Phlorotannin Extracts from Fucales Characterized by HPLC-DAD-ESI-MSn: Approaches to Hyaluronidase Inhibitory Capacity and Antioxidant Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC-DAD-ESI-MSn Phlorotannins Analysis
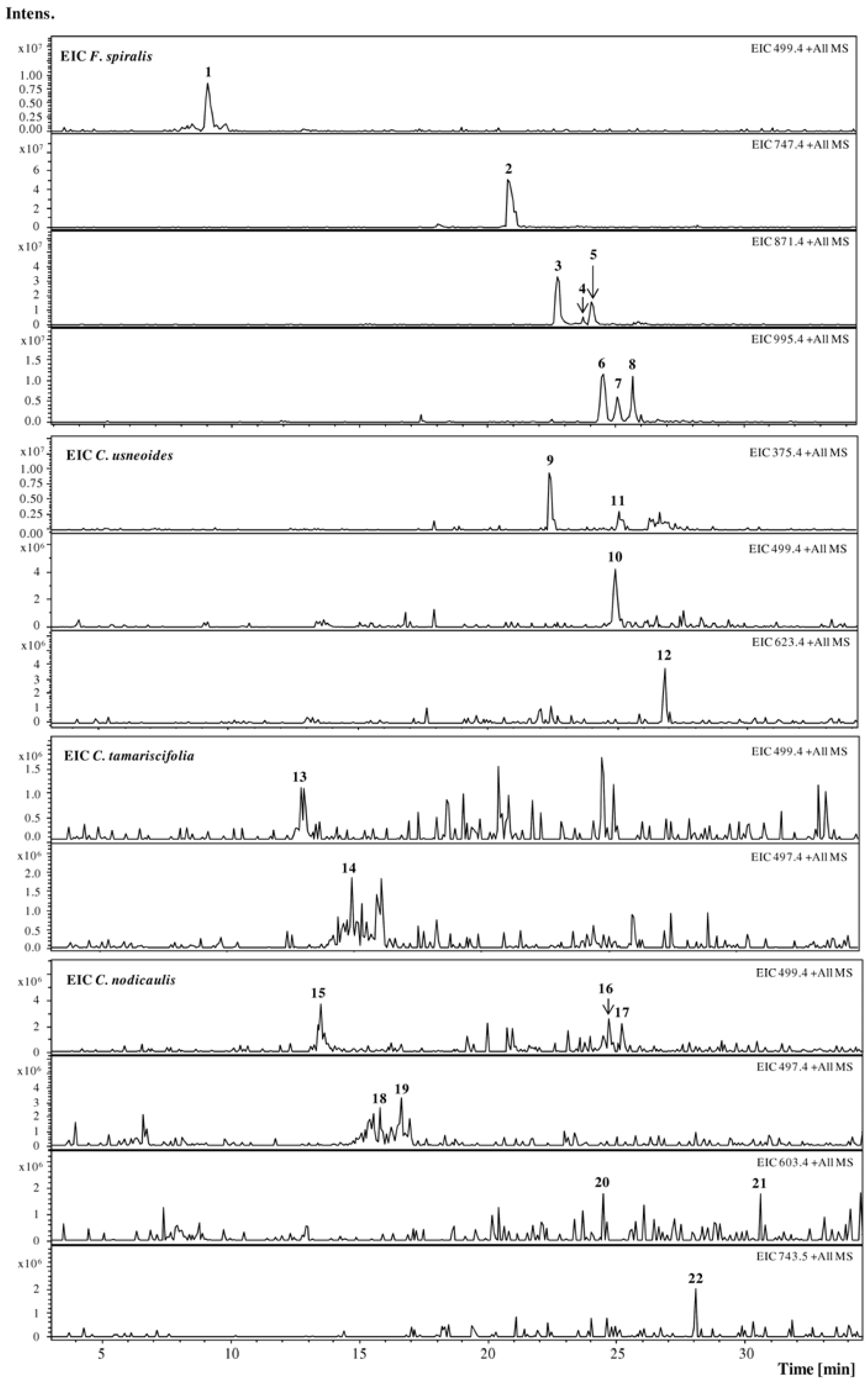
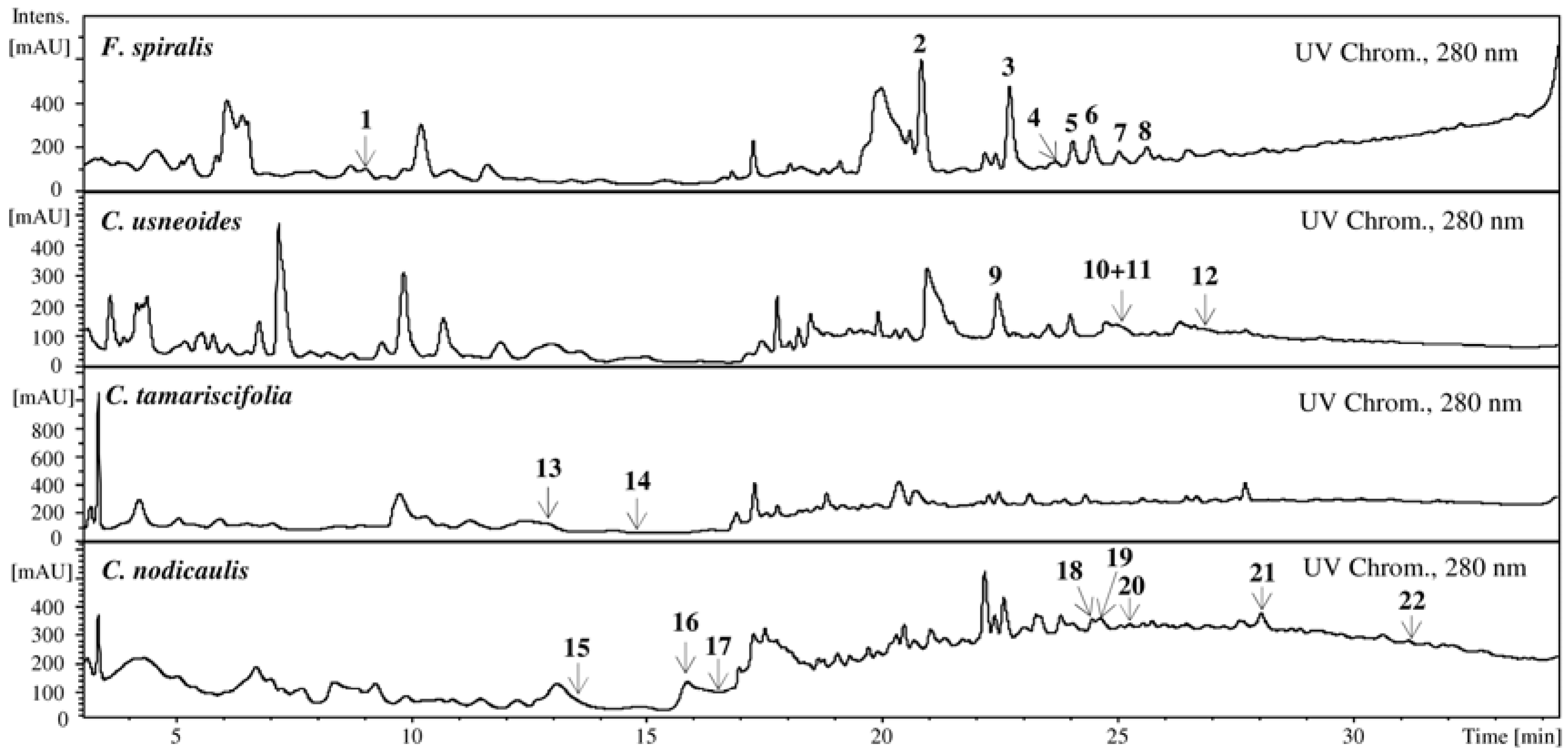
| Rt (min) | UV (nm) | [M + H]+, m/z | +MS2[M + H]+, m/z (%) | |
|---|---|---|---|---|
| 1 | 9.0 | — | 499 | 481 (80, −18), 439 (53, −18 − 42), 392 (25), 373 (35, −126), 355 (100, −126 − 18) |
| 2 | 20.8 | 248, 268sh | 747 | 729 (45, −18), 711 (70, −36), 585 (25, −126 − 36), 571 (35, −126 – 36 − 14), 220 (100) |
| 3 | 22.7 | 244, 270sh | 871 | 853 (100, −18), 835 (60, −36), 727 (30, −126 − 18), 709 (55, −126 − 36), 681 (30), 641 (12), 585 (15), 570 (7), 484 (35) |
| 4 | 23.6 | — | 871 | 853 (100, −18), 835 (76, −36), 727 (38, −126 − 18), 709 (17, −126 − 36), 603 (22), 585 (20), 517 (9), 403 (7), 310 (4) |
| 5 | 24.0 | 274sh | 871 | 853 (67, −18), 835 (100, −36), 725 (25), 709 (55, −126 − 36), 694 (58), 681 (21), 569 (17), 551 (18), 435 (20) |
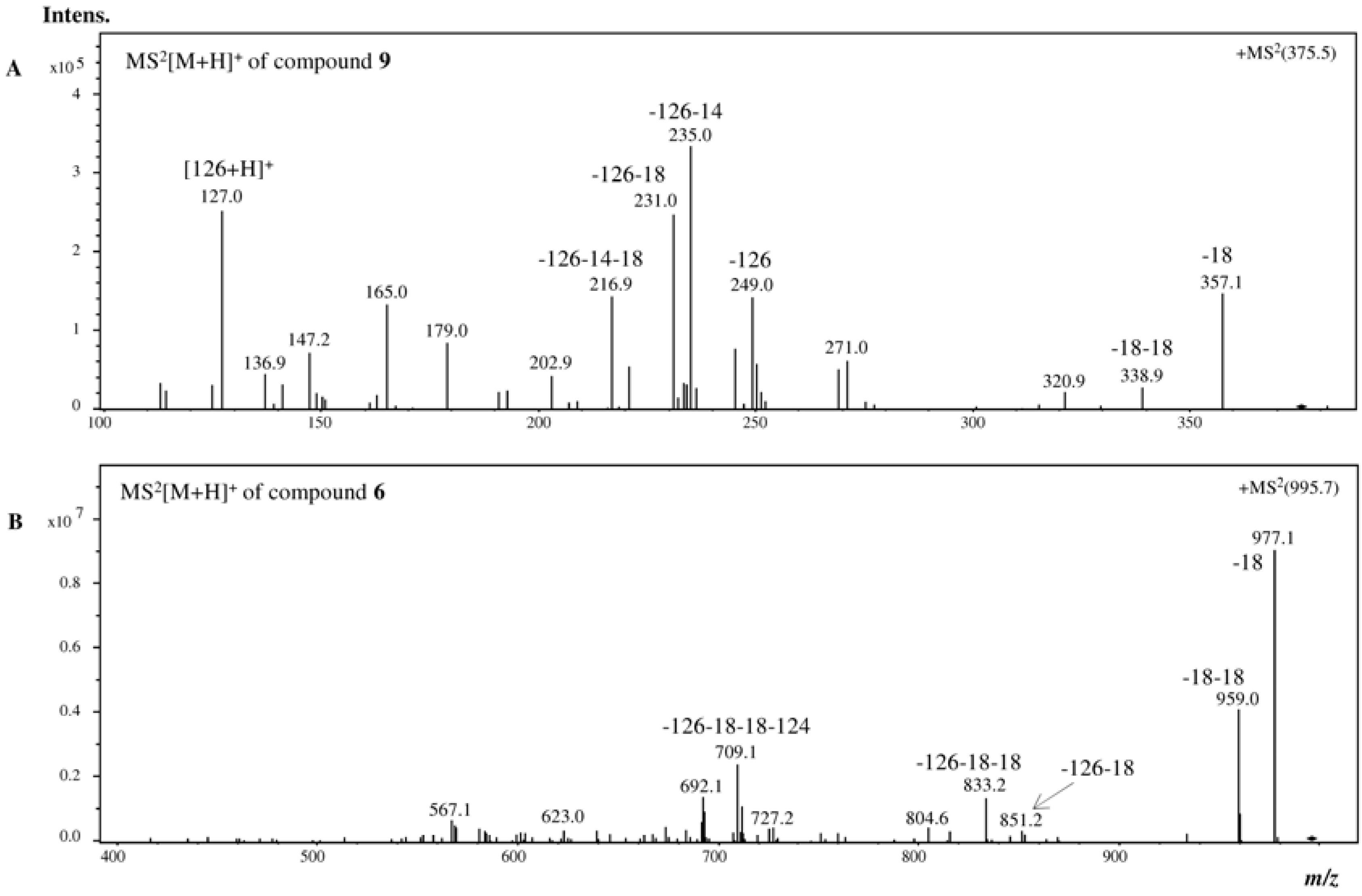
| 6 | 24.4 | 272sh | 995 | 977 (100, −18), 959 (45, −36), 851 (3, −126 − 18), 833 (15, −126 − 36), 709 (26, −126 − 124 − 36), 692 (15) |
| 7 | 25.0 | 274sh | 995 | 977 (100, −18), 959 (27, −36), 869 (7, −126), 851 (8, −126 − 18), 833 (12, −126 − 36), 709 (9, −126 − 124 − 36), 692 (6) |
| 8 | 25.6 | 274sh | 995 | 977 (100, −18), 959 (8, −36), 851 (19, −126 − 18), 833 (14, −126 − 36), 709 (12, −126 − 124 − 36), 692 (14) |
