Five New Diterpenoids from an Okinawan Soft Coral, Cespitularia sp.
Abstract
:1. Introduction
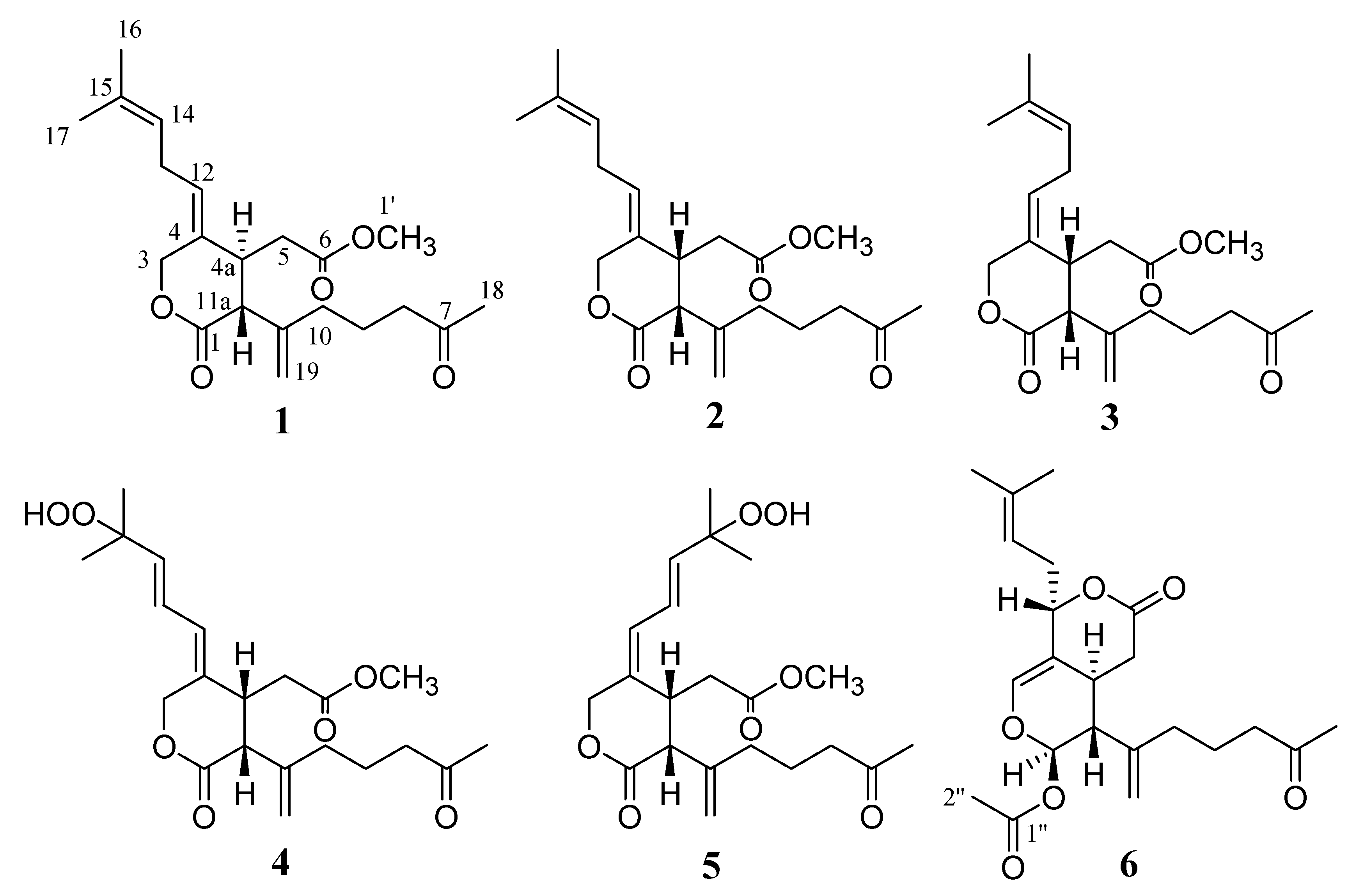
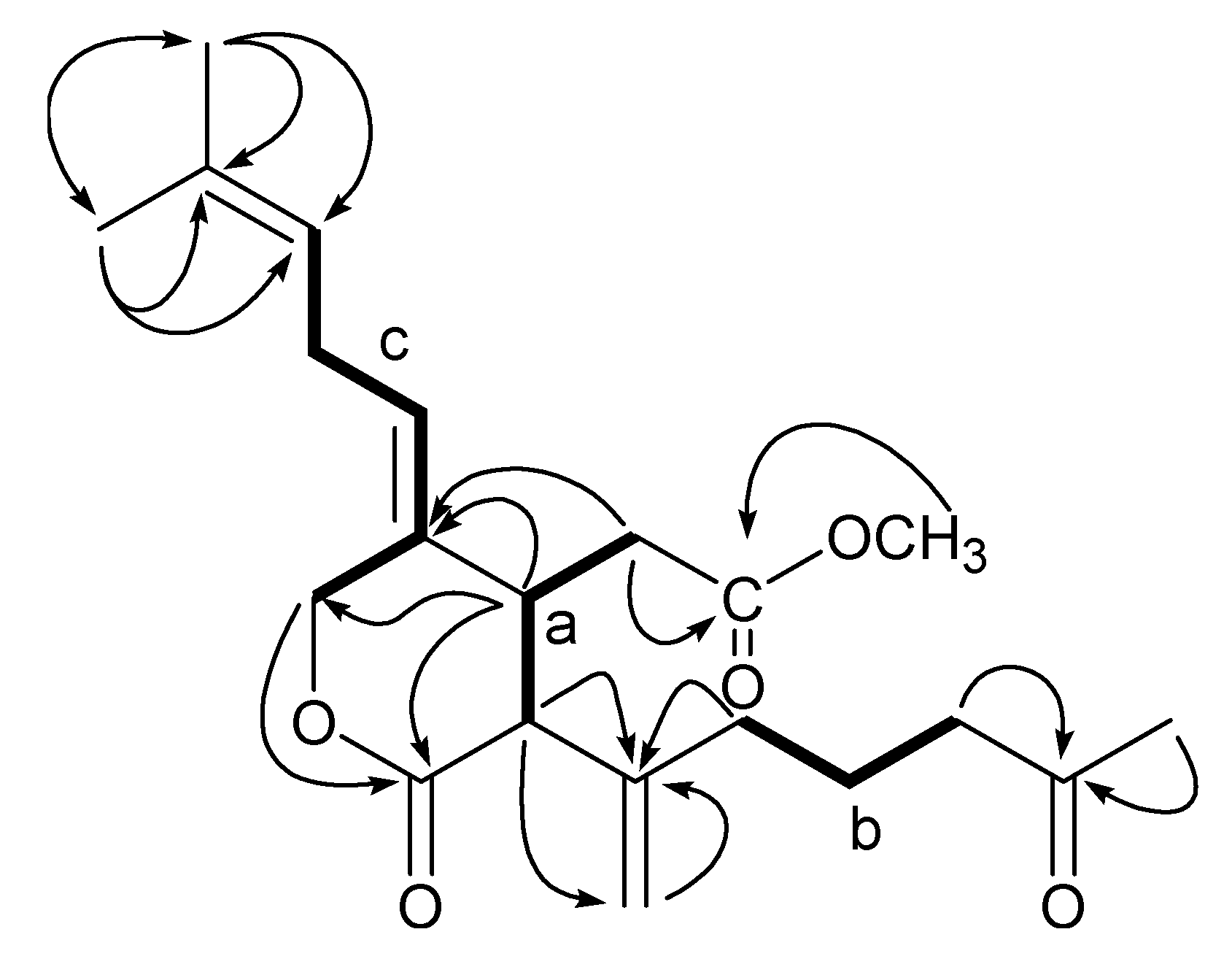
2. Results and Discussion
Structure Analysis and Characterization of Compounds 1–6
| δH (mult., J in Hz) | ||||||
|---|---|---|---|---|---|---|
| H No. | 1 | 2 | 3 | 4 | 5 | 6 |
| 1 | 5.94 (d, 7.5) | |||||
| 3 | 4.91 (s) | 4.89 (m) | 4.60 (d, 12.9) | 5.03 (s) | 4.94 (d, 14.0) | 6.38 (s) |
| 4.87 (d, 12.9) | 4.70 (d, 14.0) | |||||
| 4a | 3.12 (ddd, 11.6, 5.2, 4.3) | 3.12 (br q, 6.4) | 3.34 (br q, 6.1) | 3.22 (br q, 6.2) | 3.69 (br q, 6.0) | 2.72 (m) |
| 5 | 2.55 (dd, 16.7, 4.3) | 2.51 (dd, 16.1, 6.9) | 2.54 (dd, 16.3, 7.6) | 2.55 (dd, 16.5, 6.6) | 2.57 (dd, 16.0, 5.9) | 2.29 (dd, 18.6, 12.5) |
| 2.58 (dd, 16.7, 5.2) | 2.56 (dd, 16.1, 6.5) | 2.56 (dd, 16.3, 4.7) | 2.60 (dd, 16.5, 6.5) | 2.62 (dd, 16.0, 6.9) | 2.76 (dd, 18.6, 6.9) | |
| 8 | 2.43 (t, 7.3) | 2.47 (m) | 2.48 (m) | 2.48 (m) | 2.50 (m) | 2.44 (t, 7.0) |
| 9 | 1.71 (m) | 1.81 (m) | 1.79 (m) | 1.78 (m) | 1.80 (m) | 1.73 (q, 7.0) |
| 10 | 2.02 (m) | 2.08 (m) | 2.09 (m) | 2.08 (m) | 2.10 (m) | 1.79 (m) |
| 2.02 (m) | ||||||
| 11a | 3.10 (d, 11.6) | 3.38 (d, 6.4) | 3.51 (d, 6.1) | 3.43 (d, 6.2) | 3.50 (d, 6.0) | 2.20 (t, 8.0) |
| 12 | 5.46 (t, 7.4) | 5.41 (tq, 7.4, 1.6) | 5.50 (t, 7.0) | 6.07 (d, 11.0) | 6.10 (d, 11.1) | 4.75 (t, 7.5) |
