. Rev. 2016, 96, 873–909. [Google Scholar] [CrossRef] [PubMed]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B.; Johnstone, E.; Dorais, J.; Silver, B.; Peterson, C.M.; Hotaling, J. Female infertility, infertility-associated diagnoses, and comorbidities: A review. J. Assist. Reprod. Genet. 2017, 34, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.; Doherty, D.A. The potential implications of a PCOS diagnosis on a woman’s long-term health using data linkage. J. Clin. Endocrinol. Metab. 2015, 100, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Stepto, N.K.; Cassar, S.; Joham, A.E.; Hutchison, S.K.; Harrison, C.L.; Goldstein, R.F.; Teede, H.J. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum. Reprod. 2013, 28, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Panico, A.; Messina, G.; Lupoli, G.A.; Lupoli, R.; Cacciapuoti, M.; Moscatelli, F.; Esposito, T.; Villano, I.; Valenzano, A.; Monda, V.; et al. Quality of life in overweight (obese) and normal-weight women with polycystic ovary syndrome. Patient Prefer. Adherence 2017, 11, 423–429. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alur-Gupta, S.; Chemerinski, A.; Liu, C.; Lipson, J.; Allison, K.; Sammel, M.D.; Dokras, A. Body-image distress is increased in women with polycystic ovary syndrome and mediates depression and anxiety. Fertil. Steril. 2019, 112, 930–938.e1. [Google Scholar] [CrossRef]
- Shishehgar, F.; Tehrani, F.R.; Vahidi, S. The effects of weight loss on health-related quality of life in obese women with PCOS and controls. BMC Womens Health 2023, 23, 532. [Google Scholar] [CrossRef]
- Blauschmidt, S.; Greither, T.; Lampe, K.; Köller, S.; Kaltwaßer, P.; Behre, H.M. Dipeptidyl peptidase 4 serum activity and concentration are increased in women with polycystic ovary syndrome. Clin. Endocrinol. 2017, 87, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, B.; Gürbüz, A.S.; Durak, Z.E.; Öztürk, H.S. Dipeptidyl peptidase-4 and adenosine deaminase enzyme levels in polycystic ovary syndrome. Gynecol. Endocrinol. 2019, 35, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Braga, L.D.D.C.; Godoy-Matos, A.F.; Siciliano, P.O.; Corrêa, J.O.D.A.; Carvalho, D.P. Is DPP4 activity increased in PCOS? Diabetes Metab. Syndr. 2018, 12, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, S.; Eroglu Altinova, A.; Elgun, S.; Yalcin, M.M.; Aktas Yilmaz, B.; Ozkan, C.; Akturk, M.; Balos Toruner, F. Serum activities of dipeptidyl peptidase-4 and adenosine deaminase in polycystic ovary syndrome: Association with obesity. Gynecol. Endocrinol. 2019, 35, 714–718. [Google Scholar] [CrossRef]
- Luo, Y.; Cui, C.; Han, X.; Wang, Q.; Zhang, C. The role of miRNAs in polycystic ovary syndrome with insulin resistance. J. Assist. Reprod. Genet. 2021, 38, 289–304. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef]
- Cortez, M.A.; Bueso-Ramos, C.; Ferdin, J.; Lopez-Berestein, G.; Sood, A.K.; Calin, G.A. MicroRNAs in body fluids—The mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011, 8, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xu, P.; Wang, J.; Zhang, C. The role of MiRNA in polycystic ovary syndrome (PCOS). Gene 2019, 706, 91–96. [Google Scholar] [CrossRef]
