Distinguishing Health Benefits of Eicosapentaenoic and Docosahexaenoic Acids
Abstract
:1. Recommendations for Daily Intake of n-3 PUFAs
| Health Organization | Country | Recommendation | Ref. |
|---|---|---|---|
| National Heart Foundation | Australia | 0.5 g/day EPA + DHA plus 2 g/day ALA to lower the risk of coronary heart disease; 1.0 g/day EPA + DHA plus 2 g/day ALA for patients with documented coronary heart disease; 1.2–4.0 g/day EPA + DHA for patients with elevated serum triglyceride levels. | [22] |
| American Heart Association | USA | ≥2 fish meals/week plus oils rich in ALA in subjects without coronary heart disease; 1.0 g/day EPA + DHA for patients with documented coronary heart disease; 2.0–4.0 g/day EPA + DHA for patients with elevated serum triglyceride levels. | [34] |
| World Health Organization | International | 0.2–0.5 g/day EPA+DHA to prevent coronary heart disease and ischemic stroke. | [35] |
| American Psychiatric Association | USA | 1.0 g/day EPA + DHA for treatment of affective disorders. | [23] |
| 1. National Health and Medical Research Council. 2. The Cancer Council Australia. | 1. Australia & New Zealand.2. Australia | Adequate Intake for EPA + DHA + DPA: 0.09 g/day (women ≥ 19 years), 0.16 g/day (men ≥ 19 years). ALA: 0.8 g/day (women ≥ 19 years), 1.3 g/day (men ≥ 19 years). Intake of EPA + DHA + DPA to reduce risk of chronic disease: 0.43 g/day (women), 0.61 g/day (men). | [36,37] |
| Scientific Advisory Committee on Nutrition | UK | General nutrition, at least 0.45 g/day LC n-3 PUFAs. | [19] |
| French Agency for Food, Environmental and Occupational Health & Safety | France | General nutrition, 0.25 g/day EPA; 0.25 g/day DHA, 1% of energy intake ALA. | [20] |
| Health Council of the Netherlands | Netherlands | General nutrition, 0.45 g/day fish fatty acids. | [21] |
| International Society for the Study of Fatty Acids and Lipids | International | Cardiovascular health, ≥0.5 g/day EPA + DHA. General nutrition, 0.7% of energy intake ALA. | [38] |
2. Evidence for Differential Responses to EPA and DHA
| Gene expression | Study | Summary of differences | Ref. |
|---|---|---|---|
| Genes regulating inflammation, the cell cycle, apoptosis | Jurkat T cells, 12.5 μM DHA (n = 3) or EPA (n = 3) for 1 day (compared to untreated cells). | CD27 ligand: DHA no change, EPA ↑.
Fibronectin I: DHA ↑, EPA no change. Insulin receptor: DHA ↑, EPA no change. Microsomal Glutathione S-transferase I: DHA no change, EPA ↓. Cyclin-dependent kinase 4 inhibitor 2: DHA ↑, EPA no change. Phospholipase A2: DHA ↑, EPA no change. c-abl proto-oncogene: DHA ↑, EPA ↓. Glutathione S-transferase A1: DHA ↑, EPA ↓. Breast cancer type 2 susceptibility protein: DHA ↑, EPA ↓. | [41] |
| Cytokine mRNA expression | Lipopolysaccharide-stimulated human THP-1 macrophages.
100 μM DHA (n = 5–6) or EPA (n = 5–6) for 2 days. | Inhibition of TNFα, IL-1β, IL-6 mRNA: DHA > EPA.
Cytoplasmic IκBα protein: DHA ↑, EPA no change. | [43] |
| UDP-glucuronosyl transferase IAI (UGTIAI) mRNA expression | Human hepatoma HepG2 cells, 50 μM DHA (n = 3) or EPA (n = 3) for 1 day (compared to vehicle). Some cells co-treated with 10 μM vitamin E. | UGTIAI mRNA: DHA no change (but ↓ with vitamin E), EPA ↓ (but ↑ to control levels with vitamin E). | [44] |
| Cannabinoid receptor 2 (CB2) and NAPE-PLD mRNA expression | MC3T3-E1 osteoblast-like cells.
10 μM DHA (n = 3–4) or EPA (n = 3–4) for 3 days (compared to vehicle). | CB2 mRNA: DHA no change, EPA ↓.
NAPE-PLD mRNA: DHA no change, EPA ↓. | [45] |
| PPARγ and adiponectin mRNA | 3T3-L1 adipocytes.
125 μM DHA (n = 3) or EPA (n = 3) for 1 day (compared to vehicle). | PPARγ mRNA: DHA ↑, EPA no change.
Adiponectin mRNA: DHA ↑, EPA no change. | [46] |
| CYP2J2 mRNA expression | Human umbilical vein endothelial cells.
1, 10 μM DHA (n = 5) or EPA (n = 5) for 1 day (compared to vehicle). | CYP2J2 mRNA: DHA no change, EPA ↑. | [47] |
| Serum lipoproteins and LDL particle size | Overweight, non-smoking, mildly hyperlipidemic men.
DB, RD, PC. 4 g/day DHA (n = 17) or EPA (n = 19) for 6 weeks. | HDL2-cholesterol: DHA ↑, EPA no change.
HDL3-cholesterol: DHA no change, EPA ↓. LDL particle size: DHA ↑, EPA: no change. | [48] |
| Serum lipoproteins | Non-smoking, healthy men.
DB, RD, PC. 3.6 g/day DHA (n = 72), 3.8 g/day EPA (n = 75) for 7 weeks. | HDL-cholesterol: DHA ↑, EPA no change.
Apolipoprotein A–I: DHA no change, EPA ↓. Total cholesterol: DHA no change, EPA ↓. Accumulation into serum phospholipids: DHA > EPA. Δ6-Desaturation activity: DHA ↓, EPA no change. Δ5-Desaturation activity: DHA ↓, EPA ↑. | [49] |
| Serum lipoproteins | Non-smoking normolipidemic men and women.
B, RD. 2.3 g/day DHA (n = 25) or 2.2 g/day EPA (n = 25) for 6 weeks. | HDL cholesterol: DHA ↑, EPA no change. | [50] |
| LDL particle size | Non-smoking hypertensive diabetic men and postmenopausal women.
DB, RD, PC. 4 g/day DHA (n = 18) or EPA (n = 17) for 6 weeks. | LDL particle size: DHA ↑, EPA no change. | [51] |
| Triglyceride formation | Rat liver microsomes.
5–20 µM DHA-CoA (n = 4) or EPA-CoA (n = 4) for 10 min. | Triglyceride formation: DHA-CoA > EPA-CoA. | [52] |
| Lipid peroxidation | Rat C6 Glioblastoma cells.
100 μM DHA (n = 3) or EPA (n = 3) for 1–3 days. | Thiobarbituric acid production: DHA > EPA. | [53] |
| Endothelial cell migration | Cultured H5V endothelial cells.