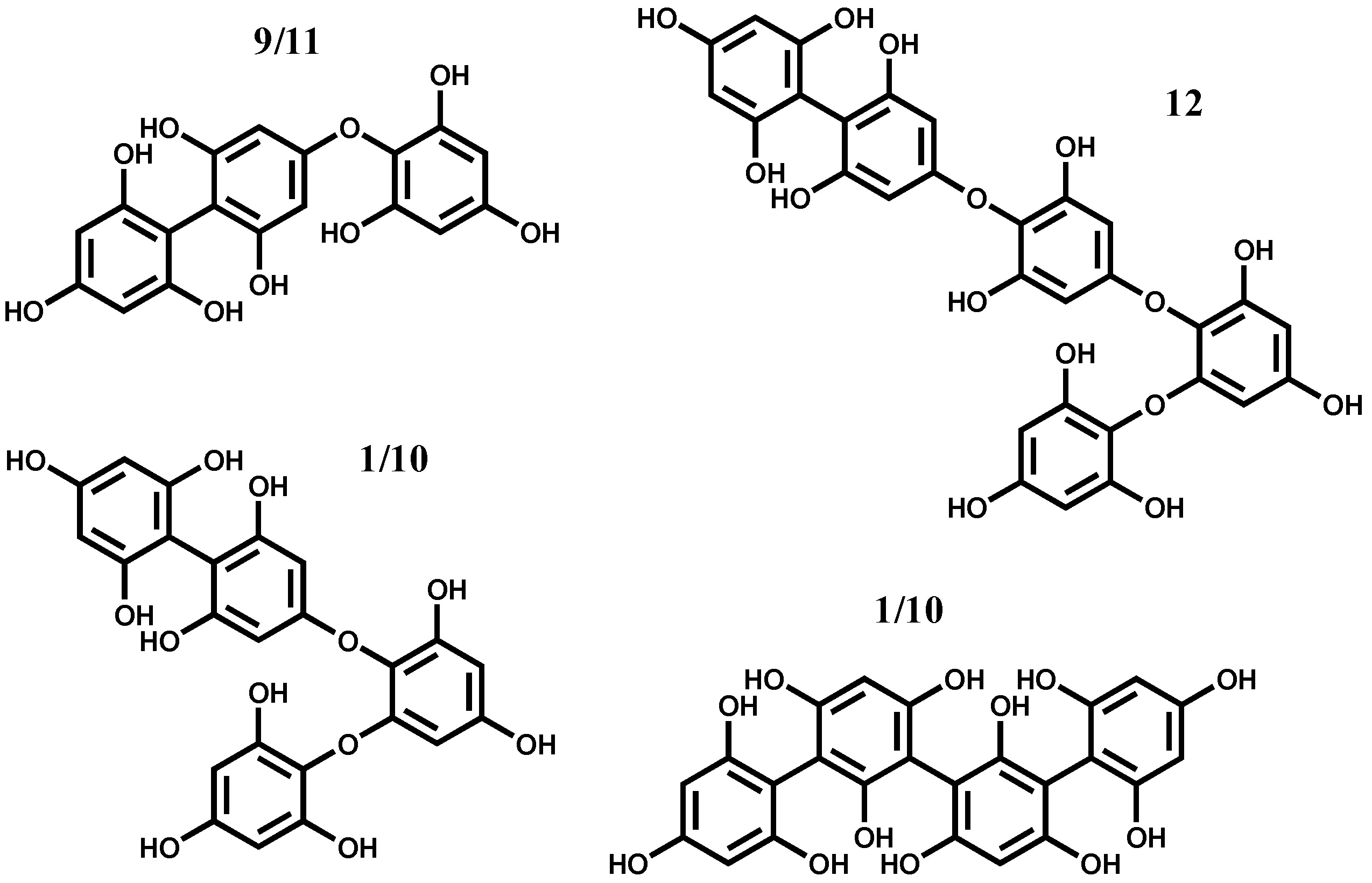
| 9 | 22.5 | 270 | 375 | 357 (44, −18), 339 (2, −36), 249 (42, −126), 235 (100, −126 − 14), 231 (74, −126 − 18), 217 (43), 127 (75, floroglc + H) |
| 10 | 25.0 | — | 499 | 481 (80, −18), 453 (18, −18 − 28), 419 (70), 372 (33, −127), 359 (30, −126 − 14), 355 (100, −126 − 18), 301 (42), 179 (43) |
| 11 | 25.2 | — | 375 | 357 (23, −18), 339 (7, −36), 249 (40, −126), 235 (100, −126 − 14), 317 (33), 127 (57, floroglc + H) |
| 12 | 26.8 | — | 623 | 605 (33, −18), 577 (18, −18 − 28), 523 (50), 497 (2, −126), 483 (12, −126 − 14), 479 (10, −126 − 18), 337 (44), 231 (100) |
| 13 | 13.0 | — | 499 | 481 (49, −18), 411 (29), 397 (28), 395 (25), 359 (25, −162 − 14), 356 (17), 250 (16), 231 (100), 166 (33) |
| 14 | 14.9 | — | 497 | 479 (9, −18), 462 (24), 451 (30, −18 − 28), 386 (48), 368 (70), 351 (34), 258 (100), 240 (38), 222 (37) |
| 15 | 13.5 | — | 499 | 481 (70, −18), 373 (24, −126), 355 (70, −126 − 18), 341 (52, −126 − 18 − 14), 291 (33), 272 (70), 249 (100) |
| 16 | 15.8 | — | 497 | 479 (100, −18), 463 (24), 451 (31, −18 − 28), 368 (8), 351 (45), 298 (14), 258 (41) |
| 17 | 16.7 | — | 497 | 479 (83, −18), 462 (100), 452 (8), 435 (18), 385 (32), 368 (74), 351 (23), 258 (53) |
| 18 | 24.5 | — | 603 | 559 (100, −44), 482 (94), 464 (78) |
| 19 | 24.7 | — | 499 | 481 (100, −18), 368 (38), 247 (25), 242 (25) |
| 20 | 25.2 | — | 499 | 462 (9), 368(100), 334 (12) |
