Dietary Acrylamide Induces Depression via SIRT3-Mediated Mitochondrial Oxidative Injury: Evidence from Multi-Omics and Mendelian Randomization
Abstract
1. Introduction
2. Materials and Methods
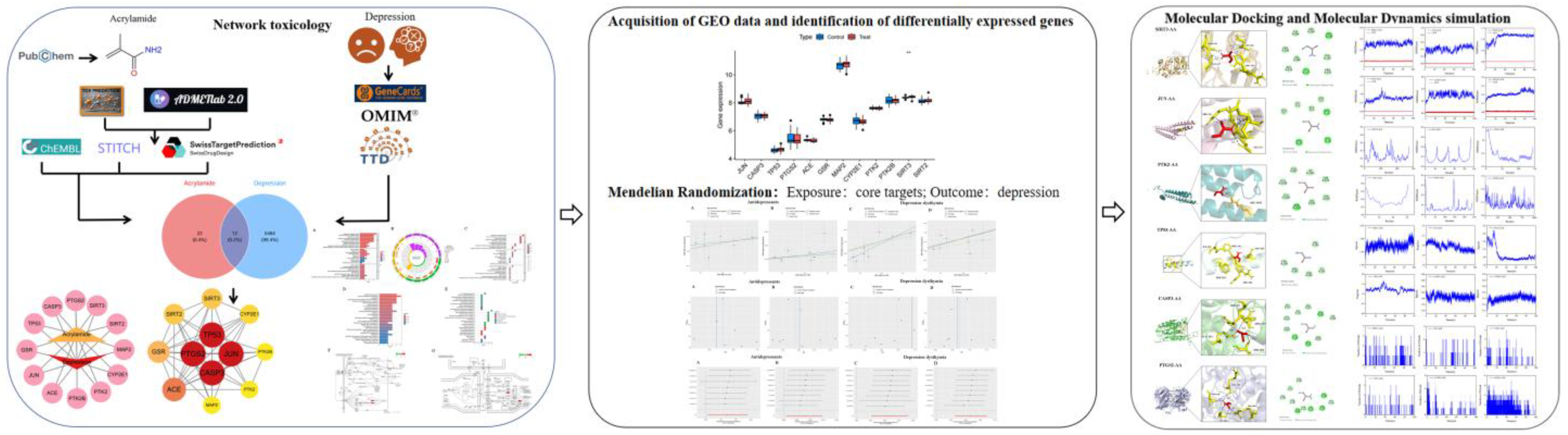
2.1. Target Construction and Toxicity of ACR
2.2. Identification of Putative Protein Targets for ACR
2.3. Collection of Depression-Related Genes
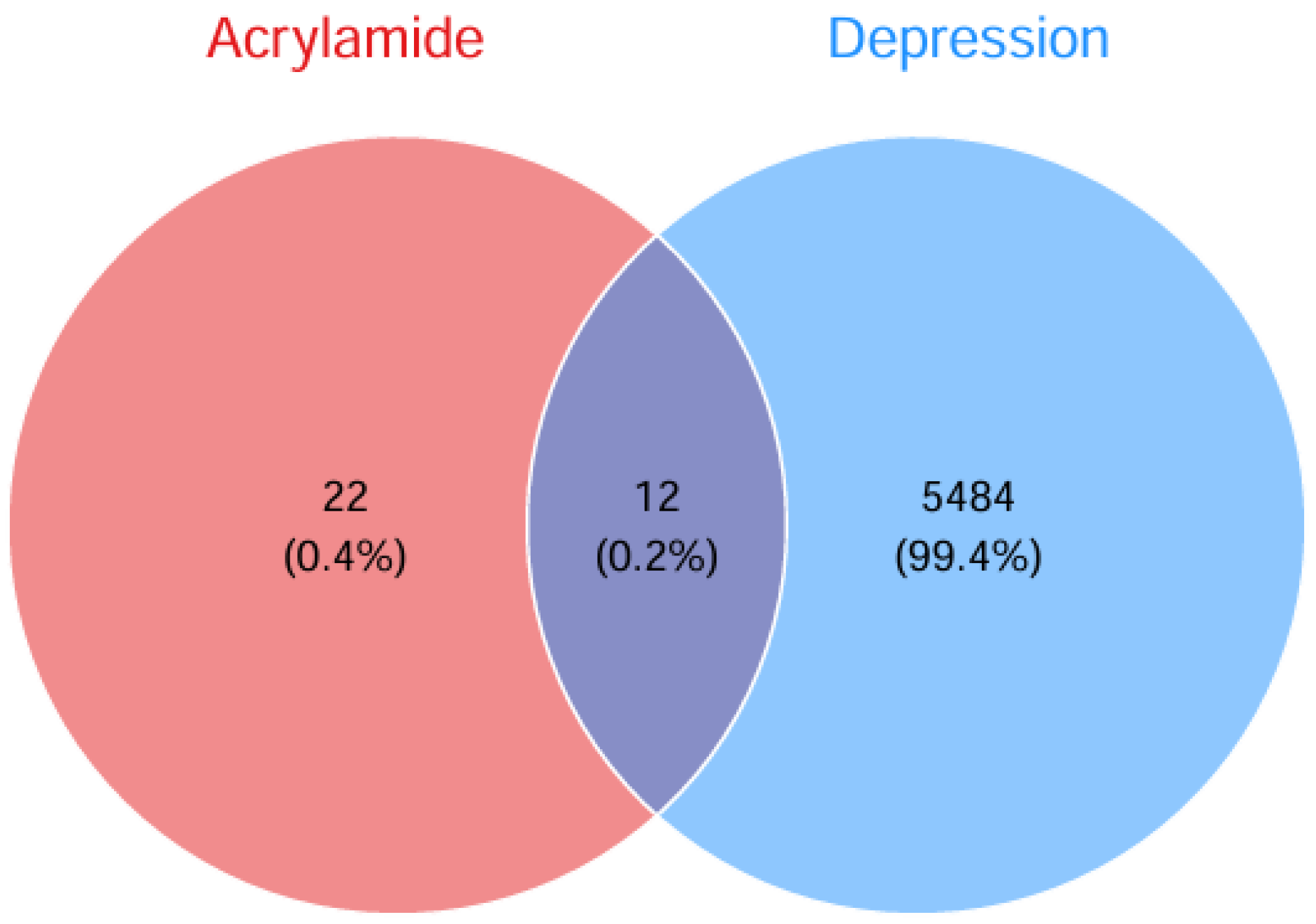
2.4. Identification of Shared Targets Between ACR and Depression
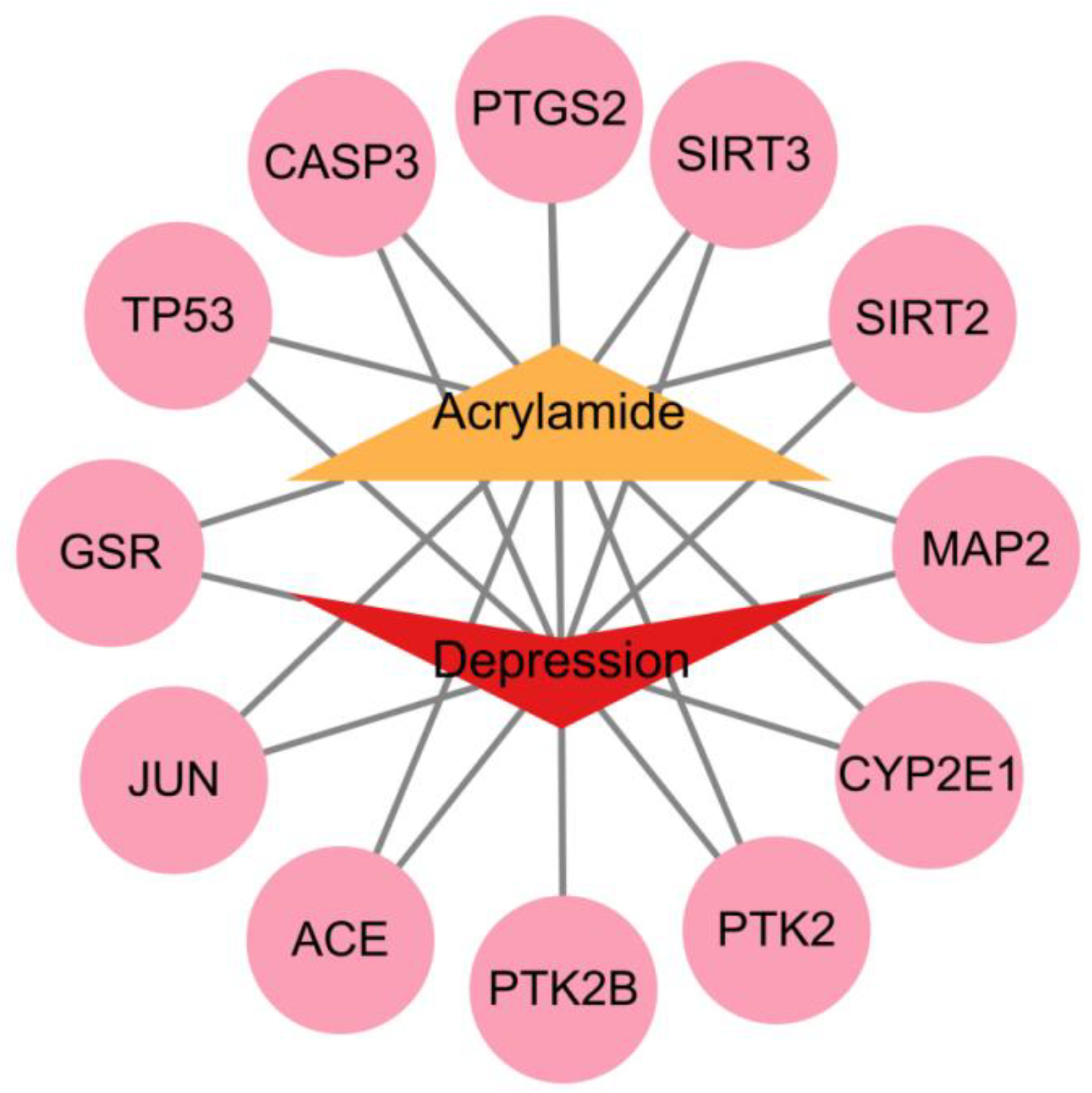
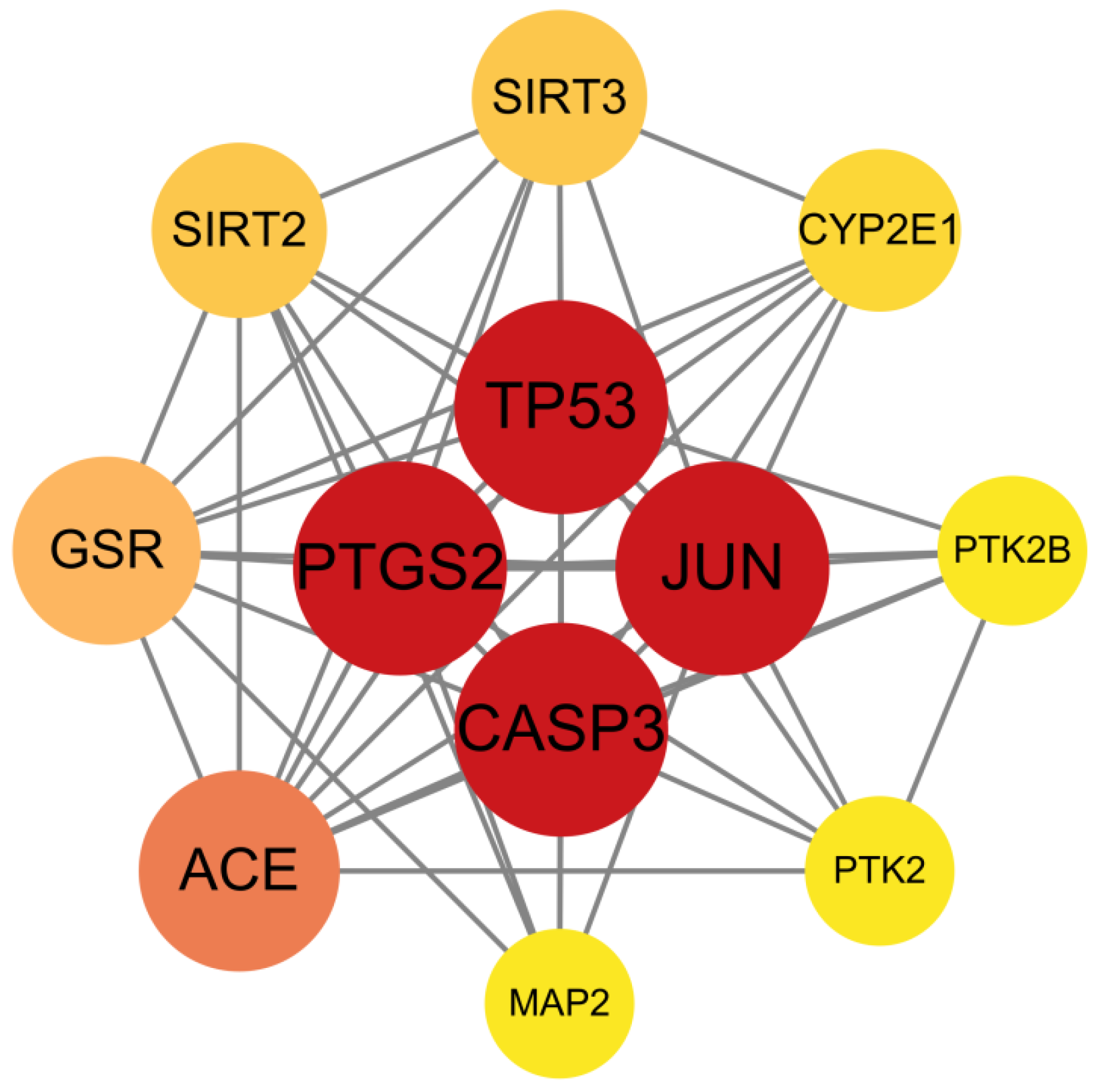
2.5. ACR-Depression Core Target Identification and PPI Network Construction
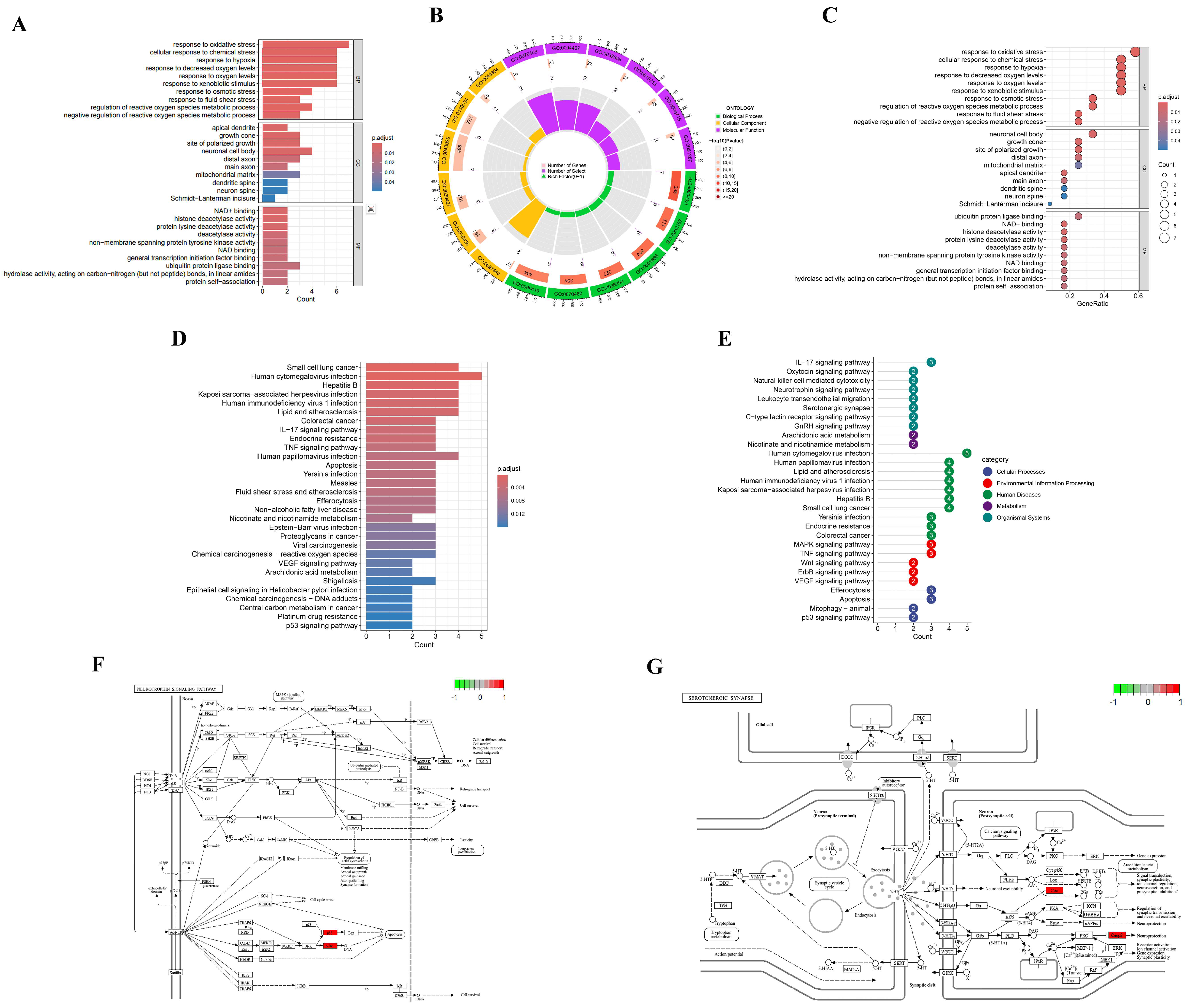
2.6. GO and KEGG Pathway Enrichment Analyses
2.7. Molecular Docking
2.8. Molecular Dynamics Simulation
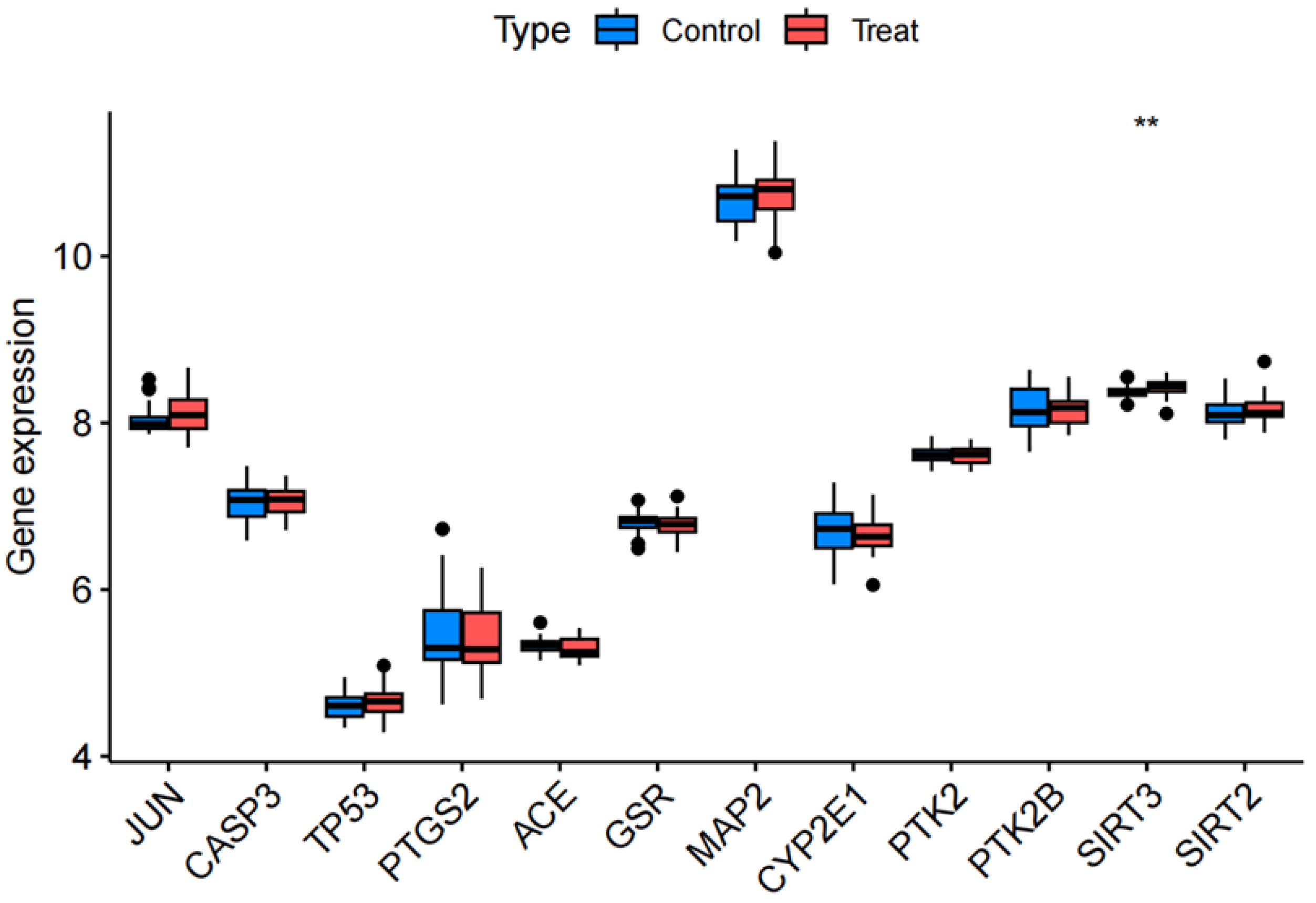
2.9. Random Sample Gene Expression Validation
2.10. GWAS Summary Statistics of Depression and Core Protein
2.11. Target-Specific Molecular Docking and Dynamics for SIRT3 and PTK2
3. Results
3.1. In Silico Evaluation of ACR Toxicity and ADMET Properties
3.2. Network Toxicology Analysis of ACR Against Depression-Associated Targets
3.3. Screening of Key Targets and Construction and Analysis of the Protein–Protein Interaction
3.4. GO and KEGG Pathway Enrichment Analysis
3.5. Randomized Dataset Validation of Core Genes
3.6. Investigating the Causal Relationship Between ACR with Depression Using Mendelian Randomization
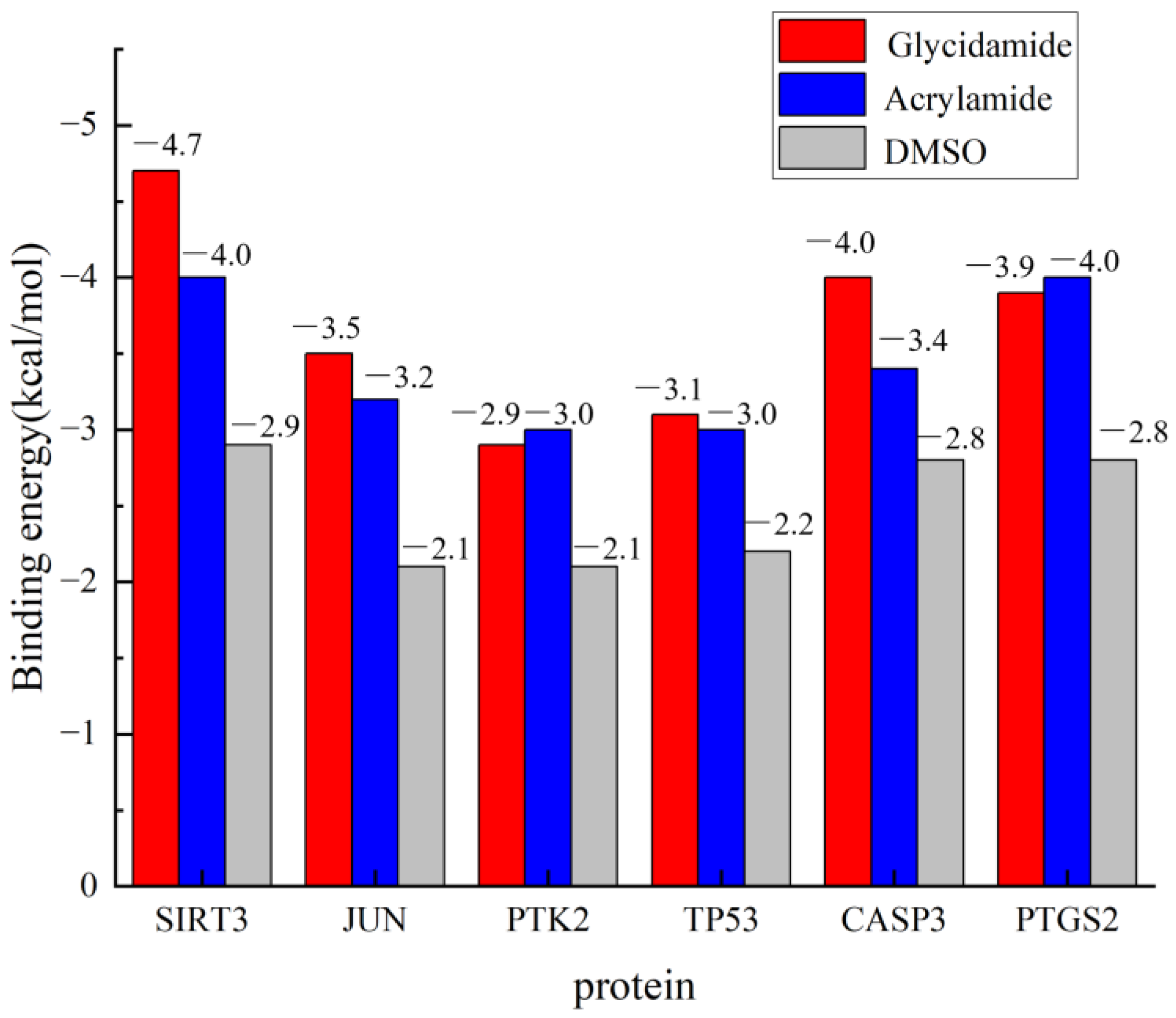
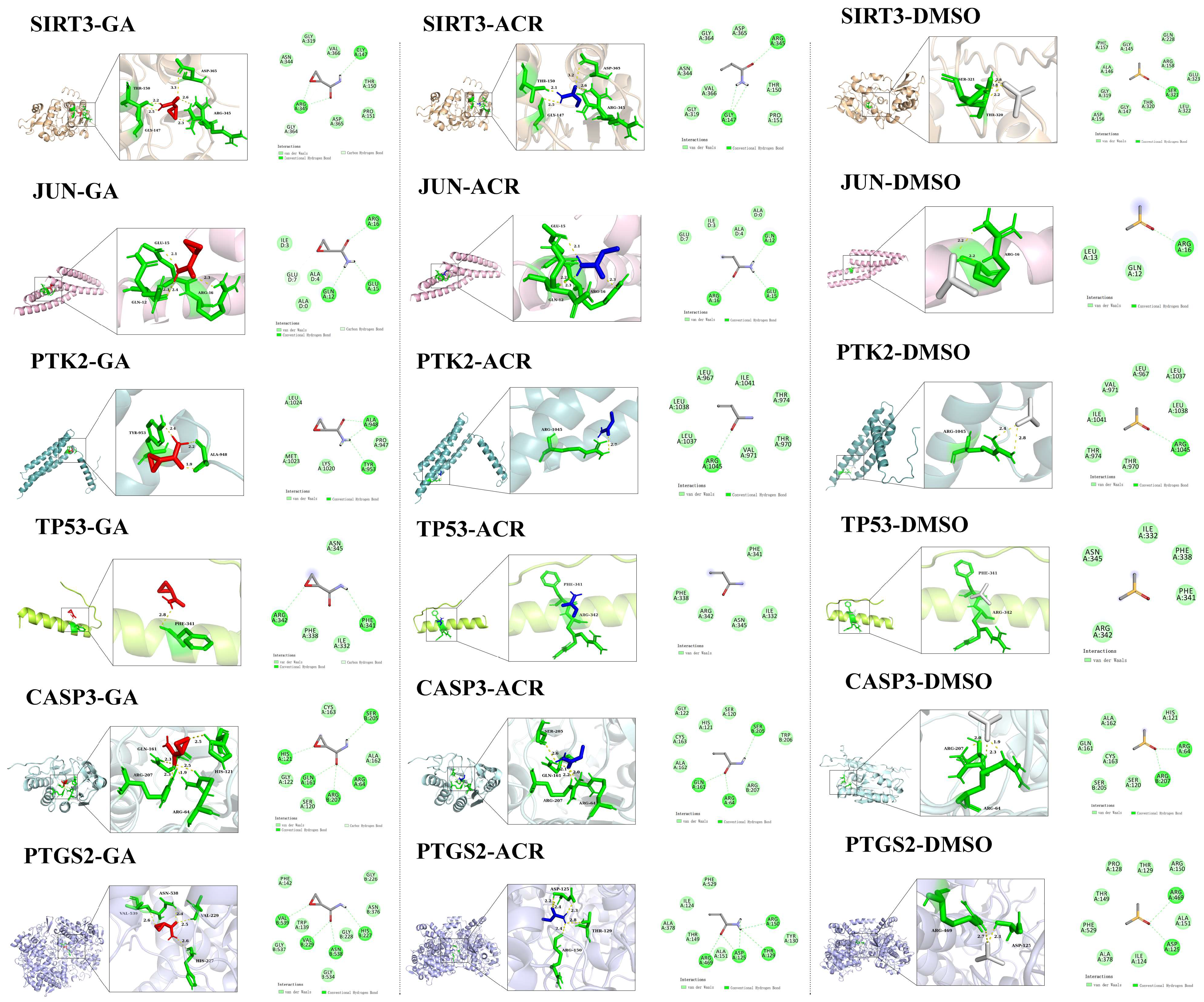
3.7. Molecular Docking of ACR, Its Metabolite GA, and Negative Control DMSO with Core Targets
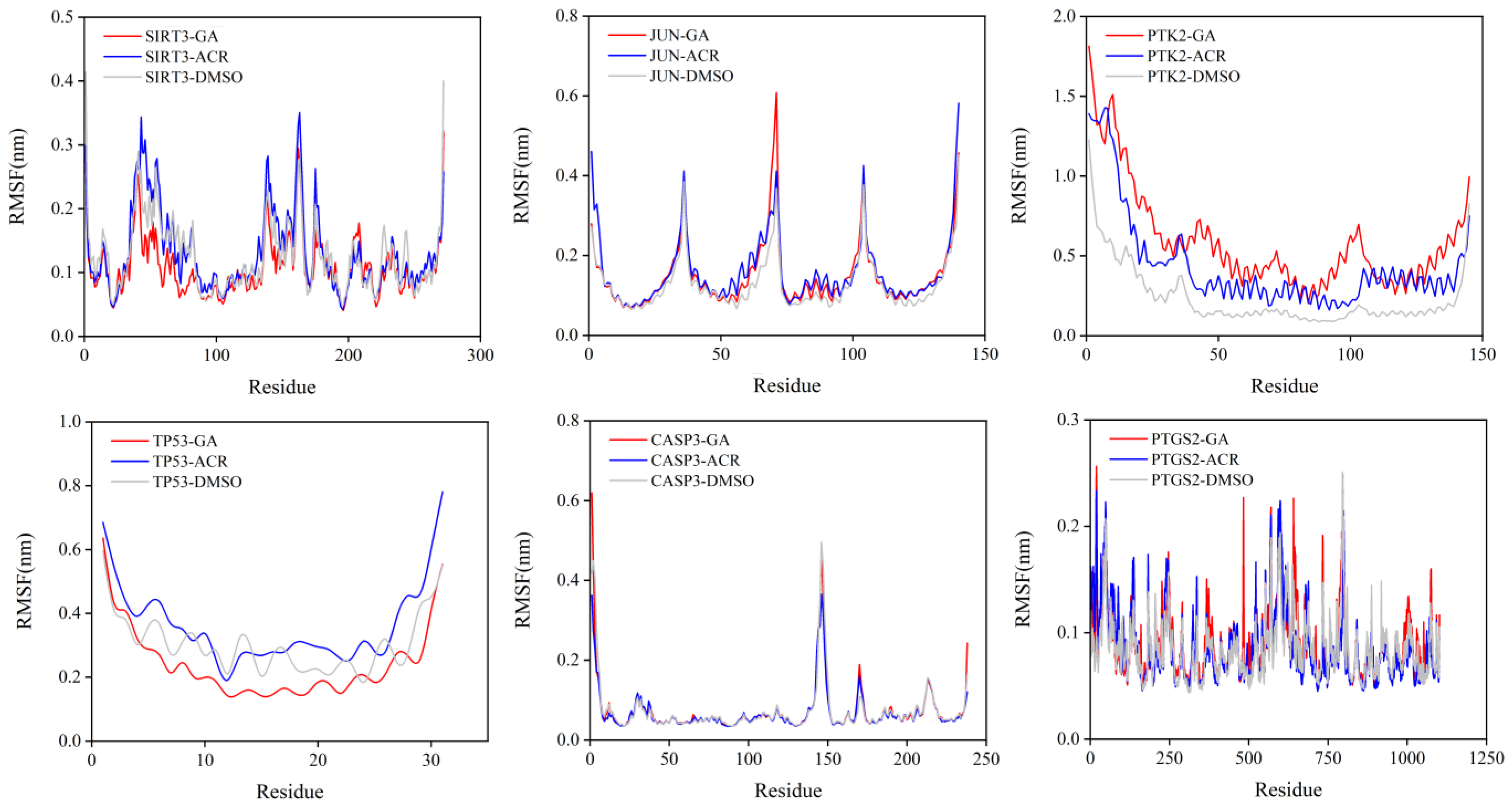
3.8. Molecular Dynamics Simulation of the Core Targets
4. Discussion
4.1. Mitochondrial Gatekeeper: SIRT3 as the Primary Metabolic Redox Target of ACR
