Preliminary Structure-Activity Relationship on Theonellasterol, a New Chemotype of FXR Antagonist, from the Marine Sponge Theonella swinhoei
Abstract
:1. Introduction
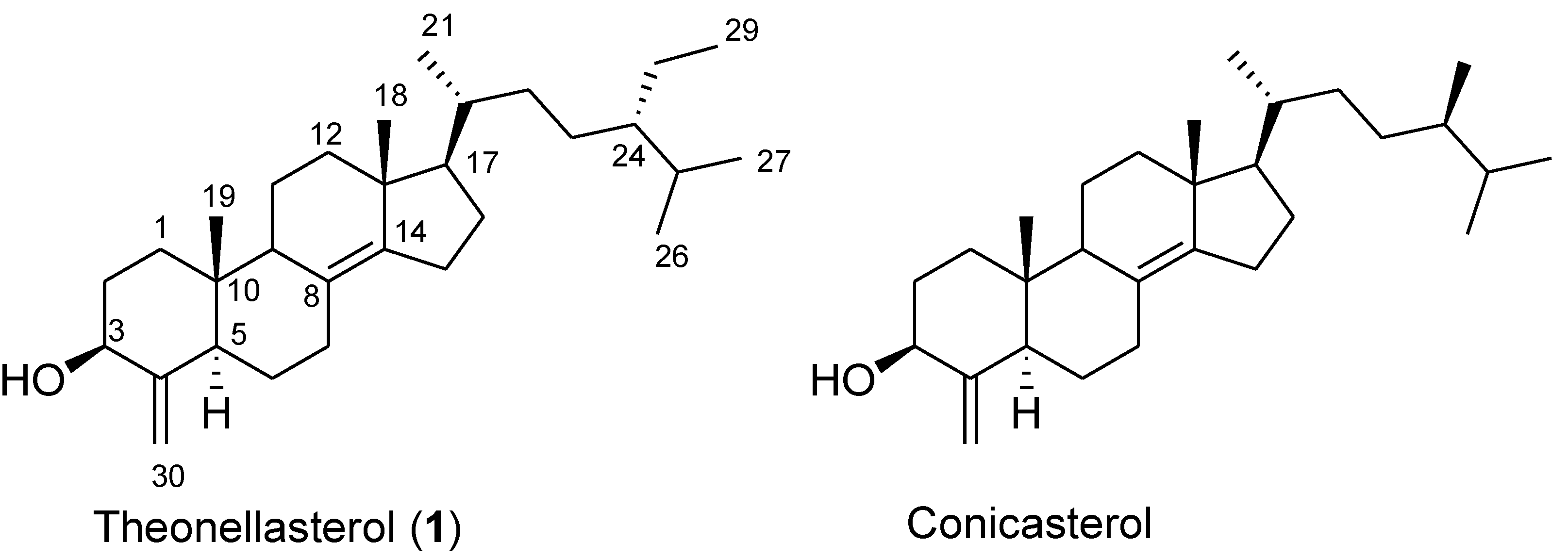
2. Results and Discussion
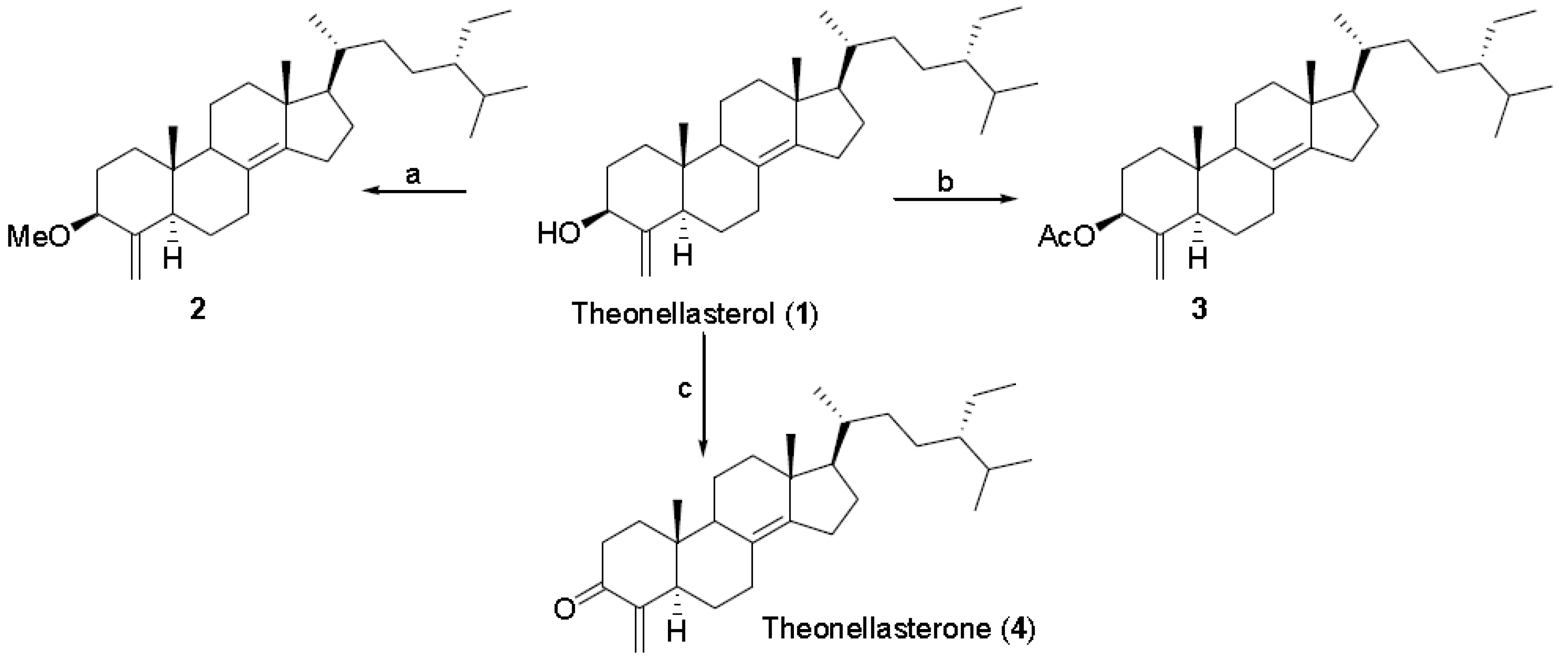

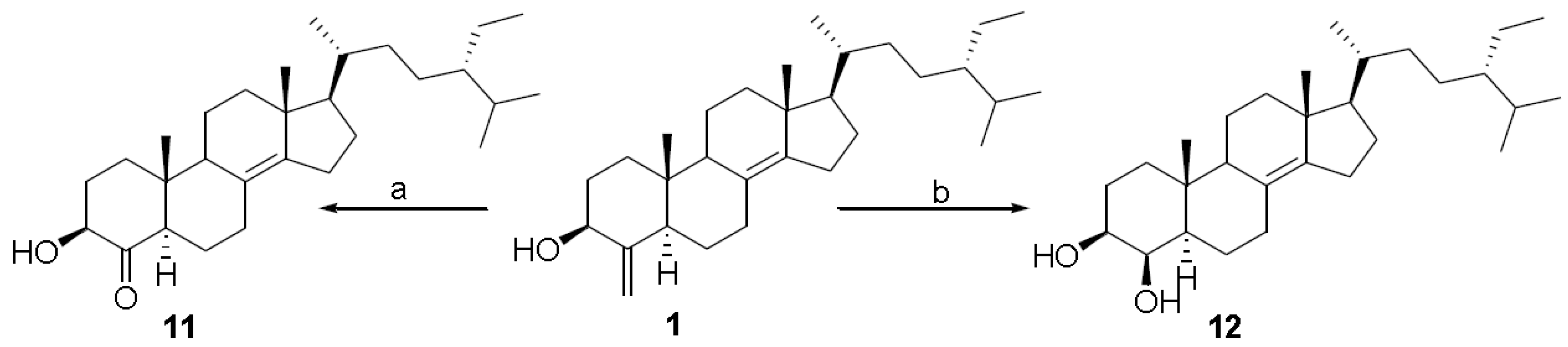

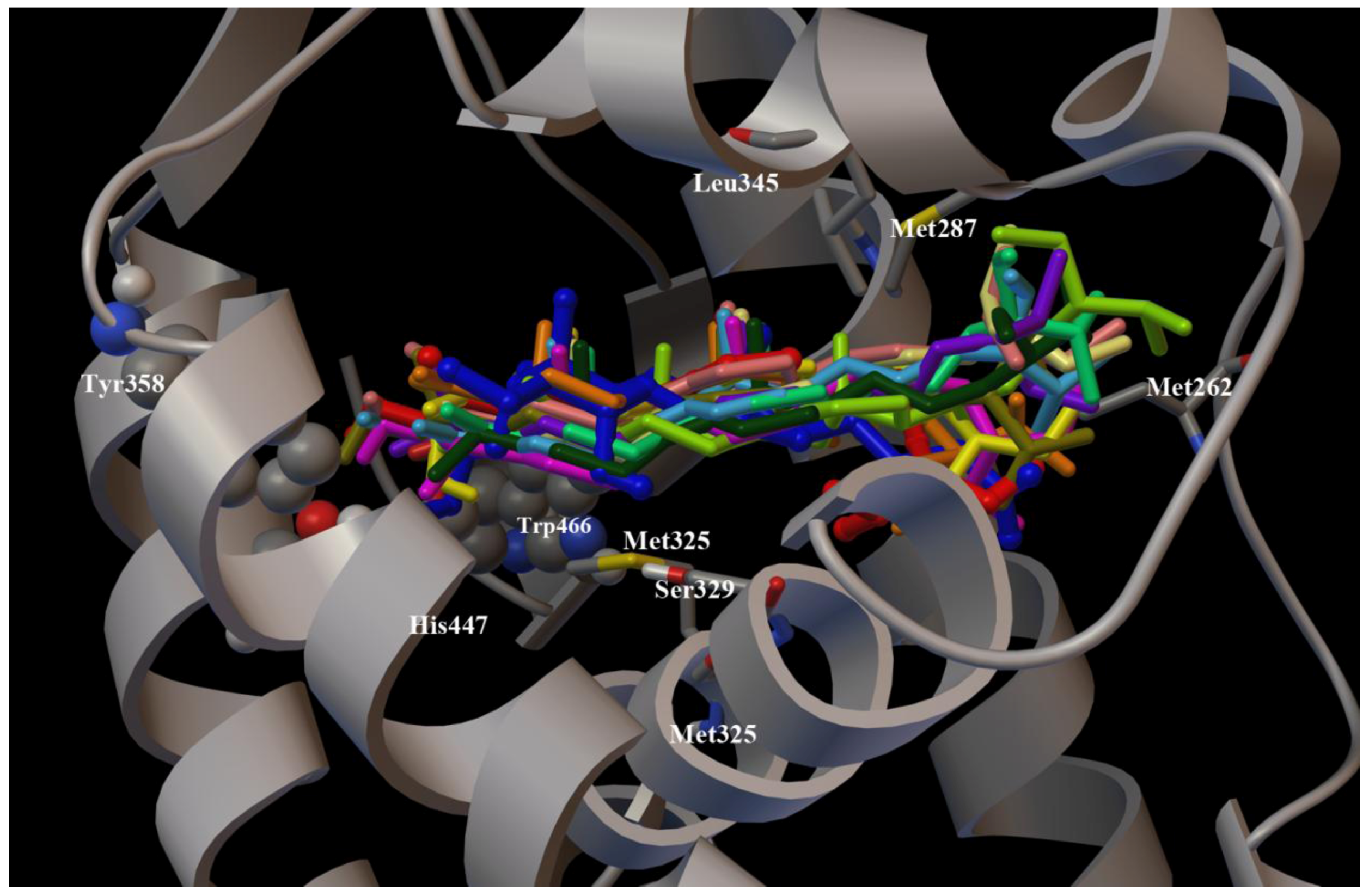
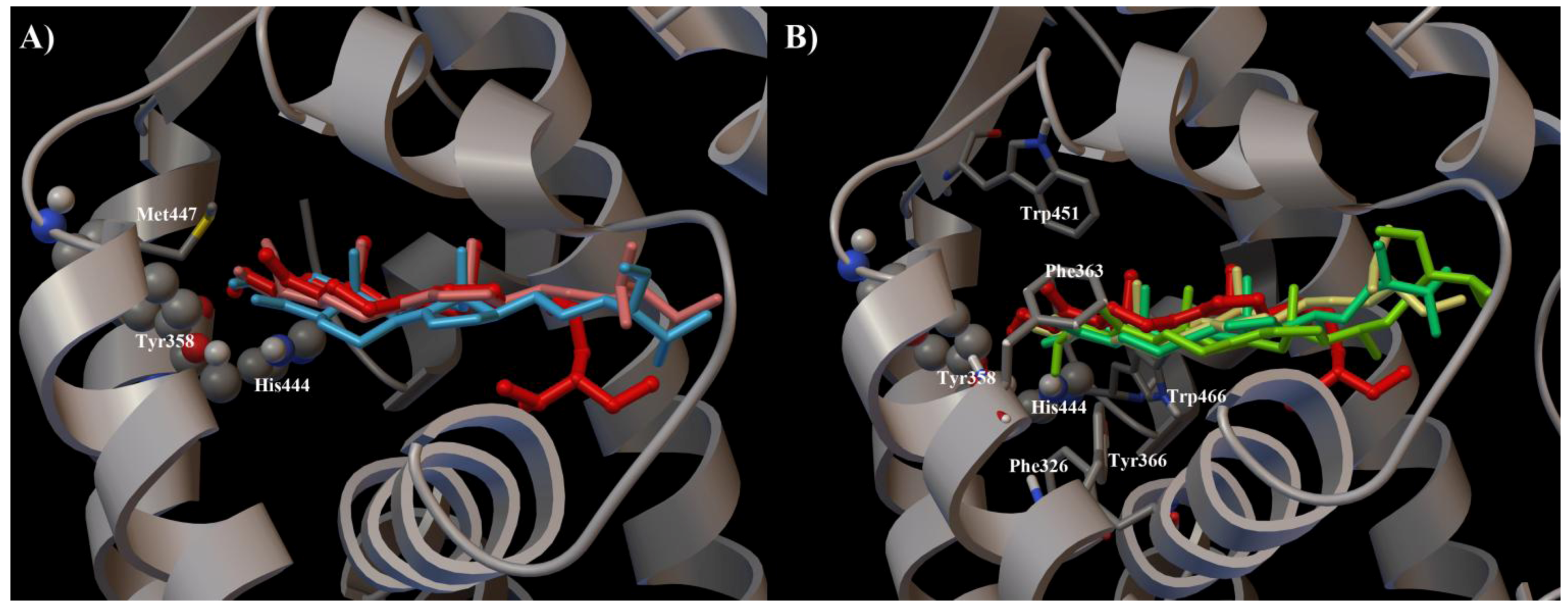
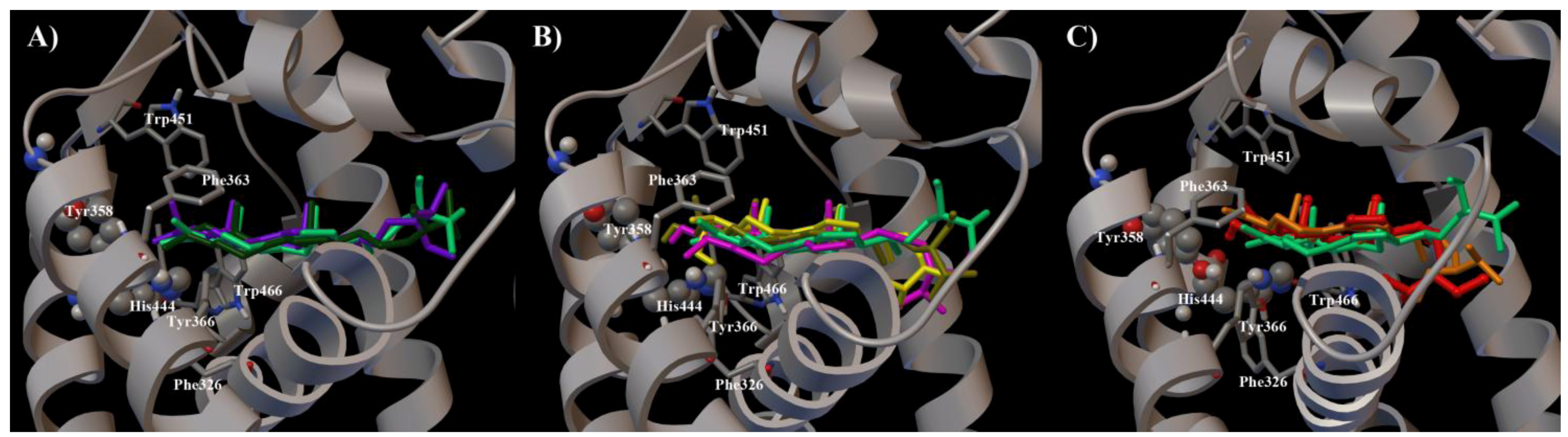
3. Experimental Section
3.1. General
3.2. Sponge Material and Isolation Procedures for Theonellasterol (1)
3.3. Synthetic Procedures
3.3.1. 3β-O-Methyl-theonellasterol 2
3.3.2. 3β-O-Acetyl-theonellasterol 3
3.3.3. Theonellasterone 4
3.3.4. (24S)-24-Ethyl-4β-methyl-5α-cholestan-3β-ol 5
3.3.5. (24S)-24-Ethyl-4β-methyl-5α-cholestan-3-one 6 and (24S)-24-Ethyl-4α-methyl-5α-cholestan-3-one 7
3.3.5.1. (24S)-24-Ethyl-4β-methyl-5α-cholestan-3-one 6
3.3.5.2. (24S)-24-Ethyl-4α-methyl-5α-cholestan-3-one 7
3.3.6. (24S)-24-Ethyl-4α-methyl-5α-cholestan-3β-ol 8 and (24S)-24-Ethyl-4α-methyl-5α-cholestan-3α-ol 9
3.3.6.1. (24S)-24-Ethyl-4α-methyl-5α-cholestan-3β-ol 8
3.3.6.2. (24S)-24-Ethyl-4α-methyl-5α-cholestan-3α-ol 9
3.3.7. (24S)-24-Ethyl-4-methyl-5α-cholest-3-ene 10
3.3.8. (24S)-24-Ethyl-3β-hydroxyl-5α-cholest-4-one 11
3.3.9. (24S)-24-Ethyl-5α-cholestan-3β,4β-diol 12
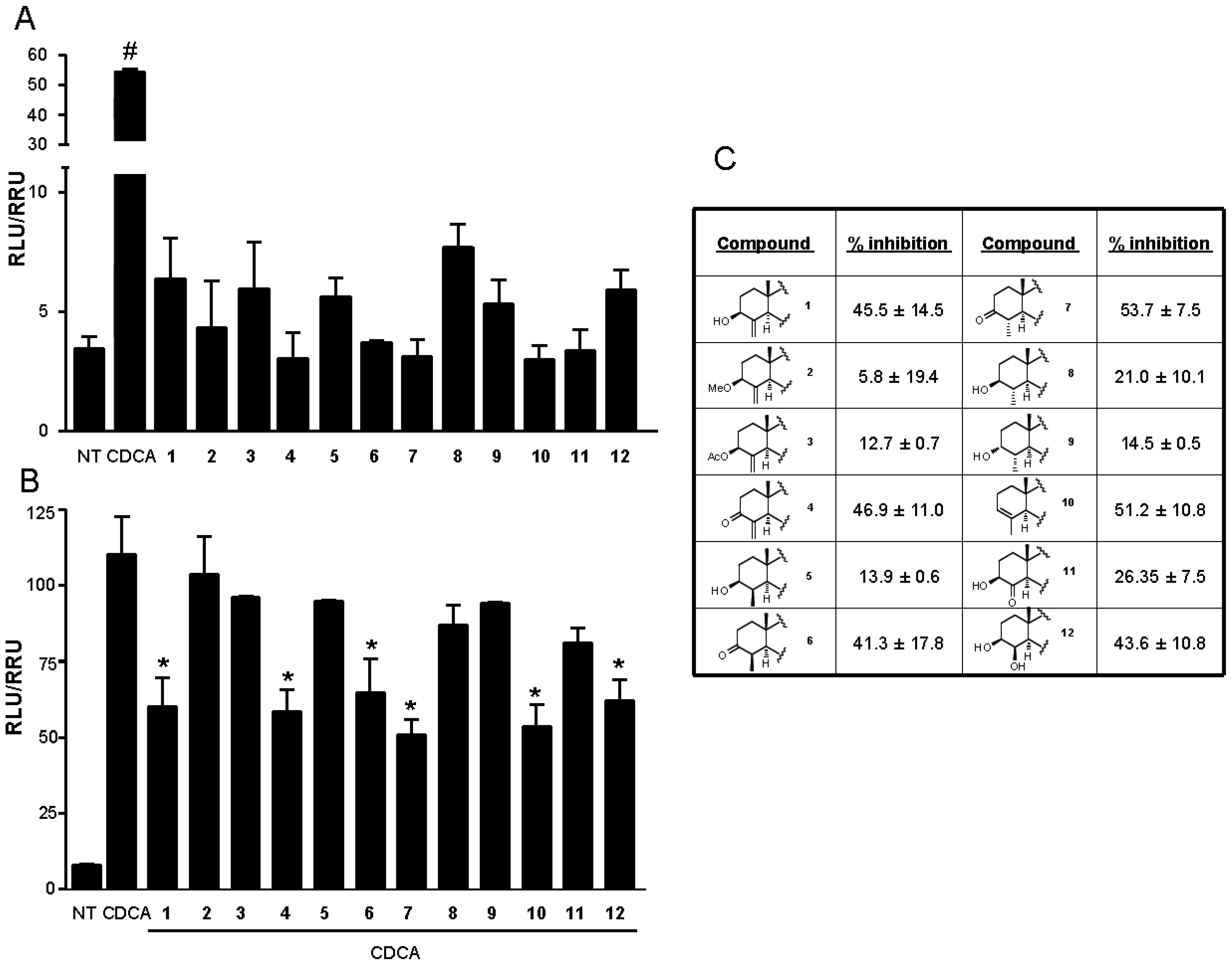
3.4. In Vitro FXR Transactivations
3.5. Computational Details
4. Conclusions
Acknowledgments