| 21 | 28.1 | — | 743 | 725 (45, −18), 715 (80, −28), 701 (97, −42), 685 (10), 633 (100), 600 (33) |
| 22 | 30.5 | — | 603 | 585 (100, −18), 523 (15), 458 (17), 368 (56), 301 (30) |
2.2. Antioxidant Activity
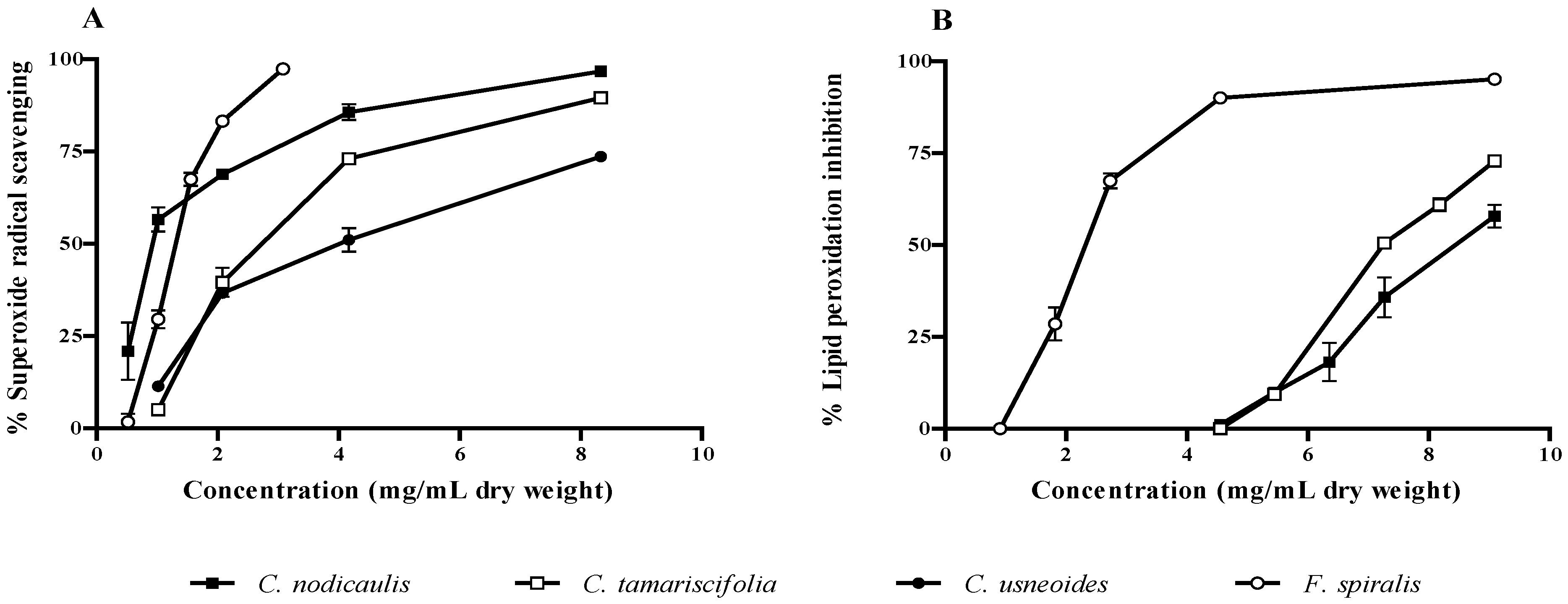
| Samples | Assays | |||||
|---|---|---|---|---|---|---|
| Superoxide radical scavenging | Lipid peroxidation inhibition | HAase inhibition | ||||
| C. tamariscifolia | 2.73 (0.30) | 7.55 (0.02) | 3.3 (0.12) | |||
| C. usneoides | 4.02 (0.68) | >9.10 | 2.51 (0.22) | |||
| F. spiralis | 1.30 (0.04) | 2.32 (0.08) | 0.73 (0.06) | |||
| C. nodicaulis | 0.93 (0.11) | 8.52 (0.08) | 1.34 (0.04) | |||
2.3. Inhibitory Effect of Brown Algae Phlorotannins on HAase
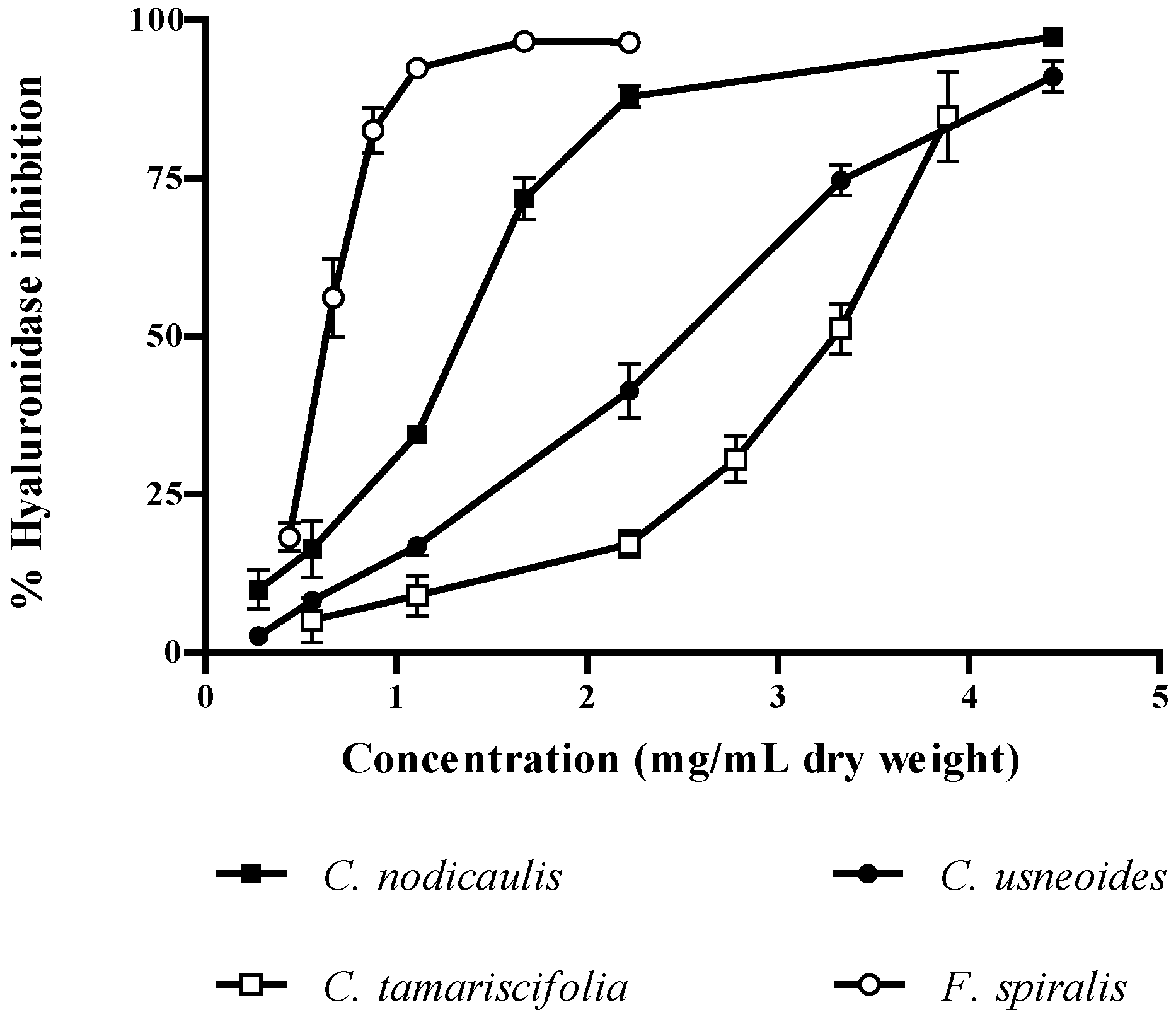
3. Experimental Section
3.1. Standards and Reagents
3.2. Samples
3.3. Extraction and Purification
3.4. HPLC-DAD-ESI-MSn Analyses
3.5. Antioxidant Activity
3.5.1. Superoxide Radical Scavenging Assay
3.5.2. Lipid Peroxidation Inhibition Assay
3.6. HAase Inhibition Assay
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Fallarero, A.; Peltoketo, A.; Loikkanen, J.; Tammela, P.; Vidal, A.; Vuorela, P. Effects of the aqueous extract of Bryothamnion triquetrum on chemical hypoxia and aglycemia-induced damage in GT1–7 mouse hypothalamic immortalized cells. Phytomedicine 2006, 13, 240–245. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS One 2012, 7, e31145. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ. Toxicol. Pharmacol. 2011, 32, 325–335. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G. Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar]