4.2. Signaling Orchestrators: JUN/PTK2 as Genetic Risk Factors Potentially Activated by Oxidative Stress
4.3. Apoptotic Network: TP53/CASP3 as Potential Effectors of Oxidative Neuronal Injury
4.4. Neuroinflammatory Hub: PTGS2 as a Downstream Mediator of Oxidative Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tamanna, N.; Mahmood, N. Food Processing and Maillard Reaction Products: Effect on Human Health and Nutrition. Int. J. Food Sci. 2015, 2015, 526762. [Google Scholar] [CrossRef]
- Hauser, C.; Müller, U.; Sauer, T.; Augner, K.; Pischetsrieder, M. Maillard reaction products as antimicrobial components for packaging films. Food Chem. 2014, 145, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Lindenmeier, M.; Faist, V.; Hofmann, T. Structural and functional characterization of pronyl-lysine, a novel protein modification in bread crust melanoidins showing in vitro antioxidative and phase I/II enzyme modulating activity. J. Agric. Food Chem. 2002, 50, 6997–7006. [Google Scholar] [CrossRef]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef]
- Fennell, T.R.; Friedman, M.A. Comparison of acrylamide metabolism in humans and rodents. Adv. Exp. Med. Biol. 2005, 561, 109–116. [Google Scholar] [CrossRef]
- Goempel, K.; Tedsen, L.; Ruenz, M.; Bakuradze, T.; Schipp, D.; Galan, J.; Eisenbrand, G.; Richling, E. Biomarker monitoring of controlled dietary acrylamide exposure indicates consistent human endogenous background. Arch. Toxicol. 2017, 91, 3551–3560. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.; Cheng, Y.; Chen, W.; Fang, W. Negative Association between Acrylamide Exposure and Metabolic Syndrome Markers in Adult Population. Int. J. Environ. Res. Public Health 2021, 18, 11949. [Google Scholar] [CrossRef]