References
- Kho, E.; Imagawa, D.K.; Rohmer, M.; Kashman, Y.; Djerassi, C. Sterols in marine invertebrates. 22. Isolation and structure elucidation of conicasterol and theonellasterol, two new 4-methylene sterols from the Red Sea sponges Theonella conica and Theonella swinhoei. J. Org. Chem. 1981, 46, 1836–1839. [Google Scholar]
- Festa, C.; De Marino, S.; Sepe, V.; Monti, M.C.; Luciano, P.; D’Auria, M.V.; Debitus, C.; Bucci, M.; Vellecco, V.; Zampella, A. Perthamides C and D, two new potent anti-inflammatory cyclopeptides from a Solomon Lithistid sponge Theonella swinhoei. Tetrahedron 2009, 65, 10424–10429. [Google Scholar]
- Margarucci, L.; Monti, M.C.; Mencarelli, A.; Fiorucci, S.; Riccio, R.; Zampella, A.; Casapullo, A. Heat shock proteins as key biological targets of the marine natural cyclopeptide perthamide C. Mol. BioSyst. 2012, 8, 1412–1417. [Google Scholar] [CrossRef]
- Sepe, V.; D’Auria, M.V.; Bifulco, G.; Ummarino, R.; Zampella, A. Concise synthesis of AHMHA unit in perthamide C. Structural and stereochemical revision of perthamide C. Tetrahedron 2010, 66, 7520–7526. [Google Scholar]
- Festa, C.; De Marino, S.; Sepe, V.; D’Auria, M.V.; Bifulco, G.; Andres, R.; Terencio, M.C.; Paya, M.; Debitus, C.; Zampella, A. Perthamides C-F, potent human antipsoriatic cyclopeptides. Tetrahedron 2011, 67, 7780–7786. [Google Scholar] [CrossRef]