- Audibert, L.; Fauchon, M.; Blanc, N.; Hauchard, D.; Ar Galla, E. Phenolic compounds in the brown seaweed Ascophyllum nodosum: Distribution and radical-scavenging activities. Phytochem. Anal. 2010, 21, 399–405. [Google Scholar] [CrossRef]
- Liu, H.; Gu, L. Phlorotannins from brown algae (Fucus vesiculosus) inhibited the formation of advanced glycation endproducts by scavenging reactive carbonyls. J. Agric. Food Chem. 2012, 60, 1326–1334. [Google Scholar]
- Li, Y.X.; Wijesekara, I.; Li, Y.; Kim, S.K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Target, N.M.; Arnold, T.M. Effects of secondary metabolites on digestion in marine herbivores. In Marine Chemical Ecology; McClintock, J.B., Baker, B.J., Eds.; CRC Press: Florida, FL, USA, 2001; pp. 391–411. [Google Scholar]
- Ragan, M.A.; Glombitza, K. Phlorotannins, brown algal polyphenols. In Progress in Phycological Research; Round, F.E., Chapman, D.J., Eds.; Biopress: Bristol, UK, 1986; pp. 129–241. [Google Scholar]
- Samee, H.; Li, Z.X.; Lin, H.; Khalid, J.; Guo, Y.C. Anti-allergic effects of ethanol extracts from brown seaweeds. J. Zhejiang Univ. Sci. B 2009, 10, 147–153. [Google Scholar]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. J. Appl. Phycol. 2008, 20, 705–711. [Google Scholar] [CrossRef]