| 13 | 2.64 (m) | 2.65 (m) | 2.75 (m) | 6.21 (dd, 15.3, 11.0) | 6.53(dd, 15.4, 11.1) | 2.43 (m) |
| 2.52 (m) | ||||||
| 14 | 4.98 (br t, 7.0) | 5.00 (br t, 7.0) | 5.03 (br t, 7.0) | 5.85 (d, 15.3) | 5.85 (d, 15.4) | 5.80 (t, 7.5) |
| 16 | 1.60 (s) | 1.60 (s) | 1.63 (s) | 1.36 (s) | 1.36 (s) | 1.61 (s) |
| 17 | 1.68 (s) | 1.69 (s) | 1.70 (s) | 1.56 (s) | 1.55 (s) | 1.70 (s) |
| 18 | 2.13 (s) | 2.14 (s) | 2.13 (s) | 2.14 (s) | 2.14 (s) | 2.12 (s) |
| 19a | 5.09 (s) | 4.94 (s) | 4.95 (s) | 4.95 (s) | 4.96 (s) | 4.91 (s) |
| 19b | 5.13 (s) | 5.07 (s) | 5.07 (s) | 5.08 (s) | 5.08 (s) | 5.01 (s) |
| 1′ | 3.61 (s) | 3.66 (s) | 3.65 (s) | 3.68 (s) | 3.66 (s) | |
| 2″ | 2.08 (s) | |||||
| δc | ||||||
|---|---|---|---|---|---|---|
| C No. | 1 | 2 | 3 | 4 | 5 | 6 |
| 1 | 170.9 (C) | 171.8 (C) | 171.8 (C) | 171.4 (C) | 172.0 (C) | 92.9 (CH) |
| 3 | 66.8 (CH2) | 66.7 (CH2) | 72.0 (CH2) | 66.7 (CH2) | 71.5 (CH2) | 137.7 (CH) |
| 4 | 129.0 (C) | 129.8 (C) | 130.5 (C) | 132.2 (C) | 132.2 (C) | 110.4 (C) |
| 4a | 39.7 (CH) | 38.2 (CH) | 33.5 (CH) | 38.3 (CH) | 33.7 (CH) | 31.1 (CH) |
| 5 | 34.0 (CH2) | 38.9 (CH2) | 37.8 (CH2) | 38.8 (CH2) | 38.6 (CH2) | 34.9 (CH2) |
| 6 | 172.4 (C) | 171.9 (C) | 172.0 (C) | 171.7 (C) | 171.8 (C) | 169.9 (C) |
| 7 | 208.5 (C) | 208.7 (C) | 208.8 (C) | 208.8 (C) | 210.0 (C) | 208.1 (C) |
| 8 | 43.0 (CH2) | 42.9 (CH2) | 42.9 (CH2) | 42.9 (CH2) | 42.8 (CH2) | 42.9 (CH2) |