- Vitale, S.G.; Fulghesu, A.M.; Mikuš, M.; Watrowski, R.; D’Alterio, M.N.; Lin, L.-T.; Shah, M.; Reyes-Muñoz, E.; Sathyapalan, T.; Angioni, S. The Translational Role of miRNA in Polycystic Ovary Syndrome: From Bench to Bedside-A Systematic Literature Review. Biomedicines 2022, 10, 1816. [Google Scholar] [CrossRef]
- Cioffi, M.; Vallespinos-Serrano, M.; Trabulo, S.M.; Fernandez-Marcos, P.J.; Firment, A.N.; Vazquez, B.N.; Vieira, C.R.; Mulero, F.; Camara, J.A.; Cronin, U.P.; et al. MiR-93 Controls Adiposity via Inhibition of Sirt7 and Tbx3. Cell Rep. 2015, 12, 1594–1605. [Google Scholar] [CrossRef]
- Nunez Lopez, Y.O.; Garufi, G.; Seyhan, A.A. Altered levels of circulating cytokines and microRNAs in lean and obese individuals with prediabetes and type 2 diabetes. Mol. Biosyst. 2016, 13, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Vonhögen, I.G.C.; Mohseni, Z.; Winkens, B.; Xiao, K.; Thum, T.; Calore, M.; da Costa Martins, P.A.; de Windt, L.J.; Spaanderman, M.E.A.; Ghossein-Doha, C. Circulating miR-216a as a biomarker of metabolic alterations and obesity in women. Noncoding RNA Res. 2020, 5, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.H.; Ahn, J.; Um, M.Y.; Jung, C.H.; Jung, S.E.; Ha, T.Y. Circulating microRNA expression profiling in young obese Korean women. Nutr. Res. Pract. 2020, 14, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, F.; Catellani, C.; Lazzeroni, P.; Sartori, C.; Nicoli, A.; Amarri, S.; La Sala, G.B.; Street, M.E. MiRNAs Regulating Insulin Sensitivity Are Dysregulated in Polycystic Ovary Syndrome (PCOS) Ovaries and Are Associated with Markers of Inflammation and Insulin Sensitivity. Front. Endocrinol. 2019, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Bai, Z.; Han, W.; Zhang, J.; Meng, H.; Bi, J.; Ma, X.; Han, S.; Zhang, Z. Identification of suitable reference genes for qPCR analysis of serum microRNA in gastric cancer patients. Dig. Dis. Sci. 2012, 57, 897–904. [Google Scholar] [CrossRef]
- Xiang, M.; Zeng, Y.; Yang, R.; Xu, H.; Chen, Z.; Zhong, J.; Xie, H.; Xu, Y.; Zeng, X. U6 is not a suitable endogenous control for the quantification of circulating microRNAs. Biochem. Biophys. Res. Commun. 2014, 454, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pratama, M.Y.; Pascut, D.; Tamini, S.; Minocci, A.; Tiribelli, C.; Grugni, G.; Sartorio, A. Circulating microRNA Associated to Different Stages of Liver Steatosis in Prader-Willi Syndrome and Non-Syndromic Obesity. J. Clin. Med. 2020, 9, 1123. [Google Scholar] [CrossRef]
- Agostini, S.; Mancuso, R.; Citterio, L.A.; Mihali, G.A.; Arosio, B.; Clerici, M. Evaluation of serum miRNAs expression in frail and robust subjects undergoing multicomponent exercise protocol (VIVIFRAIL). J. Transl. Med. 2023, 21, 67. [Google Scholar] [CrossRef]
- Barber, J.L.; Zellars, K.N.; Barringhaus, K.G.; Bouchard, C.; Spinale, F.G.; Sarzynski, M.A. The Effects of Regular Exercise on Circulating Cardiovascular-related MicroRNAs. Sci. Rep. 2019, 9, 7527. [Google Scholar] [CrossRef]