- Kohen, R. Skin antioxidants: Their role in aging and in oxidative stress—New approaches for their evaluation. Biomed. Pharmacother. 1999, 53, 181–192. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Invest. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef]
- Muckerjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Hwang, H.J. Skin elasticity and sea polyphenols. Seanol Sci. Centre Rev. 2010, 1, 1–10. [Google Scholar]
- Kang, K.A.; Zhang, R.; Piao, M.J.; Ko, D.O.; Wang, Z.H.; Lee, I.K.; Kim, B.J.; Shin, T.; Park, J.W.; Lee, L.H.; Yoo, B.S.; Hyun, J.W. Inhibitory effects of triphlorethol-A on MMP-1 induced by oxidative stress in human keratinocytes via ERK and AP-1 inhibition. J. Toxicol. Environ. Health A 2008, 71, 992–999. [Google Scholar] [CrossRef]
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; Park, N.K.; Kim, H.R. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar]
- Nagayama, K.; Iwamura, Y.; Shibata, T.; Hirayama, I.; Nakamura, T. Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J. Antimicrob. Chemother. 2002, 50, 889–893. [Google Scholar] [CrossRef]
- Cérantola, S.; Florian, B.; Erwan, A.G.; Deslandes, E. Co-occurrence and antioxidant activities of fucol and fucophlorethol classes of polymeric phenols in Fucus spiralis. Bot. Mar. 2006, 49, 347–351. [Google Scholar]
- Glombitza, K.W.; Rosener, H.U.; Müller, D. Bifuhalol und diphlorethol aus Cystoseira tamariscifolia. Phytochemistry 1975, 14, 1115–1116. [Google Scholar]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar]