- Rifai, L.; Saleh, F.A. A Review on Acrylamide in Food: Occurrence, Toxicity, and Mitigation Strategies. Int. J. Toxicol. 2020, 39, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Trus, M.; Atlas, D. Thiol-Based Redox Molecules: Potential Antidotes for Acrylamide Toxicity. Antioxidants 2024, 13, 1431. [Google Scholar] [CrossRef]
- Ma, Z.; Ma, L.; Zhang, Y. Acrylamide Neurotoxicity Studies in Caenorhabditis elegans Model. Antioxidants 2025, 14, 641. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.; Yan, H.; Zhao, P.; Yuan, Y. Allicin Attenuates Mitochondrial Calcium (Ca(2+)) Overload Induced by Acrylamide in Hepatocytes via the NAD(+)/SIRT3-FoxO3 Axis. J. Agric. Food Chem. 2025, 73, 22794–22807. [Google Scholar] [CrossRef]
- Kopp, E.K.; Sieber, M.; Kellert, M.; Dekant, W. Rapid and sensitive HILIC-ESI-MS/MS quantitation of polar metabolites of acrylamide in human urine using column switching with an online trap column. J. Agric. Food Chem. 2008, 56, 9828–9834. [Google Scholar] [CrossRef]
- Ahn, J.S.; Castle, L.; Clarke, D.B.; Lloyd, A.S.; Philo, M.R.; Speck, D.R. Verification of the findings of acrylamide in heated foods. Food Addit. Contam. 2002, 19, 1116–1124. [Google Scholar] [CrossRef]
- Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [CrossRef]
- Lu, B.; Lin, L.; Su, X. Global burden of depression or depressive symptoms in children and adolescents: A systematic review and meta-analysis. J. Affect. Disord. 2024, 354, 553–562. [Google Scholar] [CrossRef]
- Fitzpatrick, C.; Lemieux, A.; Smith, J.; West, G.L.; Bohbot, V.; Asbridge, M. Is adolescent internet use a risk factor for the development of depression symptoms or vice-versa? Psychol. Med. 2023, 53, 6773–6779. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Vryonidis, E.; Joensen, A.; Törnqvist, M. Hemoglobin adducts of acrylamide in human blood—What has been done and what is next? Food Chem. Toxicol. 2022, 161, 112799. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Larini, L.; Mannella, R.; Leporini, D. Langevin stabilization of molecular-dynamics simulations of polymers by means of quasisymplectic algorithms. J. Chem. Phys. 2007, 126, 104101. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Sakamoto, J.; Tanii, H. Neurotoxicity of acrylamide and related compounds and their effects on male gonads in mice. Arch. Toxicol. 1981, 47, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Ullah, A.; Zhang, R.; Sun, Y.; Li, J.; Kou, G. Network Pharmacology and Molecular Modeling Techniques in Unraveling the Underlying Mechanism of Citri Reticulatae Pericarpium aganist Type 2 Diabetic Osteoporosis. Nutrients 2024, 16, 220. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, biochemistry, and safety of acrylamide. A review. J. Agric. Food Chem. 2003, 51, 4504–4526. [Google Scholar] [CrossRef]
- Hemgesberg, M.N.; Bonck, T.; Merz, K.H.; Sun, Y.; Schrenk, D. Crystal structure of glycidamide: The mutagenic and genotoxic metabolite of acrylamide. Acta Crystallogr. E Crystallogr. Commun. 2016, 72, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.O.; Yildizbayrak, N.; Aydin, Y.; Erkan, M. Evidence of acrylamide- and glycidamide-induced oxidative stress and apoptosis in Leydig and Sertoli cells. Hum. Exp. Toxicol. 2017, 36, 1225–1235. [Google Scholar] [CrossRef]
- Wan, S.; Yu, L.; Yang, Y.; Liu, W.; Shi, D.; Cui, X.; Song, J.; Zhang, Y.; Liang, R.; Chen, W.; et al. Exposure to acrylamide and increased risk of depression mediated by inflammation, oxidative stress, and alkaline phosphatase: Evidence from a nationally representative population-based study. J. Affect. Disord. 2024, 367, 434–441. [Google Scholar] [CrossRef]