- Sathyapalan, T.; David, R.; Gooderham, N.J.; Atkin, S.L. Increased expression of circulating miRNA-93 in women with polycystic ovary syndrome may represent a novel, non-invasive biomarker for diagnosis. Sci. Rep. 2015, 5, 16890. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Heneidi, S.; Lee, J.-M.; Layman, L.C.; Stepp, D.W.; Gamboa, G.M.; Chen, B.-S.; Chazenbalk, G.; Azziz, R. miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes 2013, 62, 2278–2286. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, J.; Li, L.; Chen, Y.; Chen, X.; Zhao, X.; Yang, D. MicroRNA-93 promotes ovarian granulosa cells proliferation through targeting CDKN1A in polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 2015, 100, E729–E738. [Google Scholar] [CrossRef] [PubMed]
- Monfared, Y.K.; Honardoost, M.; Cea, M.; Gholami, S.; Mirzaei-Dizgah, I.; Hashemipour, S.; Sarookhani, M.R.; Farzam, S.A. Circulating salivary and serum miRNA-182, 320a, 375 and 503 expression levels in type 2 diabetes. J. Diabetes Metab. Disord. 2022, 21, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Villard, A.; Marchand, L.; Thivolet, C.; Rome, S. Diagnostic Value of Cell-free Circulating MicroRNAs for Obesity and Type 2 Diabetes: A Meta-analysis. J. Mol. Biomark. Diagn. 2015, 6, 251. [Google Scholar] [CrossRef]
- Flowers, E.; Aouizerat, B.E.; Abbasi, F.; Lamendola, C.; Grove, K.M.; Fukuoka, Y.; Reaven, G.M. Circulating microRNA-320a and microRNA-486 predict thiazolidinedione response: Moving towards precision health for diabetes prevention. Metabolism 2015, 64, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Wang, H.; Yan, C.-Y.; Gao, X.-F.; Ling, X.-J. Deregulation of RUNX2 by miR-320a deficiency impairs steroidogenesis in cumulus granulosa cells from polycystic ovary syndrome (PCOS) patients. Biochem. Biophys. Res. Commun. 2017, 482, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, Y.; Wang, X.; Liu, Y.; Yang, L. MicroRNA-320a inhibition decreases insulin-induced KGN cell proliferation and apoptosis by targeting PCGF1. Mol. Med. Rep. 2017, 16, 5706–5712. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.-Y.; Ou, H.-S.; Feng, S.-D.; Zhang, X.-Y.; Tuo, Q.-H.; Chen, L.-X.; Zhu, B.-Y.; Gao, Z.-P.; Tang, C.-K.; Yin, W.-D.; et al. CHANGES IN microRNA (miR) profile and effects of miR-320 in insulin-resistant 3T3-L1 adipocytes. Clin. Exp. Pharmacol. Physiol. 2009, 36, e32–e39. [Google Scholar] [CrossRef]
- Lendvai, G.; Jármay, K.; Karácsony, G.; Halász, T.; Kovalszky, I.; Baghy, K.; Wittmann, T.; Schaff, Z.; Kiss, A. Elevated miR-33a and miR-224 in steatotic chronic hepatitis C liver biopsies. World J. Gastroenterol. 2014, 20, 15343–15350. [Google Scholar] [CrossRef]
- Bacon, S.; Engelbrecht, B.; Schmid, J.; Pfeiffer, S.; Gallagher, R.; McCarthy, A.; Burke, M.; Concannon, C.; Prehn, J.H.M.; Byrne, M.M. MicroRNA-224 is Readily Detectable in Urine of Individuals with Diabetes Mellitus and is a Potential Indicator of Beta-Cell Demise. Genes 2015, 6, 399–416. [Google Scholar] [CrossRef]