- Festa, C.; De Marino, S.; D’Auria, M.V.; Monti, M.C.; Bucci, M.; Vellecco, V.; Debitus, C.; Zampella, A. Anti-Inflammatory cyclopeptides from the marine sponge Theonella swinhoei. Tetrahedron 2012, 68, 2851–2857. [Google Scholar] [CrossRef]
- Festa, C.; De Marino, S.; Sepe, V.; D’Auria, M.V.; Bifulco, G.; Debitus, C.; Bucci, M.; Vellecco, V.; Zampella, A. Solomonamides A and B, new anti-inflammatory peptides from Theonella swinhoei. Org. Lett. 2011, 13, 1532–1535. [Google Scholar] [CrossRef]
- Festa, C.; De Marino, S.; D’Auria, M.V.; Bifulco, G.; Renga, B.; Fiorucci, S.; Petek, S.; Zampella, A. Solomonsterols A and B from Theonella swinhoei. The first example of C-24 and C-23 sulfated sterols from a marine source endowed with a PXR agonistic activity. J. Med. Chem. 2011, 54, 401–405. [Google Scholar]
- Sepe, V.; Ummarino, R.; D’Auria, M.V.; Mencarelli, A.; D’Amore, C.; Renga, B.; Zampella, A.; Fiorucci, S. Total synthesis and pharmacological characterization of solomonsterol A, a potent marine pregnane-X-receptor agonist endowed with anti-inflammatory activity. J. Med. Chem. 2011, 54, 4590–4599. [Google Scholar] [CrossRef]
- Sepe, V.; Ummarino, R.; D’Auria, M.V.; Renga, B.; Fiorucci, S.; Zampella, A. The first total synthesis of solomonsterol B, a marine pregnane X receptor agonist. Eur. J. Org. Chem. 2012, 27, 5187–5194. [Google Scholar]