- Fairhead, V.A.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Variation in phlorotannin content within two species of brown macroalgae (Desmarestia anceps and D. menziesii) from the Western Antarctic Peninsula. Polar Biol. 2005, 28, 680–686. [Google Scholar]
- Stern, J.L.; Hagerman, A.E.; Winter, F.C.; Estes, J.A. A new assay for quantifying brown algal phlorotannins and comparisons to previous methods. J. Chem. Ecol. 1996, 22, 1273–1293. [Google Scholar]
- Steevensz, A.J.; MacKinnon, S.L.; Hankinson, R.; Craft, C.; Connan, S.; Stengel, D.B.; Melansona, J.E. Profiling phlorotannins in brown macroalgae by Liquid Chromatography-High Resolution Mass Spectrometry. Phytochem. Anal. 2012, 23, 547–553. [Google Scholar] [CrossRef]
- Parys, S.; Kehraus, S.; Krick, A.; Glombitza, K.W.; Carmeli, S.; Klimo, K.; Gerhäuser, C.; König, G.M. In vitro chemoprotective potential of fucophlorethols from the brown alga Fucus vesiculosus L. by anti-oxidant activity and inhibition of selected cytochrome P450 enzymes. Phytochemistry 2012, 71, 221–229. [Google Scholar]
- Glombitza, K.W.; Keusgen, M.; Hauperich, S. Fucophlorethols from the brown algae Sargassum spinuligerum and Cystophora torulosa. Phytochemistry 1997, 46, 1417–1422. [Google Scholar]
- Sugiura, Y.; Matsuda, K.; Yamada, Y.; Nishikawa, M.; Shioya, K.; Katsuzaki, H.; Imai, K.; Amano, H. Isolation of a new anti-allergic phlorotannin, phlorofucofuroeckol-B, from an edible brown alga Eisenia arborea. Biosci. Biotechnol. Biochem. 2006, 70, 2807–2811. [Google Scholar] [CrossRef]