- Vahdat-Lasemi, M.; Hosseini, S.; Jajarmi, V.; Kazemi, B.; Salehi, M. Intraovarian injection of miR-224 as a marker of polycystic ovarian syndrome declines oocyte competency and embryo development. J. Cell. Physiol. 2019, 234, 13858–13866. [Google Scholar] [CrossRef]
- Grieco, G.E.; Besharat, Z.M.; Licata, G.; Fignani, D.; Brusco, N.; Nigi, L.; Formichi, C.; Po, A.; Sabato, C.; Dardano, A.; et al. Circulating microRNAs as clinically useful biomarkers for Type 2 Diabetes Mellitus: miRNomics from bench to bedside. Transl. Res. 2022, 247, 137–157. [Google Scholar] [CrossRef]
- Florijn, B.W.; Duijs, J.M.G.J.; Klaver, M.; Kuipers, E.N.; Kooijman, S.; Prins, J.; Zhang, H.; Sips, H.C.M.; Stam, W.; Hanegraaf, M.; et al. Estradiol-driven metabolism in transwomen associates with reduced circulating extracellular vesicle microRNA-224/452. Eur. J. Endocrinol. 2021, 185, 539–552. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, L.; Yu, Q.; Zhang, Y.; Yan, L.; Chen, Z.-J. The estrogen-regulated lncRNA H19/miR-216a-5p axis alters stromal cell invasion and migration via ACTA2 in endometriosis. Mol. Hum. Reprod. 2019, 25, 550–561. [Google Scholar] [CrossRef]
- Guan, G.-Y.; Wei, N.; Song, T.; Zhao, C.; Sun, Y.; Pan, R.-X.; Zhang, L.-L.; Xu, Y.-Y.; Dai, Y.-M.; Han, H. miR-448-3p alleviates diabetic vascular dysfunction by inhibiting endothelial-mesenchymal transition through DPP-4 dysregulation. J. Cell. Physiol. 2020, 235, 10024–10036. [Google Scholar] [CrossRef]
- Tang, M.; Wang, Q.; Wang, K.; Wang, F. Mesenchymal stem cells-originated exosomal microRNA-152 impairs proliferation, invasion and migration of thyroid carcinoma cells by interacting with DPP4. J. Endocrinol. Investig. 2020, 43, 1787–1796. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, D.; Zhang, Y.; Wang, J.; Liu, L.; Zhao, Y. miR-124-3p relieves allergic rhinitis by inhibiting dipeptidyl peptidase-4. Int. Immunopharmacol. 2021, 101, 108279. [Google Scholar] [CrossRef]
- Rashad, N.M.; Ateya, M.A.-M.; Saraya, Y.S.; Elnagar, W.M.; Helal, K.F.; Lashin, M.E.-B.; Abdelrhman, A.A.; Alil, A.E.; Yousef, M.S. Association of miRNA—320 expression level and its target gene endothelin-1 with the susceptibility and clinical features of polycystic ovary syndrome. J. Ovarian Res. 2019, 12, 39. [Google Scholar] [CrossRef]
- Long, W.; Zhao, C.; Ji, C.; Ding, H.; Cui, Y.; Guo, X.; Shen, R.; Liu, J. Characterization of serum microRNAs profile of PCOS and identification of novel non-invasive biomarkers. Cell. Physiol. Biochem. 2014, 33, 1304–1315. [Google Scholar] [CrossRef]
- Soyman, Z.; Durmus, S.; Ates, S.; Simsek, G.; Sozer, V.; Kundaktepe, B.P.; Kurtulus, D.; Gelisgen, R.; Sal, V.; Uzun, H. Circulating mir-132, mir-146a, mir-222, and mir-320 expression in differential diagnosis of women with polycystic ovary syndrome. Acta Endocrinol. (Buchar.) 2022, 18, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Deswal, R.; Dang, A.S. Dissecting the role of micro-RNAs as a diagnostic marker for polycystic ovary syndrome: A systematic review and meta-analysis. Fertil. Steril. 2020, 113, 661–669.e2. [Google Scholar] [CrossRef]