- Sepe, V.; Ummarino, R.; D’Auria, M.V.; Lauro, G.; Bifulco, G.; D’Amore, C.; Renga, B.; Fiorucci, S.; Zampella, A. Modification in the side chain of solomonsterol A: Discovery of cholestan disulfate as a potent pregnane-X-receptor agonist. Org. Biomol. Chem. 2012, 10, 6350–6362. [Google Scholar] [CrossRef]
- De Marino, S.; Festa, C.; D’Auria, M.V.; Cresteil, T.; Debitus, C.; Zampella, A. Swinholide J, a potent cytotoxin from the marine sponge Theonella swinhoei. Mar. Drugs 2011, 9, 1133–1141. [Google Scholar] [CrossRef]
- De Marino, S.; Ummarino, R.; D’Auria, M.V.; Chini, M.G.; Bifulco, G.; Renga, B.; D’Amore, C.; Fiorucci, S.; Debitus, C.; Zampella, A. Theonellasterols and conicasterols from Theonella swinhoei. Novel marine natural ligands for human nuclear receptors. J. Med. Chem. 2011, 54, 3065–3075. [Google Scholar] [CrossRef]
- De Marino, S.; Sepe, V.; D’Auria, M.V.; Bifulco, G.; Renga, B.; Petek, S.; Fiorucci, S.; Zampella, A. Towards new ligands of nuclear receptors. Discovery of malaitasterol A, an unique bis-secosterol from marine sponge Theonella swinhoei. Org. Biomol. Chem. 2011, 9, 4856–4862. [Google Scholar]
- Sepe, V.; Ummarino, R.; D’Auria, M.V.; Chini, M.G.; Bifulco, G.; Renga, B.; D’Amore, C.; Debitus, C.; Fiorucci, S.; Zampella, A. Conicasterol E, a small heterodimer partner sparing farnesoid X receptor modulator endowed with a pregnane X receptor agonistic activity, from the marine sponge Theonella swinhoei. J. Med. Chem. 2012, 55, 84–93. [Google Scholar] [CrossRef]