- Zubia, M.; Fabre, M.S.; Kerjean, V.; Lann, K.; Stiger-Pouvreau, V.; Fauchon, M.; Deslandes, E. Antioxidant and antitumoural activities of some Phaeophyta from Britany coasts. Food Chem. 2009, 116, 693–701. [Google Scholar] [CrossRef]
- Sousa, C.; Valentão, P.; Ferreres, F.; Seabra, R.M.; Andrade, P.B. Tronchuda cabbage (Brassica oleracea L. var. costata DC): Scavenger of reactive nitrogen species. J. Agric. Food Chem. 2008, 56, 4205–4211. [Google Scholar] [CrossRef]
- Sugiura, Y.; Matsuda, K.; Yamada, Y.; Imai, K.; Kakinuma, M.; Amano, H. Radical scavenging and hyaluronidase inhibitory activities of phlorotannins from the edible brown alga Eisenia arborea. Food Sci. Technol. Res. 2008, 14, 595–598. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Valentão, P.; Pereira, J.A.; Silva, B.M.; Tavares, F.; Andrade, P.B. Ficus carica L.: Metabolic and biological screening. Food Chem. Toxicol. 2009, 47, 2841–2846. [Google Scholar]
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]
- Shimasaki, H. Diene conjugation. In Experimental Protocols for Reactive Oxygen and Nitrogen Species; Taniguchi, N., Gutteridge, J.M.C., Eds.; Press Inc.: New York, NY, USA, 2000; pp. 142–143. [Google Scholar]