100 μM DHA (n = 3) or EPA (n = 3) for 24 h. | Endothelial cell migration: DHA no change, EPA ↓. | [54] |
| Enzyme activity and membrane fluidity | Human cultured foreskin fibroblasts.
50 μM DHA (n = 6, membrane fluidity; n = 9 enzyme activity) or EPA (n = 6, membrane fluidity; n = 9 enzyme activity) for 4 days. | 5′-nucleotidase activity: DHA ↑, EPA no change.
Adenylate cyclase activity: DHA ↑, EPA no change. Fluorescence anisotropy: DHA ↑, EPA no change. | [55] |
| Mitogen signaling pathways | Jurkat T cells transfected with RasGRP.
10 μM DHA (n = 6) or EPA (n = 6) for 3 h. | Potentiation of PMA-stimulated ERK1/2 activity: DHA ↑, EPA no change *.
* Result likely linked to differential incorporation of the LC n-3 PUFAs into diacylglycerol and not different affinities of the phospholipids for RasGRP. | [56] |
| Collagen-stimulated production of platelet thromboxane | Non-smoking men and postmenopausal women with type 2 diabetes mellitus.
DB, RD, PC. 4 g/day DHA (n = 10) or EPA (n = 11) for 6 weeks. | Platelet thromboxane levels: DHA ↓, EPA no change. | [57] |
| Platelet aggregation | Healthy men and women.
1 μM DHA (n = 42) or EPA (n = 42) for 6 min. Healthy men; 1 μM DHA (n = 20) or EPA (n = 20) for 6 min (compared to ethanol control). | Aggregation to collagen; men and women: EPA ↓ > DHA ↓.
Aggregation to collagen; men only: EPA ↓ > DHA ↓. | [29] |
| Platelet aggregation | Human platelets.
100 μM 4(RS)-4-F4t-NeuroP or 15-F3t-IsoP for 5 min prior to U46619. | Reversible aggregation to U46619: 4(RS)-4-F4t-NeuroP no change, 15-F3t-IsoP ↓. Note: 4(RS)-4-F4t-NeuroP is a product derived from DHA; 15-F3t-IsoP is a product derived from EPA. | [58] |
| Mean platelet volume and platelet count | Healthy men and women.
RD, PC. 4 g/day DHA (n = 12) or EPA (n = 10) for 4 weeks. | Mean platelet volume: DHA no change, EPA ↓.
Platelet count: DHA no change, EPA ↑. | [28] |
| Reactive oxygen species | Goat cultured neutrophils.
25–200 μM DHA (n = 6) or EPA (n = 6) for 0.5–2 h. | Cytochrome C activity in resting neutrophils: DHA ↓, EPA no change (0.5 h treatment).
Cytochrome C activity in PMA-stimulated neutrophils: DHA ↓ > EPA ↓. | [59] |
| iNOS protein expression | Mouse RAW264 macrophages.
60 μM DHA (n = 3) or EPA (n = 3) for 1 day, then stimulated IFN-γ and LPS for 12 h (compared to untreated, stimulated cells). | iNOS/actin protein: DHA ↓, EPA no change. | [60] |
| Ca2+-induced opening of MPTP | Cardiac mitochondria from male Wistar rats.
B. 2.5% caloric intake DHA (n = 8–9) or EPA (n = 8–9). | MPTP opening: DHA ↓, EPA no change. | [61] |
| Ischaemia-induced cardiac arrhythmias (SHR) | Spontaneously hypertensive rats.
0.5% w/w in the diet, up to 450 mg/kg/day; DHA (n = 10) or EPA (n = 10) for 5 weeks. | Ischaemia-induced cardiac arrhythmias: DHA ↓, EPA no change. | [62] |
| Blood pressure and thromboxane-like aortic constriction (SHR) | Spontaneously hypertensive rats during the development phase of hypertension.
Blood pressure: 4.5% w/w in the diet; DHA (n = 8) or EPA (n = 8) for 12 weeks. Aortic constriction: 4.5% w/w in the diet; DHA (n = 5) or EPA (n = 5) for 12 weeks. | Blood pressure: DHA ↓ > EPA ↓.
Aortic constriction: DHA ↓ > EPA ↓. | [62] |
| Salt-loading induced proteinuria (SHR) | Salt-loaded, stroke-prone spontaneously hypertensive rats with established hypertension.
4.5% w/w in the diet; DHA (n = 7) or EPA (n = 8) for 6, 9 and 12 weeks. | Proteinuria: DHA ↓, EPA no change. | [62] |
| Forearm blood flow (Human) | Overweight, non-smoking, mildly hyperlipidemic men.
DB, RD, PC. 4 g/day DHA (n = 13) or EPA (n = 13) for 6 weeks. | Forearm blood flow: DHA ↑, EPA no change.
Noradrenaline-mediated constriction of forearm microcirculation: DHA ↓, EPA no change. | [63] |
| Blood pressure and Heart rate (Human) | Overweight, non-smoking, mildly hyperlipidemic men.
DB, RD, PC. 4 g/day DHA (n = 17) or EPA (n = 19) for 6 weeks. | Mean 24 h SBP: DHA ↓, EPA no change.
Mean 24 h DBP: DHA ↓, EPA no change. Mean day SBP: DHA ↓, EPA no change. Mean day DBP: DHA ↓, EPA no change. Mean 24 h HR: DBP: DHA ↓, EPA no change. Mean day HR: DBP: DHA ↓, EPA no change. Mean night HR: DBP: DHA ↓, EPA no change. | [26] |
| Blood pressure and QT interval (male SHR) | Spontaneously hypertensive rats.
240 mg/day DHA (n = 6) or EPA (n = 6) for 8 weeks compared to normal fat diet. | Day SBP: DHA ↓, EPA no change.
Night SBP: DHA ↓, EPA no change. Day pulse pressure: DHA ↓, EPA ↑. Night pulse pressure: DHA ↓, EPA ↑. Day QT interval: DHA ↓, EPA no change. Night QT interval: DHA ↓, EPA no change. | [27] |
| Heart rate | Non-smoking, healthy men.
DB, RD, PC. 3.6 g/day DHA (n = 72) or 3.8 g/day EPA (n = 75) for 7 weeks (compared to control) | Resting HR: DHA ↓, EPA ↑. | [42] |
| Vascular tension (Rat) | Pre-contracted rat isolated aorta (untreated).
1 nM–100 μM 4(RS)-4-F4t-NeuroP (n = 4) or 15-F3t-IsoP (n = 4) dose-response curves. | Aortic contraction: 4(RS)-4-F4t-NeuroP no change, 15-F3t-IsoP ↑. Note: 4(RS)-4-F4t-NeuroP is a product derived from DHA; 15-F3t-IsoP is a product derived from EPA. | [58] |
| Antidepressant effect (Human) | Meta analysis, patients with depressive symptoms.