- Wang, A.; Wan, X.; Zhuang, P.; Jia, W.; Ao, Y.; Liu, X.; Tian, Y.; Zhu, L.; Huang, Y.; Yao, J.; et al. High fried food consumption impacts anxiety and depression due to lipid metabolism disturbance and neuroinflammation. Proc. Natl. Acad. Sci. USA 2023, 120, e2221097120. [Google Scholar] [CrossRef]
- Parisien, M.; Lima, L.V.; Dagostino, C.; El-Hachem, N.; Drury, G.L.; Grant, A.V.; Huising, J.; Verma, V.; Meloto, C.B.; Silva, J.R.; et al. Acute inflammatory response via neutrophil activation protects against the development of chronic pain. Sci. Transl. Med. 2022, 14, eabj9954. [Google Scholar] [CrossRef]
- Anamika; Khanna, A.; Acharjee, P.; Acharjee, A.; Trigun, S.K. Mitochondrial SIRT3 and neurodegenerative brain disorders. J. Chem. Neuroanat. 2019, 95, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Lin, Y.; Lei, Q.; Guan, K.L.; Zhao, S.; Xiong, Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011, 12, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.S.; Moreira, J.B.; Costa, M.; Rodrigues, R.S.; Sebastião, A.M.; Xapelli, S.; Solá, S. The Mitochondrial Antioxidant Sirtuin3 Cooperates with Lipid Metabolism to Safeguard Neurogenesis in Aging and Depression. Cells 2021, 11, 90. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Xu, T. Aerobic Exercise Improves Depressive-like Behavior in CUMS-Induced Rats via the SIRT3/ROS/NLRP3 Signaling Pathway. Life 2023, 13, 1711. [Google Scholar] [CrossRef]