- Muckenschnabela, I.; Bernhardta, G.; Sprussa, T.; Dietlb, B.; Buschauera, A. Quantitation of hyaluronidases by the Morgan-Elson reaction: Comparison of the enzyme activities in the plasma of tumor patients and healthy volunteers. Cancer Lett. 1998, 131, 13–20. [Google Scholar] [CrossRef]
- Samples Availability: Available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin Extracts from Fucales Characterized by HPLC-DAD-ESI-MSn: Approaches to Hyaluronidase Inhibitory Capacity and Antioxidant Properties. Mar. Drugs 2012, 10, 2766-2781. https://doi.org/10.3390/md10122766
Ferreres F, Lopes G, Gil-Izquierdo A, Andrade PB, Sousa C, Mouga T, Valentão P. Phlorotannin Extracts from Fucales Characterized by HPLC-DAD-ESI-MSn: Approaches to Hyaluronidase Inhibitory Capacity and Antioxidant Properties. Marine Drugs. 2012; 10(12):2766-2781. https://doi.org/10.3390/md10122766
Chicago/Turabian StyleFerreres, Federico, Graciliana Lopes, Angel Gil-Izquierdo, Paula B. Andrade, Carla Sousa, Teresa Mouga, and Patrícia Valentão. 2012. "Phlorotannin Extracts from Fucales Characterized by HPLC-DAD-ESI-MSn: Approaches to Hyaluronidase Inhibitory Capacity and Antioxidant Properties" Marine Drugs 10, no. 12: 2766-2781. https://doi.org/10.3390/md10122766
APA StyleFerreres, F., Lopes, G., Gil-Izquierdo, A., Andrade, P. B., Sousa, C., Mouga, T., & Valentão, P. (2012). Phlorotannin Extracts from Fucales Characterized by HPLC-DAD-ESI-MSn: Approaches to Hyaluronidase Inhibitory Capacity and Antioxidant Properties. Marine Drugs, 10(12), 2766-2781. https://doi.org/10.3390/md10122766