- Chini, M.G.; Jones, C.R.; Zampella, A.; D’Auria, M.V.; Renga, B.; Fiorucci, S.; Butts, C.P.; Bifulco, G. Quantitative NMR-derived interproton distances combined with quantum mechanical calculations of 13C chemical shifts in the stereochemical determination of conicasterol F, a nuclear receptor ligand from Theonella swinhoei. J. Org. Chem. 2012, 77, 1489–1496. [Google Scholar] [CrossRef]
- De Marino, S.; Ummarino, R.; D’Auria, M.V.; Chini, M.G.; Bifulco, G.; D’Amore, C.; Renga, B.; Mencarelli, A.; Petek, S.; Fiorucci, S.; Zampella, A. 4-Methylenesterols from Theonella swinhoei sponge are natural pregnane-X-receptor agonists and farnesoid-X-receptor antagonists that modulate innate immunity. Steroids 2012, 77, 484–495. [Google Scholar] [CrossRef]
- Fiorucci, S.; Distrutti, E.; Bifulco, G.; D’Auria, M.V.; Zampella, A. Marine sponge steroids as nuclear receptor ligands. Trends Pharmacol. Sci. 2012, 33, 591–601. [Google Scholar] [CrossRef]
- Pellicciari, R.; Costantino, G.; Fiorucci, S. Farnesoid X receptor: From structure to potential clinical applications. J. Med. Chem. 2005, 48, 5383–5403. [Google Scholar] [CrossRef]
- Fiorucci, S.; Rizzo, G.; Donini, A.; Distrutti, E.; Santucci, L. Targeting farnesoid X receptor for liver and metabolic disorders. Trends Mol. Med. 2007, 13, 298–309. [Google Scholar] [CrossRef]
- Sonoda, J.; Xie, W.; Rosenfeld, J.M.; Barwick, J.L.; Guzelian, P.S.; Evans, R.M. Regulation of a xenobiotic sulfonation cascade by nuclear pregnane X receptor (PXR). Proc. Natl. Acad. Sci. USA 2002, 99, 13801–13806. [Google Scholar]