- Krentowska, A.; Ponikwicka-Tyszko, D.; Łebkowska, A.; Adamska, A.; Sztachelska, M.; Milewska, G.; Hryniewicka, J.; Wołczyński, S.; Kowalska, I. Serum expression levels of selected microRNAs and their association with glucose metabolism in young women with polycystic ovary syndrome. Pol. Arch. Intern. Med. 2024, 134, 16637. [Google Scholar] [CrossRef]
- Scalici, E.; Traver, S.; Mullet, T.; Molinari, N.; Ferrières, A.; Brunet, C.; Belloc, S.; Hamamah, S. Circulating microRNAs in follicular fluid, powerful tools to explore in vitro fertilization process. Sci. Rep. 2016, 6, 24976. [Google Scholar] [CrossRef] [PubMed]
- Sang, Q.; Yao, Z.; Wang, H.; Feng, R.; Wang, H.; Zhao, X.; Xing, Q.; Jin, L.; He, L.; Wu, L.; et al. Identification of microRNAs in human follicular fluid: Characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J. Clin. Endocrinol. Metab. 2013, 98, 3068–3079. [Google Scholar] [CrossRef]
- Yuan, D.; Luo, J.; Sun, Y.; Hao, L.; Zheng, J.; Yang, Z. PCOS follicular fluid derived exosomal miR-424-5p induces granulosa cells senescence by targeting CDCA4 expression. Cell. Signal. 2021, 85, 110030. [Google Scholar] [CrossRef]
- Song, P.; Chen, X.; Zhang, P.; Zhou, Y.; Zhou, R. miR-200b/MYBL2/CDK1 suppresses proliferation and induces senescence through cell cycle arrest in ovine granulosa cells. Theriogenology 2023, 207, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Zhang, M.; Zhao, S.; Lu, M.; Lin, L.; Chen, L.; Gao, W.; Li, W.; Shang, J.; Zhou, J.; et al. EIF4A3-Induced Exosomal circLRRC8A Alleviates Granulosa Cells Senescence Via the miR-125a-3p/NFE2L1 axis. Stem Cell Rev. Rep. 2023, 19, 1994–2012. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Takayama, K.; Katayama, S.; Urano, T.; Horie-Inoue, K.; Ikeda, K.; Takahashi, S.; Kawazu, C.; Hasegawa, A.; Ouchi, Y.; et al. miR-148a is an androgen-responsive microRNA that promotes LNCaP prostate cell growth by repressing its target CAND1 expression. Prostate Cancer Prostatic Dis. 2010, 13, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Jalava, S.E.; Urbanucci, A.; Latonen, L.; Waltering, K.K.; Sahu, B.; Jänne, O.A.; Seppälä, J.; Lähdesmäki, H.; Tammela, T.L.J.; Visakorpi, T. Androgen-regulated miR-32 targets BTG2 and is overexpressed in castration-resistant prostate cancer. Oncogene 2012, 31, 4460–4471. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Liu, C.; Mu, X.; Cheng, S. Hyperglycemia inhibition of endothelial miR-140-3p mediates angiogenic dysfunction in diabetes mellitus. J. Diabetes Complicat. 2019, 33, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Świderska, E.; Podolska, M.; Strycharz, J.; Szwed, M.; Abramczyk, H.; Brożek-Płuska, B.; Wróblewski, A.; Szemraj, J.; Majsterek, I.; Drzewoski, J.; et al. Hyperglycemia Changes Expression of Key Adipogenesis Markers (C/EBPα and PPARᵞ)and Morphology of Differentiating Human Visceral Adipocytes. Nutrients 2019, 11, 1835. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Xu, G.; Jing, G.; Chen, J.; Shalev, A. Human Glucagon Expression Is under the Control of miR-320a. Endocrinology 2021, 162, bqaa238. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Katsushima, K.; Shinjo, K.; Hatanaka, A.; Ohka, F.; Suzuki, S.; Naiki-Ito, A.; Soga, N.; Takahashi, S.; Kondo, Y. Histone Deacetylase Inhibition in Prostate Cancer Triggers miR-320-Mediated Suppression of the Androgen Receptor. Cancer Res. 2016, 76, 4192–4204. [Google Scholar] [CrossRef] [PubMed]