DB, RD, PC. 28 studies. | Treatment of depression: EPA > DHA | [4] |
| Alzheimer disease (AD; Human) | 815 subjects unaffected by AD; 65–94 years. Analysis of fish consumption using food frequency questionnaire; follow-up at 3.9 years. | Risk AD: DHA ↓, EPA no change. | [3] |
3. Factors Contributing to Differential Responses to EPA and DHA
3.1. Regulation of Transcription Factors
3.2. Receptor-Mediated Effects of EPA and DHA
3.3. Incorporation of EPA and DHA into Phospholipids
3.4. LC n-3 PUFA Metabolites
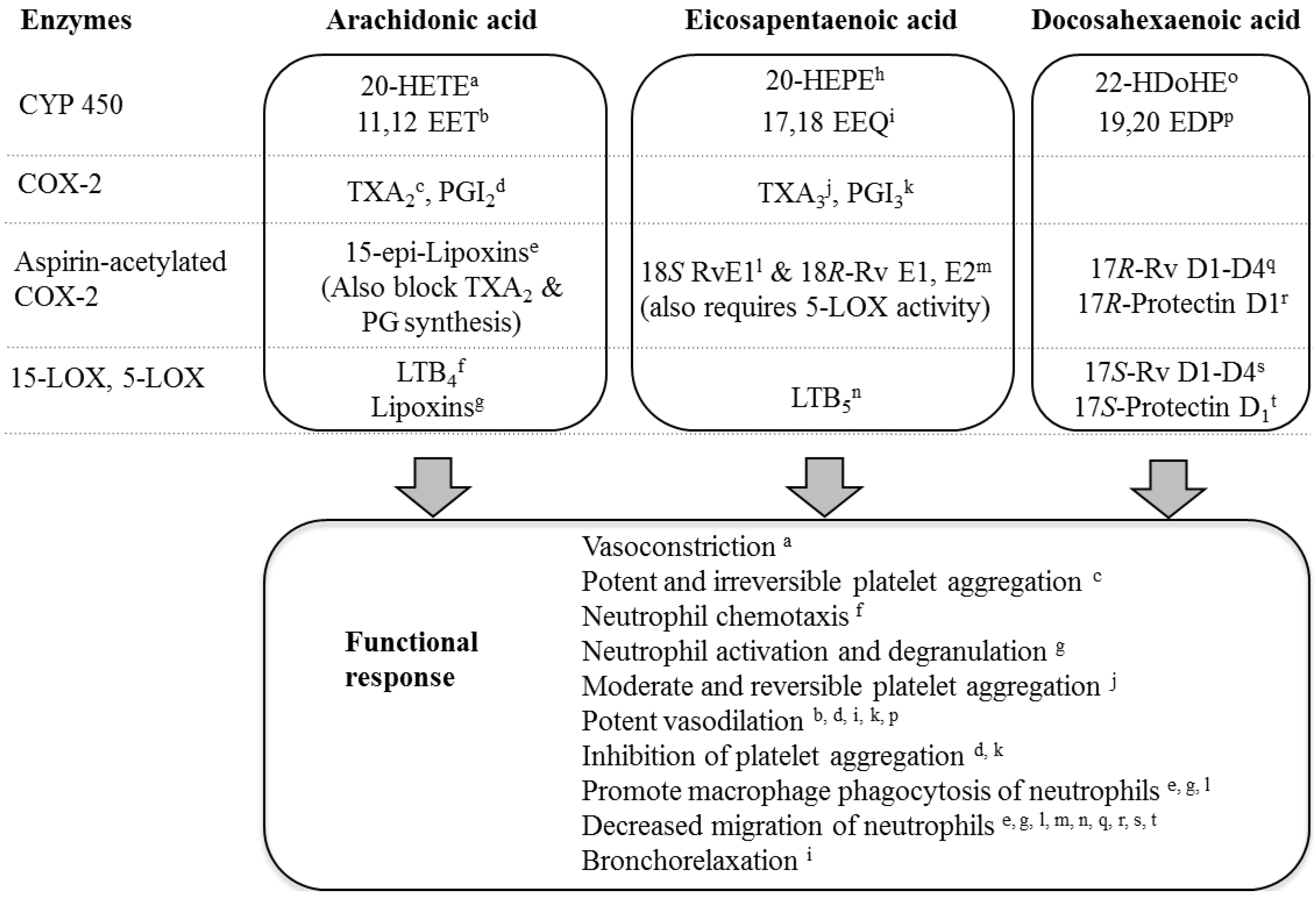
4. Conclusions
- Samples Availability: Available from the authors.
References
- Fortin, P.R.; Lew, R.A.; Liang, M.H.; Wright, E.A.; Beckett, L.A.; Chalmers, T.C.; Sperling, R.I. Validation of a meta-analysis: the effects of fish oil in rheumatoid arthritis. J. Clin. Epidemiol. 1995, 48, 1379–1390. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Giltay, E.J.; Grobbee, D.E.; Donders, A.R.T.; Kok, F.J. Blood pressure response to fish oil supplementation: Metaregression analysis of randomized trials. J. Hypertens. 2002, 20, 1493–1499. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer Disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef]
- Martins, J.G. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: Evidence from a meta-analysis of randomized controlled trials. J. Am. Coll. Nutr. 2009, 28, 525–542. [Google Scholar]
- Kwak, S.M.; Myung, S.-K.; Lee, Y.J.; Seo, H.G. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease. A meta-analysis of randomized, double-blind, placebo-controlled trials. Arch. Intern. Med. 2012, 172, 686–694. [Google Scholar] [CrossRef]
- Rizos, E.C.; Nitzani, E.E.; Bika, E.; Kostapanos, M.S.; Elisaf, M.S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012, 308, 1024–1033. [Google Scholar] [CrossRef]
- Burr, M.L.; Fehily, A.M.; Gilbert, J.F.; Rogers, S.; Holliday, R.M.; Sweetnam, P.M.; Elwood, P.C.; Deadman, N.M. Effects of changes in fat, and fibre intakes on death and myocardial reinfarction: Diet and reinfarction trial (DART). Lancet 1989, 2, 757–761. [Google Scholar]
- GISSI-Prevenzione Investigators. Dietary supplemenatation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [CrossRef]
- Kromhout, D. Omega-3 fatty acids and coronary heart disease. The final verdict? Curr. Opin. Lipidol. 2012, 23. [Google Scholar] [CrossRef]
- Laufs, U.; Schirmer, S.H. Margarines supplemented with low dose n-3 fatty acids are not effective in secondary prevention. Eur. Heart J. 2012, 33, 1555–1557. [Google Scholar] [CrossRef]
- O’Sullivan, T.A.; Ambrosini, G.; Beilin, L.J.; Mori, T.A.; Oddy, W.H. Dietary intake and food sources of fatty acids in Australian adolescents. Nutrition 2011, 27, 153–159. [Google Scholar] [CrossRef]
- Gibson, R.A.; Neumann, M.A.; Lien, E.L.; Boyd, K.A.; Tu, W.C. Docosahexaenoic acid synthesis from alpha-linolenic acid is inhibited by diets high in polyunsaturated fatty acids. Prostaglandins Leukot. Essent. Fatty Acids 2012. [Google Scholar]
- Burdge, G.C.; Finnegan, Y.E.; Minihane, A.M.; Williams, C.M.; Wootton, S.A. Effect of altered dietary n-3 fatty acid intake upon plasma lipid fatty acid composition, conversion of [13C]α-linolenic acid to longer-chain fatty acids and partitioning towards β-oxidation in older men. Br. J. Nutr. 2003, 90, 311–321. [Google Scholar] [CrossRef]
- Singer, P.; Berger, I.; Wirth, M.; Gödicke, W.; Jaeger, W.; Voigt, S. Slow desaturation and elongation of linoleic and α-linolenic acids as a rationale of eicosapentaenoic acid-rich diet to lower blood pressure and serum lipids in normal, hypertensive and hyperlipidemic subjects. Prostaglandins Leukot. Med. 1986, 24, 173–193. [Google Scholar] [CrossRef]
- Block, R.C.; Harris, W.S.; Pottala, J.V. Determinants of blood cell omega-3 fatty acid content. Open Biomark. J. 2008, 1, 1–6. [Google Scholar] [CrossRef]
- Astorg, P.; Arnault, N.; Czernichow, S.; Noisette, N.; Galan, P.; Hercberg, S. Dietary intakes and food sources of n- and n-3 PUFA in French adult men and women. Lipids 2004, 39, 527–535. [Google Scholar] [CrossRef]
- Howe, P.; Meyer, B.; Record, S.; Baghurst, K. Dietary intake of long-chain ω-3 polyunsaturated fatty acids: Contribution of meat sources. Nutrition 2006, 22, 47–53. [Google Scholar] [CrossRef]