| 9 | 21.5 (CH2) | 21.5 (CH2) | 21.6 (CH2) | 21.5 (CH2) | 21.9 (CH2) | 21.5 (CH2) |
| 10 | 34.1 (CH2) | 33.0 (CH2) | 33.3 (CH2) | 33.0 (CH2) | 33.2 (CH2) | 34.9 (CH2) |
| 11 | 143.0 (C) | 143.3 (C) | 143.4 (C) | 143.0 (C) | 143.1 (C) | 145.1 (C) |
| 11a | 56.1 (CH) | 52.4 (CH) | 51.9 (CH) | 52.2 (CH) | 52.3 (CH) | 48.8 (CH) |
| 12 | 128.5 (CH) | 127.9 (CH) | 129.6 (CH) | 127.0 (CH) | 128.0 (CH) | 79.5 (CH) |
| 13 | 26.8 (CH2) | 26.7 (CH2) | 27.1 (CH2) | 123.7 (CH) | 123.6 (CH) | 35.1 (CH2) |
| 14 | 120.9 (CH) | 121.0 (CH) | 120.8 (CH) | 139.9 (CH) | 141.1 (CH) | 117.9 (CH) |
| 15 | 133.5 (C) | 133.5 (C) | 133.7 (C) | 82.3 (C) | 82.2 (C) | 136.0 (C) |
| 16 | 17.9 (CH3) | 17.9 (CH3) | 18.0 (CH3) | 24.4 (CH3) | 25.1 (CH3) | 18.2 (CH3) |
| 17 | 25.8 (CH3) | 25.2 (CH3) | 25.8 (CH3) | 24.5 (CH3) | 24.4 (CH3) | 25.9 (CH3) |
| 18 | 30.1 (CH3) | 30.1 (CH3) | 30.1 (CH3) | 30.2 (CH3) | 30.2 (CH3) | 30.1 (CH3) |
| 19 | 115.6 (CH2) | 114.5 (CH2) | 114.5 (CH2) | 114.7 (CH2) | 115.0 (CH2) | 113.7 (CH2) |
| 1′ | 52.0 (CH3) | 51.9 (CH3) | 52.0 (CH3) | 52.1 (CH3) | 52.2 (CH3) | |
| 1″ | 169.3 (C) | |||||
| 2″ | 20.9 (CH3) | |||||
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Materials
3.3. Extraction and Compounds Isolation
4. Conclusion
Acknowledgments
Conflict of Interest
References
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2002, 19, 1–48. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef]