| Total Cohort (n = 358 1,2) Median (Range) | Non-PCOS Subgroup (n = 214) Median (Range) | PCOS Subgroup (n = 136) Median (Range) | p (Student’s t-Test) | |
|---|---|---|---|---|
| Age (years) | 32 (19–46) | 33 (20–46) | 30 (19–40) | <0.001 |
| BMI | 23.5 (17.0–47.8) | 22.9 (17.0–44.6) | 25.3 (17.4–47.8) | <0.001 |
| LH (IU/L) | 5.06 (0.23–59.9) | 7.94 (0.23–68.5) | 11.6 (3.75–59.9) | 0.14 |
| FSH (IU/L) | 6.07 (1.17–108.0) | 6.46 (1.74–108.0) | 5.39 (1.17–12.1) | 0.03 |
| LH:FSH | 1.57 (0.38–5.91) | 1.07 (0.38–3.75) | 2.20 (0.83–5.91) | 0.15 |
| Estradiol (pmol/L) | 1221.5 (18.4–7465) | 233 (18.4–1776) | 194 (73.96–7465) | 0.08 |
| Prolactin (mIU/L) | 245.0 (107–543) | 269 (107–543) | 221 (116–396) | 0.05 |
| HOMA-IR | 1.79 (0.28–18.19) | 1.38 (0.28–13.83) | 2.47 (0.41–18.19) | <0.001 |
| Total n (Percentage) | Non-PCOS n (Percentage) | PCOS n (Percentage) | p (ChiSquare Test) | |
| hyperandrogenism | 97 (27.7%) | 14 (6.5%) | 83 (61.0%) | <0.001 |
| dysmenorrhoe | 104 (29.7%) | 18 (8.4%) | 86 (63.2%) | <0.001 |
| polycystic ovaries in ultrasound | 78 (22.3%) | 5 (2.3%) | 73 (53.7%) | <0.001 |
| MicroRNA | Correlation with | rs | p | N |
|---|---|---|---|---|
| miR-93 | DPP4_concS 1 | −0.281 | <0.001 | 197 |
| miR-320a | 0.519 | <0.001 | 347 | |
| miR-216 | 0.277 | <0.001 | 285 | |
| miR-224 | 0.243 | <0.001 | 335 | |
| miR-320a | miR-186 | 0.402 | <0.001 | 192 |
| miR-93 | 0.519 | <0.001 | 347 | |
| miR-216a | 0.251 | <0.001 | 285 | |
| miR-224 | 0.425 | <0.001 | 335 | |
| miR-216a | Estradiol | 0.271 | <0.001 | 186 |
| DPP4_act 2 | −0.256 | 0.002 | 151 | |
| DPP4_concS 1 | −0.303 | <0.001 | 147 | |
| miR-148a | 0.274 | <0.001 | 220 | |
| miR-224 | Estradiol | −0.277 | <0.001 | 214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogt, S.; Handke, D.; Behre, H.M.; Greither, T. Decreased Serum Levels of the Insulin Resistance-Related microRNA miR-320a in Patients with Polycystic Ovary Syndrome. Curr. Issues Mol. Biol. 2024, 46, 3379-3393. https://doi.org/10.3390/cimb46040212
Vogt S, Handke D, Behre HM, Greither T. Decreased Serum Levels of the Insulin Resistance-Related microRNA miR-320a in Patients with Polycystic Ovary Syndrome. Current Issues in Molecular Biology. 2024; 46(4):3379-3393. https://doi.org/10.3390/cimb46040212
Chicago/Turabian StyleVogt, Sarina, Diana Handke, Hermann M. Behre, and Thomas Greither. 2024. "Decreased Serum Levels of the Insulin Resistance-Related microRNA miR-320a in Patients with Polycystic Ovary Syndrome" Current Issues in Molecular Biology 46, no. 4: 3379-3393. https://doi.org/10.3390/cimb46040212
APA StyleVogt, S., Handke, D., Behre, H. M., & Greither, T. (2024). Decreased Serum Levels of the Insulin Resistance-Related microRNA miR-320a in Patients with Polycystic Ovary Syndrome. Current Issues in Molecular Biology, 46(4), 3379-3393. https://doi.org/10.3390/cimb46040212