- Guo, G.L.; Lambert, G.; Negishi, M.; Ward, J.M.; Brewer, H.B.J.; Kliewer, S.A.; Gonzalez, F.J.; Sinal, C.J. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J. Biol. Chem. 2003, 278, 45062–45071. [Google Scholar]
- Fiorucci, S.; Cipriani, S.; Mencarelli, A.; Baldelli, F.; Bifulco, G.; Zampella, A. Farnesoid X receptor agonist for the treatment of liver and metabolic disorders: Focus on 6-ethyl-CDCA. Mini Rev. Med. Chem. 2011, 11, 753–762. [Google Scholar] [CrossRef]
- Kakizaki, S.; Takizawa, D.; Tojima, H.; Horiguchi, N.; Yamazaki, Y.; Mori, M. Nuclear receptors CAR and PXR; therapeutic targets for cholestatic liver disease. Front Biosci. 2011, 17, 2988–3005. [Google Scholar]
- Fiorucci, S.; Zampella, A.; Distrutti, E. Development of FXR, PXR and CAR agonists and antagonists for treatment of liver disorders. Curr. Top. Med. Chem. 2012, 6, 605–624. [Google Scholar]
- Poupon, R.E.; Bonnand, A.M.; Chrétien, Y.; Poupon, R. Ten-Year survival in ursodeoxycholic acid-treated patients with primary biliary cirrhosis. Hepatology 1999, 29, 1668–1671. [Google Scholar] [CrossRef]
- Parés, A.; Caballería, L.; Rodés, J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology 2006, 130, 715–720. [Google Scholar] [CrossRef]