- Ueda, K.; Hu, Y. Haterumalide, B: A new cytotoxicmacrolide from an Okinawan ascidian Lissoclinum sp. Tetrahedron Lett. 1999, 40, 6305–6308. [Google Scholar] [CrossRef]
- Takada, N.; Sato, H.; Suenaga, K.; Arimoto, H.; Yamada, K.; Ueda, K.; Uemura, D. Isolation and structures of haterumalides NA, NB, NC, ND, and NE, novel macrolides from an Okinawan sponge Ircinia sp. Tetrahedron Lett. 1999, 40, 6309–6312. [Google Scholar] [CrossRef]
- Kokubo, S.; Yogi, K.; Uddin, M.J.; Inuzuka, T.; Suenaga, K.; Ueda, K.; Uemura, D. Kohamaic acids A and B, novel cytotoxicsesterterpenic acids, from the marine sponge Ircinia sp. Chem. Lett. 2001, 2, 176–177. [Google Scholar]
- Kobayashi, M.; Yasuzawa, T.; Kobayashi, Y.; Kyogoku, Y.; Kitagawa, I. Alcyonolide, a novel diterpenoid from a soft coral. Tetrahedron Lett. 1981, 22, 4445–4448. [Google Scholar] [CrossRef]
- Vanderah, D.J.; Steudler, P.A.; Ciereszko, L.S.; Schmitz, F.J.; Ekstrand, J.D.; Van der Helm, D. Marine natural products. Xenicin: A diterpenoid possessing a nine-membered ring from the soft coral, Xenia elongata. J. Am. Chem. Soc. 1997, 99, 5780–5784. [Google Scholar]
- Kashman, Y.; Groweiss, A. New diterpenoids from the soft corals Xenia macrospiculata and Xenia obscuronata. J. Org. Chem. 1980, 45, 3814–3824. [Google Scholar] [CrossRef]
- Fattorusso, E.; Romano, A.; Taglialatela-Scafati, O.; Achmad, M.J.; Bavestrello, G.; Cerrano, C. Xenimanadins A–D, a family of xenicane diterpenoids from the Indonesian soft coral Xenia sp. Tetrahedron 2008, 64, 3141–3146. [Google Scholar]