- Yamagishi, K.; Iso, H.; Date, C.; Fukui, M.; Wakai, K.; Kikuchi, S.; Inaba, Y.; Tanabe, N.; Tamakoshi, A. Fish, ω-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women. J. Am. Coll. Cardiol. 2008, 52, 988–996. [Google Scholar] [CrossRef]
- Scientific Advisory Committee on Nutrition. Advice on Fish Consumption: Benefits & Risks. Available online: http://www.sacn.gov.uk/pdfs/fics_sacn_advice_fish.pdf (accessed on 5 September 2012).
- French Agency for Food, Environmental and Occupational Health & Safety. Opinion of the French Food Safety Agency Regarding the Benefits/Risks of Fish Consumption. Available online: http://www.anses.fr/Documents/NUT2008sa0123EN.pdf (accessed on 5 September 2012).
- Health Council of the Netherlands. Guidelines for a Healthy Diet. Available online: http://www.gezondheidsraad.nl/en/publications/guidelines-healthy-diet-2006-0 (accessed on 5 September 2012).
- Heart Foundation of Australia. Position Statement, 2008: Fish, Fish Oils, n-3 Polyunsaturated Fatty Acids and Cardiovascular Health. Available online: http://www.heartfoundation.org.au/ SiteCollection Documents/Fish-position-statement.pdf (accessed on 5 September 2012).
- Freeman, M.P.; Hibbeln, J.R.; Wisner, K.L.; Davis, J.M.; Mischoulon, D.; Peet, M.; Keck, P.E.; Marangell, L.B.; Richardson, A.J.; Lake, J.; Stoll, A.L. Omega-3 fatty acids: Evidence basis for treatment and future research in psychiatry. J. Clin. Psychiatry 2006, 67, 1954–1967. [Google Scholar] [CrossRef]
- McNamara, R.K. Evaluation of docosahexaenoic acid deficiency as a preventable risk factor for recurrent affective disorders: Current status, future direction, and dietary recommendations. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 223–231. [Google Scholar] [CrossRef]
- Sumich, A.; Matsudaira, T.; Gow, R.V.; Ibrahimovic, A.; Ghebremeskel, K.; Crawford, M.; Taylor, E. Resting state electroencephalographic correlates with red cell long-chain fatty acids, memory performance and age in adolescent boys with attention deficit disorder. Neuropharmacology 2009, 57, 708–714. [Google Scholar] [CrossRef]
- Mori, T.A.; Bao, D.Q.; Burke, V.; Puddey, I.B.; Beilin, L.J. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension 1999, 34, 253–260. [Google Scholar] [CrossRef]
- Rousseau-Ralliard, D.; Moreau, D.; Guilland, J.-C.; Raederstorff, D.; Grynberg, A. Docosahexaenoic acid, but not eicosapentaenoic acid, lowers ambulatory blood pressure and shortens interval QT in spontaneously hypertensive rats in vivo. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 269–277. [Google Scholar] [CrossRef]
- Park, Y.; Harris, W. EPA, but not DHA, decreases mean platelet volume in normal subjects. Lipids 2002, 37, 941–946. [Google Scholar] [CrossRef]
- Phang, M.; Garg, M.L.; Sinclair, A.J. Inhibition of platelet aggregation by omega-3 polyunsaturated fatty acids is gender specific-Redefining platelet response to fish oils. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 35–40. [Google Scholar] [CrossRef]
- Hamilton, K.; Brooks, P.; Holmes, M.; Cunningham, J.; Russell, F.D. Evaluation of the composition of omega-3 fatty acids in dietary oil supplements. Nutr. Diet. 2010, 67, 182–189. [Google Scholar] [CrossRef]
- Phang, M.; Sinclair, A.J.; Lincz, L.F.; Garg, M.L. Gender-specific inhibition of platelet aggregation following omega-3 fatty acid supplementation. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 109–114. [Google Scholar] [CrossRef]
- Salunkhe, D.; Tiwari, N.; Walujkar, S.; Bhadekar, R. Halomonas sp. nov., an EPA-producing mesophilic marine isolate from the Indian Ocean. Pol. J. Microbiol. 2011, 60, 73–78. [Google Scholar]
- Soltan, S.S.A.M.; Gibson, R.A. Levels of omega 3 fatty acids in Australian seafood. Asia Pac. J. Clin. Nutr. 2008, 17, 385–390. [Google Scholar]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Omega-3 fatty acids and cardiovascular disease. New recommendations from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 151–152. [Google Scholar] [CrossRef]
- World Health Organisation. Population Nutrient Intake Goals for Preventing Diet-Related Chronic Diseases. Available online: http://www.who.int/nutrition/topics/5_population_nutrient/en/index13.html (accessed on 5 September 2012).
- Nutrient Reference Values for Australia and New Zealand. Available online: http://www.nrv.gov.au/resources/_files/n35-fat.pdf (accessed on 5 September 2012).