- Tabassum, S.; Misrani, A.; Huang, H.; Zhang, Z.; Li, Q.; Long, C. Resveratrol Attenuates Chronic Unpredictable Mild Stress-Induced Alterations in the SIRT1/PGC1α/SIRT3 Pathway and Associated Mitochondrial Dysfunction in Mice. Mol. Neurobiol. 2023, 60, 5102–5116. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, J.; Liang, L.F.; Shen, S.Y.; Li, W.; Chen, X.R.; Li, B.; Zhang, Y.Q.; Yu, J. Sirtuin 3 Plays a Critical Role in the Antidepressant- and Anxiolytic-like Effects of Kaempferol. Antioxidants 2022, 11, 1886. [Google Scholar] [CrossRef]
- Zhu, C.; Shen, S.; Zhang, S.; Huang, M.; Zhang, L.; Chen, X. Autophagy in Bone Remodeling: A Regulator of Oxidative Stress. Front. Endocrinol. 2022, 13, 898634. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Mejia, I.C.; Pijuan, J.; Navaridas, R.; Santacana, M.; Gatius, S.; Velasco, A.; Castellà, G.; Panosa, A.; Cabiscol, E.; Pinyol, M.; et al. Oxidative stress-induced FAK activation contributes to uterine serous carcinoma aggressiveness. Mol. Oncol. 2023, 17, 98–118. [Google Scholar] [CrossRef] [PubMed]
- Coffey, E.T.; Smiciene, G.; Hongisto, V.; Cao, J.; Brecht, S.; Herdegen, T.; Courtney, M.J. c-Jun N-terminal protein kinase (JNK) 2/3 is specifically activated by stress, mediating c-Jun activation, in the presence of constitutive JNK1 activity in cerebellar neurons. J. Neurosci. 2002, 22, 4335–4345. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Pereira, C.; Moraes, J.A.; Souza, M.d.J.; Laurindo, F.R.; Arruda, M.A.; Barja-Fidalgo, C. Redox modulation of FAK controls melanoma survival--role of NOX4. PLoS ONE 2014, 9, e99481. [Google Scholar] [CrossRef]
- Pearce, M.; Garcia, L.; Abbas, A.; Strain, T.; Schuch, F.B.; Golubic, R.; Kelly, P.; Khan, S.; Utukuri, M.; Laird, Y.; et al. Association Between Physical Activity and Risk of Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry 2022, 79, 550–559. [Google Scholar] [CrossRef]