- Coll, J.C.; Kearns, P.S.; Rideout, J.A. Isolation of a novel diterpene triacetate from two soft corals of the order alcyonacea. J. Nat. Prod. 1998, 61, 835–837. [Google Scholar] [CrossRef]
- Ueda, K.; Kadekaru, T.; Siwu, E.R.O.; Kita, M.; Uemura, D. Haterumadysins A–D, sesquiterpenes from the Okinawan marine sponge Dysidea chlorea. J. Nat. Prod. 2006, 69, 1077–1079. [Google Scholar] [CrossRef]
- Samples Availability: Available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Roy, P.K.; Maarisit, W.; Roy, M.C.; Taira, J.; Ueda, K. Five New Diterpenoids from an Okinawan Soft Coral, Cespitularia sp. Mar. Drugs 2012, 10, 2741-2748. https://doi.org/10.3390/md10122741
Roy PK, Maarisit W, Roy MC, Taira J, Ueda K. Five New Diterpenoids from an Okinawan Soft Coral, Cespitularia sp. Marine Drugs. 2012; 10(12):2741-2748. https://doi.org/10.3390/md10122741
Chicago/Turabian StyleRoy, Prodip K., Wilmar Maarisit, Michael C. Roy, Junsei Taira, and Katsuhiro Ueda. 2012. "Five New Diterpenoids from an Okinawan Soft Coral, Cespitularia sp." Marine Drugs 10, no. 12: 2741-2748. https://doi.org/10.3390/md10122741
APA StyleRoy, P. K., Maarisit, W., Roy, M. C., Taira, J., & Ueda, K. (2012). Five New Diterpenoids from an Okinawan Soft Coral, Cespitularia sp. Marine Drugs, 10(12), 2741-2748. https://doi.org/10.3390/md10122741