- Cancer Council of Australia. Position Statement, 2009: Omega-3 Fatty Acids, Fish and Cancer Prevention. Available online: http://www.cancercouncil.com.au/wp-content/uploads/2010/09/Fish-Omega3-Fatty-Acids-and-Cancer-Position-Statement.pdf (accessed on 5 September 2012).
- International Society for the Study of Fatty Acids and Lipids. Intake of PUFA in Health Adults. Available online: http://www.issfal.org/statements/pufa-recommendations/statement-3 (accessed on 5 September 2012).
- Yusufi, A.N.K.; Cheng, J.; Thompson, M.A.; Walker, H.J.; Gray, C.E.; Warner, G.M.; Grande, J.P. Differential effects of low-dose docosahexaenoic acid and eicosapentaenoic acid on the regulation of mitogenic signalling pathways in mesangial cells. J. Lab. Clin. Med. 2003, 141, 318–330. [Google Scholar] [CrossRef]
- Owen, A.J.; Peter-Przyborowska, B.A.; Hoy, A.J.; McLennan, P.L. Dietary fish oil dose- and time-response effects on cardiac phospholipid fatty acid composition. Lipids 2004, 39, 955–961. [Google Scholar] [CrossRef]
- Verlengia, R.; Gorjão, R.; Kanunfre, C.C.; Bordin, S.; de Lima, T.M.; Martins, E.F.; Curi, R. Comparative effects of eicosapentaenoic acid and docosahexaenoic acid on proliferation, cytokine production, and pleiotropic gene expression in Jurkat cells. J. Nutr. Biochem. 2004, 15, 657–665. [Google Scholar] [CrossRef]
- Grimsgaard, S.; Bønaa, K.H.; Hansen, J.-B.; Myhre, E.S.P. Effects of highly purified eicosapentaenoic acid and docosahexaenoic acid on hemodynamics in humans. Am. J. Clin. Nutr. 1998, 68, 52–59. [Google Scholar]
- Weldon, S.M.; Mullen, A.C.; Loscher, C.E.; Hurley, L.A.; Roche, H.M. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J. Nutr. Biochem. 2007, 18, 250–258. [Google Scholar] [CrossRef]
- Caputo, M.; Zirpoli, H.; Torino, G.; Tecce, M.F. Selective regulation of UGT1A1 and SREBP-1c mRNA expression by docosahexaenoic, eicosapentaenoic, and arachidonic acids. J. Cell. Physiol. 2010, 226, 187–193. [Google Scholar]
- Hutchins, H.L.; Li, Y.; Hannon, K.; Watkins, B.A. Eicosapentaenoic acid decreases expression of anandamide synthesis enzyme and cannabinoid receptor 2 in osteoblast-like cells. J. Nutr. Biochem. 2011, 22, 195–200. [Google Scholar] [CrossRef]
- Oster, R.T.; Tishinsky, J.M.; Yuan, Z.; Robinson, L.E. Docosahexaenoic acid increases cellular adiponectin mRNA and secreted adiponectin protein, as well as PPARγ mRNA, in 3T3-L1 adipocytes. Appl. Physiol. Nutr. Metab. 2010, 35, 783–789. [Google Scholar] [CrossRef]
- Wang, D.; Hirase, T.; Nitto, T.; Soma, M.; Node, K. Eicosapentaenoic acid increases cytochrome P-450 2J2 gene expression and epoxyeicosatrienoic acid production via peroxisome proliferator-activated receptor γ in endothelial cells. J. Cardiol. 2009, 54, 368–374. [Google Scholar] [CrossRef]
- Mori, T.A.; Burke, V.; Puddey, I.B.; Watts, G.F.; O’Neal, D.N.; Best, J.D.; Beilin, L.J. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am. J. Clin. Nutr. 2000, 71, 1085–1094. [Google Scholar]
- Grimsgaard, S.; Bønaa, K.H.; Hansen, J.-B.; Nordøy, A. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. Am. J. Clin. Nutr. 1997, 66, 649–659. [Google Scholar]
- Egert, S.; Kannenberg, F.; Somoza, V.; Erbersdobler, H.F.; Wahrburg, U. Dietary α-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J. Nutr. 2009, 139, 861–868. [Google Scholar] [CrossRef]
- Woodman, R.J.; Mori, T.A.; Burke, V.; Puddey, I.B.; Watts, G.F.; Best, J.D.; Beilin, L.J. Docosahexaenoic acid but not eicosapentaenoic acid increases LDL particle size in treated hypertensive type 2 diabetic patients. Diabetes Care 2003, 26, 253. [Google Scholar] [CrossRef]
- Madsen, L.; Rustan, A.C.; Vaagenes, H.; Berge, K.; Dyrøy, E.; Berge, R.K. Eicosapentaenoic and docosahexaenoic acid affect mitochondrial and peroxisomal fatty acid oxidation in relation to substrate preference. Lipids 1999, 34, 951–963. [Google Scholar] [CrossRef]
- Leonardi, F.; Attorri, L.; di Benedetto, R.; di Biase, A.; Sanchez, M.; Nardini, M.; Salvati, S. Effect of arachidonic, eicosapentaenoic and docosahexaenoic acids on the oxidative status of C6 glioma cells. Free Radic. Res. 2005, 39, 865–874. [Google Scholar] [CrossRef]
- Tonutti, L.; Manzi, L.; Tacconi, M.T.; Bazzoni, G. Eicosapentaenoic acid inhibits endothelial cells migration in vitro. J. Angiogenesis Res. 2010, 2, 12. [Google Scholar] [CrossRef]
- Brown, E.R.; Subbaiah, P.V. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on human skin fibroblasts. Lipids 1994, 29, 825–829. [Google Scholar] [CrossRef]
- Madani, S.; Hichami, A.; Charkaoui-Malki, M.; Khan, N.A. Diacylglycerols containing omega 3 and omega 6 fatty acids bind to RasGRP and modulate MAP kinase activation. J. Biol. Chem. 2004, 279, 1176–1183. [Google Scholar]
- Woodman, R.J.; Mori, T.A.; Burke, V.; Puddey, I.B.; Barden, A.; Watts, G.F.; Beilin, L.J. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atherosclerosis 2003, 166, 85–93. [Google Scholar] [CrossRef]
- Barden, A.; Mas, E.; Henry, P.; Durand, T.; Galano, J.M.; Roberts, L.J.; Croft, K.D.; Mori, T.A. The effects of oxidation products of arachidonic acid and n3 fatty acids on vascular and platelet function. Free Radic. Res. 2011, 45, 469–476. [Google Scholar] [CrossRef]
- Pisani, L.F.; Lecchi, C.; Invernizzi, G.; Sartorelli, P.; Savoini, G.; Ceciliani, F. In vitro modulatory effect of ω-3 polyunsaturated fatty acid (EPA and DHA) on phagocytosis and ROS production of goat neutrophils. Vet. Immunol. Immunopathol. 2009, 131, 79–85. [Google Scholar] [CrossRef]