- Renga, B.; Mencarelli, A.; D’Amore, C.; Cipriani, S.; D’Auria, M.V.; Sepe, V.; Chini, M.G.; Monti, M.C.; Bifulco, G.; Zampella, A.; Fiorucci, S. Discovery that theonellasterol a marine sponge sterol is a highly selective FXR antagonist that protects against liver injury in cholestasis. PLoS One 2012, 7, e30443. [Google Scholar]
- Sepe, V.; Bifulco, G.; Renga, B.; D’Amore, C.; Fiorucci, S.; Zampella, A. Discovery of sulfated sterols from marine invertebrates as a new class of marine natural antagonists of farnesoid-X-receptor. J. Med. Chem. 2011, 54, 1314–1320. [Google Scholar] [CrossRef]
- D’Auria, M.V.; Sepe, V.; Zampella, A. Natural ligands for nuclear receptors: Biology and potential therapeutic applications. Curr. Top. Med. Chem. 2012, 12, 637–690. [Google Scholar] [CrossRef]
- Pellicciari, R.; Gioiello, A.; Costantino, G.; Sadeghpour, B.M.; Rizzo, G.; Meyer, U.; Parks, D.J.; Entrena-Guadix, A.; Fiorucci, S. Back door modulation of the farnesoid X receptor: Design, synthesis, and biological evaluation of a series of side chain modified chenodeoxycholic acid derivatives. J. Med. Chem. 2006, 49, 4208–4215. [Google Scholar] [CrossRef]
- Mi, L.Z.; Devarakonda, S.; Harp, J.M.; Han, Q.; Pellicciari, R.; Willson, T.M.; Khorasanizadeh, S.; Rastinejad, F. Structural basis for bile acid binding and activation of the nuclear receptor FXR. Mol. Cell 2003, 11, 1093–1100. [Google Scholar] [CrossRef]