- D’Amelio, M.; Cavallucci, V.; Cecconi, F. Neuronal caspase-3 signaling: Not only cell death. Cell Death Differ. 2010, 17, 1104–1114. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, Y.; Xiao, Y.; Qian, L.; Jiang, Y.; Hu, Y.; Liu, X. Di-Huang-Yin-Zi regulates P53/SLC7A11 signaling pathway to improve the mechanism of post-stroke depression. J. Ethnopharmacol. 2024, 319, 117226. [Google Scholar] [CrossRef]
- Rawdin, B.J.; Mellon, S.H.; Dhabhar, F.S.; Epel, E.S.; Puterman, E.; Su, Y.; Burke, H.M.; Reus, V.I.; Rosser, R.; Hamilton, S.P.; et al. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav. Immun. 2013, 31, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Fan, C.; Wang, P.; Li, Y.; Yang, M.; Yu, S.Y. Hippocampal CA1 βCaMKII mediates neuroinflammatory responses via COX-2/PGE2 signaling pathways in depression. J. Neuroinflamm. 2018, 15, 338. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Feng, Y.B.; Wang, L.; Shen, J.; Li, Y.; Fan, C.; Wang, P.; Yu, S.Y. COX-2 inhibition rescues depression-like behaviors via suppressing glial activation, oxidative stress and neuronal apoptosis in rats. Neuropharmacology 2019, 160, 107779. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Han, Y.; He, Y.; Meng, P.; Fu, Y.; Yang, H.; He, G.; Long, M.; Shi, Y. Quercetin inhibits neuronal Ferroptosis and promotes immune response by targeting lipid metabolism-related gene PTGS2 to alleviate breast cancer-related depression. Phytomedicine 2024, 130, 155560. [Google Scholar] [CrossRef]
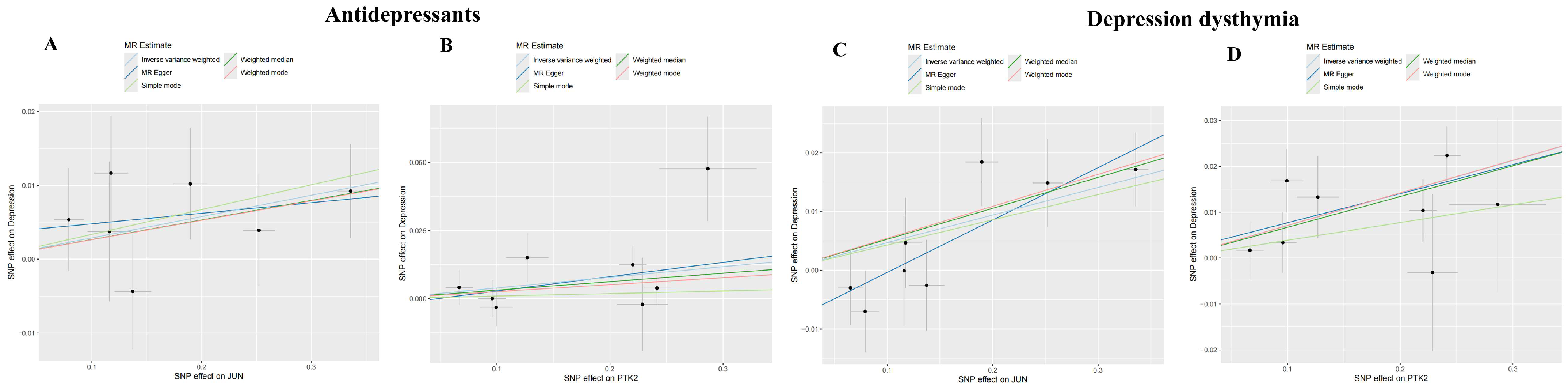

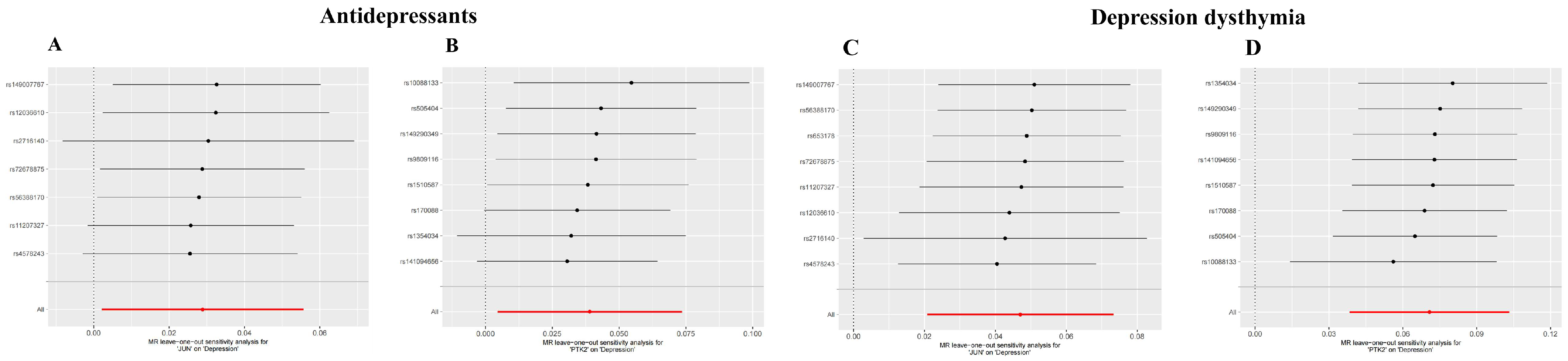




| MR Analyses | JUN OR (95% CI) | p | PTK2 OR (95% CI) | p |
|---|---|---|---|---|
| Antidepressants | ||||
| MR Egger | 1.014 (0.955–1.078) | 0.664 | 1.054 (0.969–1.147) | 0.268 |
| Weighted median | 1.027 (0.995–1.060) | 0.095 | 1.031 (0.990–1.075) | 0.140 |
| Inverse variance weighted | 1.029 (1.002–1.057) | 0.034 | 1.040 (1.005–1.076) | 0.027 |