- Komatsu, W.; Ishihara, K.; Murata, M.; Saito, H.; Shinohara, K. Docosahexaenoic acid suppresses nitric oxide production and inducible nitric oxide synthase expression in interferon-γ plus lipopolysaccharide-stimulated murine macrophages by inhibiting the oxidative stress. Free Radic. Biol. Med. 2003, 34, 1006–1016. [Google Scholar] [CrossRef]
- Khairallah, R.J.; Sparagna, G.C.; Khanna, N.; O’Shea, K.M.; Hecker, P.A.; Kristian, T.; Fiskum, G.; des Rosiers, C.; Polster, B.M.; Stanley, W.C. Dietary supplementation with docosahexaenoic acid, but not eicosapentaenoic acid, dramatically alters cardiac mitochondrial phospholipid fatty acid composition and prevents permeability transition. Biochim. Biophys. Acta 2010, 1797, 1555–1562. [Google Scholar] [CrossRef]
- McLennan, P.; Howe, P.; Abeywardena, M.; Muggli, R.; Raederstorff, D.; Mano, M.; Rayner, T.; Head, R. The cardiovascular protective role of docosahexaenoic acid. Eur. J. Pharmacol. 1996, 300, 83–89. [Google Scholar] [CrossRef]
- Mori, T.A.; Watts, G.F.; Burke, V.; Hilme, E.; Puddey, I.B.; Beilin, L.J. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation 2000, 102, 1264–1269. [Google Scholar] [CrossRef]
- Liu, R.-Z.; Graham, K.; Clubrecht, D.D.; Lai, R.; Mackey, J.; Godbout, R. A fatty acid-binding protein 7/RXRβ pathway enhances survival and proliferation in triple-negative breast cancer. J. Pathol. 2012, 228, 310–321. [Google Scholar] [CrossRef]
- Balendiran, G.K.; Schnütgen, F.; Scapin, G.; Börchers, T.; Xhong, N.; Lim, K.; Godbout, R.; Spener, F.; Sacchettini, J.C. Crystal structure and thermodynamic analysis of human brain fatty acid-binding protein. J. Biol. Chem. 2000, 275, 27045–27054. [Google Scholar]
- Edwards, I.J.; O’Flaherty, J.T. Omega-3 fatty acids and PPARγ in cancer. PPAR Res. 2008, 2008, 358052. [Google Scholar]
- Comba, A.; Lin, Y.-H.; Eynard, A.R.; Valentich, M.A.; Fernandez-Zapico, M.E.; Pasqualini, M.E. Basic aspects of tumor cell fatty acid-regulated signalling and transcription factors. Cancer Metastasis Rev. 2011, 30, 325–342. [Google Scholar] [CrossRef]
- Li, H.; Ruan, X.Z.; Powis, S.H.; Fernando, R.; Mon, W.Y.; Wheeler, D.C.; Moorhead, J.F.; Varghese, Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: Evidence for a PPAR-γ-dependent mechanism. Kidney Int. 2005, 67, 867–874. [Google Scholar] [CrossRef]
- Yamamoto, K.; Itoh, T.; Abe, D.; Shimizu, M.; Kanda, T.; Koyama, T.; Nishikawa, M.; Tamai, T.; Ooizumi, H.; Yamada, S. Identification of putative metabolites of docosahexaenoic acid as potent PPARγ agonists and antidiabetic agents. Bioorg. Med. Chem. Lett. 2005, 15, 517–522. [Google Scholar]
- Itoh, T.; Yamamoto, K. Peroxisome proliferator activated receptor γ and oxidized docosahexaenoic acids as new class of ligand. Naunyn Schmiedeberg Arch. Pharmacol. 2008, 377, 541–547. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Yacoubian, S.; Lee, C.-H.; Yang, R.; Petasis, N.; Serhan, C.N. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 1660–1665. [Google Scholar]
- Zhao, Y.; Joshi-Barve, S.; Barve, S.; Chen, L.H. Eicosapentaenoic acid prevents LPS-induced TNF-α expression by preventing NF-κB activation. J. Am. Coll. Nutr. 2004, 23, 71–78. [Google Scholar]
- Wong, S.W.; Kwon, M.-J.; Choi, A.M.K.; Kim, H.-P.; Nakahira, K.; Hwang, D.H. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J. Biol. Chem. 2009, 284, 27384–27392. [Google Scholar]
- Hirasawa, A.; Tsumaya, K.; Awaji, T.; Katsuma, S.; Adachi, T.; Yamada, M.; Sugimoto, Y.; Miyazaki, S.; Tsujimoto, G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005, 11, 90–94. [Google Scholar] [CrossRef]
- Miyauchi, S.; Hirasawa, A.; Iga, T.; Liu, N.; Itsubo, C.; Sadakane, K.; Hara, T.; Tsujimoto, G. Distribution and regulation of protein expression of the free fatty acid receptor GPR120. Naunyn Schmiedeberg Arch. Pharmacol. 2009, 379, 427–434. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.Q.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Morishita, M.; Tanak, T.; Shida, T.; Takayama, K. Usefulness of colon targeted DHA and EPA as novel diabetes medications that promote intrinsic GLP-1 secretion. J. Control. Release 2008, 132, 99–104. [Google Scholar] [CrossRef]
- Burns, R.N.; Moniri, N.H. Agonism with the omega-3 fatty acids α-linolenic acid and docosahexaenoic acid mediates phosphorylation of both the short and long isoforms of the human GPR120 receptor. Biochem. Biophys. Res. Commun. 2010, 396, 1030–1035. [Google Scholar] [CrossRef]
- Judé, S.; Martel, E.; Vincent, F.; Besson, P.; Couet, C.; Ogilvie, G.K.; Pinault, M.; de Chalendar, C.; Bougnoux, P.; Richard, S.; et al. Dietary long-chain n-3 fatty acids modify blood and cardiac phospholipids and reduce protein kinase-C-δ and protein kinase-C-ε translocation. Br. J. Nutr. 2007, 98, 1143–1151. [Google Scholar]
- O’Shea, K.M.; Khairallah, R.J.; Sparagna, G.C.; Xu, W.; Hecker, P.A.; Robillard-Frayne, I.; des Rosiers, C.; Kristian, T.; Murphy, R.C.; Fiskum, G.; et al. Dietary ω-3 fatty acids alter cardiac mitochondrial phospholipid composition and delay Ca2+-induced permeability transition. J. Mol. Cell. Cardiol. 2009, 47, 819–827. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Schneider, I.; Meyer, H.; Neubronner, J.; von Schacky, C.; Hahn, A. Incorporation of EPA and DHA into plasma phospholipids in response to different omega-3 fatty acid formulations-A comparative bioavailability study of fish oil vs. krill oil. Lipids Health Dis. 2011, 10, 145. [Google Scholar] [CrossRef]
- Holub, B.J.; Bakker, D.J.; Skeaff, C.M. Alterations in molecular species of cholesterol esters formed via plasma lecithin-cholesterol acyltransferase in human subjects consuming fish oil. Atherosclerosis 1987, 66, 11–18. [Google Scholar]