- Schmidt, A.W.; Doert, T.; Goutal, S.; Gruner, M.; Mende, F.; Kurzchalia, T.V.; Knölker, H.J. Regio- and stereospecific synthesis of cholesterol derivatives and their hormonal activity in Caenorhabditis elegans. Eur. J. Org. Chem. 2006, 16, 3687–3706. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Sanner, M.F. A programming language for software integration and development. J. Mol. Graphics 1999, 17, 57–61. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sepe, V.; Ummarino, R.; D'Auria, M.V.; Taglialatela-Scafati, O.; Marino, S.D.; D'Amore, C.; Renga, B.; Chini, M.G.; Bifulco, G.; Nakao, Y.; et al. Preliminary Structure-Activity Relationship on Theonellasterol, a New Chemotype of FXR Antagonist, from the Marine Sponge Theonella swinhoei. Mar. Drugs 2012, 10, 2448-2466. https://doi.org/10.3390/md10112448
Sepe V, Ummarino R, D'Auria MV, Taglialatela-Scafati O, Marino SD, D'Amore C, Renga B, Chini MG, Bifulco G, Nakao Y, et al. Preliminary Structure-Activity Relationship on Theonellasterol, a New Chemotype of FXR Antagonist, from the Marine Sponge Theonella swinhoei. Marine Drugs. 2012; 10(11):2448-2466. https://doi.org/10.3390/md10112448
Chicago/Turabian StyleSepe, Valentina, Raffaella Ummarino, Maria Valeria D'Auria, Orazio Taglialatela-Scafati, Simona De Marino, Claudio D'Amore, Barbara Renga, Maria Giovanna Chini, Giuseppe Bifulco, Yoichi Nakao, and et al. 2012. "Preliminary Structure-Activity Relationship on Theonellasterol, a New Chemotype of FXR Antagonist, from the Marine Sponge Theonella swinhoei" Marine Drugs 10, no. 11: 2448-2466. https://doi.org/10.3390/md10112448