| Simple mode | 1.034 (0.986–1.085) | 0.215 | 1.009 (0.943–1.080) | 0.795 |
| Weighted mode | 1.027 (0.994–1.061) | 0.163 | 1.026 (0.980–1.074) | 0.313 |
| Depression–dysthymia | ||||
| MR Egger | 1.093 (1.037–1.153) | 0.016 | 1.065 (0.989–1.148) | 0.145 |
| Weighted median | 1.054 (1.021–1.088) | 0.001 | 1.069 (1.026–1.115) | 0.0017 |
| Inverse variance weighted | 1.048 (1.021–1.076) | 0.0004 | 1.073 (1.039–1.109) | 1.808 × 10−5 |
| Simple mode | 1.044 (0.994–1.096) | 0.129 | 1.039 (0.969–1.115) | 0.315 |
| Weighted mode | 1.056 (1.020–1.093) | 0.018 | 1.074 (1.024–1.125) | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Li, S.; Liu, S.; Wang, Z. Dietary Acrylamide Induces Depression via SIRT3-Mediated Mitochondrial Oxidative Injury: Evidence from Multi-Omics and Mendelian Randomization. Curr. Issues Mol. Biol. 2025, 47, 836. https://doi.org/10.3390/cimb47100836
Zhang L, Li S, Liu S, Wang Z. Dietary Acrylamide Induces Depression via SIRT3-Mediated Mitochondrial Oxidative Injury: Evidence from Multi-Omics and Mendelian Randomization. Current Issues in Molecular Biology. 2025; 47(10):836. https://doi.org/10.3390/cimb47100836
Chicago/Turabian StyleZhang, Lele, Shun Li, Shengjie Liu, and Zhenjie Wang. 2025. "Dietary Acrylamide Induces Depression via SIRT3-Mediated Mitochondrial Oxidative Injury: Evidence from Multi-Omics and Mendelian Randomization" Current Issues in Molecular Biology 47, no. 10: 836. https://doi.org/10.3390/cimb47100836
APA StyleZhang, L., Li, S., Liu, S., & Wang, Z. (2025). Dietary Acrylamide Induces Depression via SIRT3-Mediated Mitochondrial Oxidative Injury: Evidence from Multi-Omics and Mendelian Randomization. Current Issues in Molecular Biology, 47(10), 836. https://doi.org/10.3390/cimb47100836