- Subbaiah, P.V.; Kaufman, D.; Bagdade, J.D. Incorporation of dietary n-3 fatty acids into molecular species of phosphatidyl choline and cholesteryl ester in normal human plasma. Am. J. Clin. Nutr. 1993, 58, 360–380. [Google Scholar]
- Parks, J.S.; Thuren, T.Y.; Schmitt, J.D. Inhibition of lecithin:Cholesterol acyltransferase activity by synthetic phosphatidylcholine species containing eicosapentaenoic acid or docosahexaenoic acid in the sn-2 position. J. Lipid Res. 1992, 33, 879–887. [Google Scholar]
- Murphy, M.G.; Wright, V.; Ackman, R.G.; Horackova, M. Diets enriched in menhaden fish oil, seal oil, or shark liver oil have distinct effects on the lipid and fatty-acid composition of guinea pig heart. Mol. Cell. Biochem. 1997, 177, 257–269. [Google Scholar] [CrossRef]
- Ha, Y.C.; Barter, P.J. Differences in plasma cholesteryl ester transfer activity in sixteen vertebrate species. Comp. Biochem. Physiol. 1982, 71B, 265–269. [Google Scholar]
- Shikano, M.; Masuzawa, Y.; Yazawa, K.; Takayama, K.; Kudo, I.; Inoue, K. Complete discrimination of docosahexaenoate from arachidonate by 85 kDa cytosolic phospholipase A2 during the hydrolysis of diacyl- and alkenylacylglycerophosphoethanolamine. Biochim. Biophys. Acta 1994, 1212, 211–216. [Google Scholar] [CrossRef]
- Chaudry, A.A.; Wahle, K.W.; McClinton, S.; Moffat, L.E. Arachidonic acid metabolism in benign and malignant prostatic tissue in vitro: Effects of fatty acids and cyclooxygenase inhibitors. Int. J. Cancer 1994, 57, 176–180. [Google Scholar] [CrossRef]
- Raederstorff, D.; Pantze, M.; Bachmann, H.; Moser, U. Anti-inflammatory properties of docosahexaenoic and eicosapentaenoic acids in phorbol-ester-induced mouse ear inflammation. Int. Arch. Allergy Immunol. 1996, 111, 284–290. [Google Scholar] [CrossRef]
- Payan, D.G.; Wong, M.Y.; Chernov-Rogan, T.; Valone, F.H.; Pickett, W.C.; Blake, V.A.; Gold, W.M.; Goetzl, E.J. Alterations in human leukocyte function induced by ingestion of eicosapentaenoic acid. J. Clin. Immunol. 1986, 6, 402–410. [Google Scholar] [CrossRef]
- Hung, P.; Kaku, S.; Yunoki, S.; Ohkura, K.; Gu, J.-Y.; Ikeda, I.; Sugano, M.; Yazawa, K.; Yamada, K. Dietary effect of EPA-rich and DHA-rich fish oils on the immune function of Sprague-Dawley rats. Biosci. Biotechnol. Bochem. 1999, 63, 135–140. [Google Scholar] [CrossRef]
- Hawcroft, G.; Loadman, P.M.; Belluzzi, A.; Hull, M.A. Effect of eicosapentaenoic acid on E-type prostaglandin synthesis and EP4 receptor signalling in human colorectal cancer cells. Neoplasia 2010, 12, 618–627. [Google Scholar]
- Pehowich, D.J. Dietary n-3 fatty acids alter angiotensin-induced contraction and 1,2-diacylglycerol fatty acid composition in thoracic aortas from diabetic rats. Prostaglandins Leukot. Essent. Fatty Acids 1998, 58, 301–309. [Google Scholar] [CrossRef]
- Hichami, A.; Morin, C.; Rousseau, E.; Khan, N.A. Diacylglycerol-containing docosahexaenoic acid in acyl chain modulates airway smooth muscle tone. Am. J. Respir. Cell Mol. Biol. 2005, 33, 378–386. [Google Scholar] [CrossRef]
- Fowler, K.H.; McMurray, D.N.; Fan, Y.-Y.; Aukema, H.M.; Chapkin, R.S. Purified dietary n-3 polyunsaturated fatty acids alter diacylglycerol mass and molecular species composition in concanavalin A-stimulated murine splenocytes. Biochim. Biophys. Acta 1993, 1210, 89–96. [Google Scholar]
- Madani, S.; Hichami, A.; Legrand, A.; Belleville, J.; Khan, N.A. Implication of acyl chain of diacylglycerols in activation of different isoforms of protein kinase C. FASEB J. 2001, 15, 2595–2601. [Google Scholar] [CrossRef]
- Maddipati, K.R.; Zhou, S.-L. Stability and analysis of eicosanoids and docosanoids in tissue culture media. Prostaglandins Other Lipid Mediat. 2011, 94, 59–72. [Google Scholar] [CrossRef]
- Harmon, S.D.; Fang, X.; Kaduce, T.L.; Hu, S.; Gopal, V.R.; Falck, J.R.; Spector, A.A. Oxygenation of ω-3 fatty acids by human cytochrome P450 4F3B: Effect on 20-hydroxyeicosatetraenoic acid production. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 169–177. [Google Scholar] [CrossRef]
- Arnold, C.; Markovic, M.; Blossey, K.; Wallukat, G.; Fischer, R.; Dechend, R.; Konkel, A.; von Schacky, C.; Luft, F.C.; Muller, D.N.; et al. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of ω-3 fatty acids. J. Biol. Chem. 2010, 285, 32720–32733. [Google Scholar]
- Konkel, A.; Schunck, W.-H. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim. Biophys. Acta 2011, 1814, 210–222. [Google Scholar]
- Shearer, G.C.; Harris, W.S.; Pedersen, T.L.; Newman, J.W. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J. Lipid Res. 2010, 51, 2074–2081. [Google Scholar] [CrossRef]
- Oh, S.F.; Pillai, P.S.; Recchiuti, A.; Yang, R.; Serhan, C.N. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J. Clin. Invest. 2011, 121, 569–581. [Google Scholar] [CrossRef]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.-L. A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef]
- Hong, S.; Gronert, K.; Devchand, P.R.; Moussignac, R.-L.; Serhan, C.N. Novel Docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. J. Biol. Chem. 2003, 278, 14677–14687. [Google Scholar]
- Sun, Y.-P.; Oh, S.F.; Uddin, J.; Yang, R.; Gotlinger, K.; Campbell, E.; Colgan, S.P.; Petasis, N.A.; Serhan, C.N. Resolvin D1 and its aspirin-triggered 17R epimer: Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J. Biol. Chem. 2007, 282, 9323–9334. [Google Scholar]
- Ye, D.; Zhang, D.; Oltman, C.; Dellsperger, K.; Lee, H.-C.; Vanrollins, M. Cytochrome P-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J. Pharmacol. Exp. Ther. 2002, 303, 768–776. [Google Scholar] [CrossRef]
- Hercule, H.C.; Salanova, B.; Essin, K.; Honeck, H.; Falck, J.R.; Sausbier, M.; Ruth, P.; Schunck, W.-H.; Luft, F.C.; Gollasch, M. The vasodilator 17,18-epoxyeicosatetraenoic acid targets the pore-forming BKα channel subunit in rodents. Exp. Physiol. 2007, 92, 1067–1076. [Google Scholar] [CrossRef]
- Fredman, G.; Serhan, C.N. Specialized proresolving mediator targets for RvE1 and RvD1 in peripheral blood and mechanisms of resolution. Biochem. J. 2011, 437, 185–197. [Google Scholar] [CrossRef]
- Wang, J.-S.; Singh, H.; Zhang, F.; Ishizuka, T.; Deng, H.; Kemp, R.; Wolin, M.S.; Hintze, T.H.; Abraham, N.G.; Nasjletti, A.; Laniado-Schwartzman, M. Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ. Res. 2006, 98, 962–969. [Google Scholar] [CrossRef]
- Cheng, J.; Ou, J.-S.; Singh, H.; Falxk, J.R.; Narsimhaswany, D.; Pritchard, K.A.; Laniado-Schwartzman, M. 20-hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1018–H1026. [Google Scholar] [CrossRef]
- Campbell, W.B.; Gebremedhin, D.; Pratt, P.F.; Harder, D.R. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 1996, 78, 415–423. [Google Scholar] [CrossRef]
- Fisslthaler, B.; Popp, R.; Kiss, L.; Potente, M.; Harder, D.R.; Fleming, I.; Busse, R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 1999, 401, 493–497. [Google Scholar] [CrossRef]
- Wada, M.; DeLong, C.J.; Hong, Y.H.; Rieke, C.J.; Song, I.; Sidhu, R.S.; Yuan, C.; Warnock, M.; Schmaier, A.H.; Yokoyama, C.; et al. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived substrates and products. J. Biol. Chem. 2007, 282, 22254–22266. [Google Scholar]
- Needleman, P.; Raz, A.; Minkes, M.S.; Ferrendelli, J.A.; Sprecher, H. Triene prostaglandins: Prostacyclin and thromboxane biosynthesis and unique biological properties. Proc. Natl. Acad. Sci. USA 1979, 76, 944–948. [Google Scholar] [CrossRef]
- Takano, T.; Clish, C.B.; Gronert, K.; Petasis, N.; Serhan, C.N. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J. Clin. Invest. 1998, 101, 819–826. [Google Scholar] [CrossRef]
- Lee, T.H.; Mencia-Huerta, J.-M.; Shih, C.; Corey, E.J.; Lewis, R.A.; Austen, K.F. Characterization and biologic properties of 5,12-dihydroxy derivatives of eicosapentaenoic acid, including leukotriene B5 and the double lipoxygenase product. J. Biol. Chem. 1984, 259, 2383–2389. [Google Scholar]
- Goldman, D.W.; Pickett, W.C.; Goetzl, E.J. Human neutrophil chemotactic and degranulating activities of leukotriene B5 (LTB5) derived from eicosapentaenoic acid. Biochem. Biophys. Res. Commun. 1983, 117, 282–288. [Google Scholar] [CrossRef]
- Serhan, C.N.; Hamberg, M.; Samuelsson, B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc. Natl. Acad. Sci. USA 1984, 81, 5335–5339. [Google Scholar] [CrossRef]
- Serhan, C.N. A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Histochem. Cell Biol. 2004, 122, 305–321. [Google Scholar] [CrossRef]
- Morin, C.; Sirois, M.; Echave, V.; Rizcallah, E.; Rousseau, E. Relaxing effects of 17(18)-EpETE on arterial and airway smooth muscles in human lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, L130–L139. [Google Scholar]
- Tjonahen, E.; Oh, S.F.; Siegelman, J.; Elangovan, S.; Percarpio, K.B.; Hong, S.; Arita, M.; Serhan, C.N. Resolvin E2: Identification and anti-inflammatory actions: Pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem. Biol. 2006, 13, 1193–1202. [Google Scholar] [CrossRef]
- Serhan, C.N.; Fredman, G.; Yang, R.; Karamnov, S.; Belayev, L.S.; Bazan, N.G.; Zhu, M.; Winkler, J.W.; Petasis, N.A. Novel proresolving aspirin-triggered DHA pathway. Chem. Biol. 2011, 18, 976–987. [Google Scholar] [CrossRef]
- Serhan, C.N.; Gotlinger, K.; Hong, S.; Lu, Y.; Siegelman, J.; Baer, T.; Yang, R.; Colgan, S.P.; Petasis, N.A. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: Assignments of dihydroxy-containing docosatrienes. J. Immunol. 2006, 176, 1848–1859. [Google Scholar]
- Schwarz, D.; Kisselev, P.; Chernogolov, A.; Schunck, W.-H.; Roots, I. Human CYP1A1 variants lead to differential eicosapentaenoic acid metabolite patterns. Biochem. Biophys. Res. Commun. 2005, 336, 779–783. [Google Scholar] [CrossRef]
- Arita, M.; Ohira, T.; Sun, Y.-P.; Elangovan, S.; Chiang, N.; Serhan, C.N. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 2007, 178, 3912–3917. [Google Scholar]
- Titos, E.; Rius, B.; González-Périz, A.; López-Vicario, C.; Morán-Salvador, E.; Martínez-Clemente, M.; Arroyo, V.; Clária, J. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J. Immunol. 2011, 187, 5408–5418. [Google Scholar] [CrossRef]
- Bannenberg, G.L.; Chiang, N.; Ariel, A.; Arita, M.; Tjonahen, E.; Gotlinger, K.H.; Hong, S.; Serhan, C.N. Molecular circuits of resolution: Formation and actions of resolvins and protectins. J. Immunol. 2005, 174, 4345–4355. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Russell, F.D.; Bürgin-Maunder, C.S. Distinguishing Health Benefits of Eicosapentaenoic and Docosahexaenoic Acids. Mar. Drugs 2012, 10, 2535-2559. https://doi.org/10.3390/md10112535
Russell FD, Bürgin-Maunder CS. Distinguishing Health Benefits of Eicosapentaenoic and Docosahexaenoic Acids. Marine Drugs. 2012; 10(11):2535-2559. https://doi.org/10.3390/md10112535
Chicago/Turabian StyleRussell, Fraser D., and Corinna S. Bürgin-Maunder. 2012. "Distinguishing Health Benefits of Eicosapentaenoic and Docosahexaenoic Acids" Marine Drugs 10, no. 11: 2535-2559. https://doi.org/10.3390/md10112535





