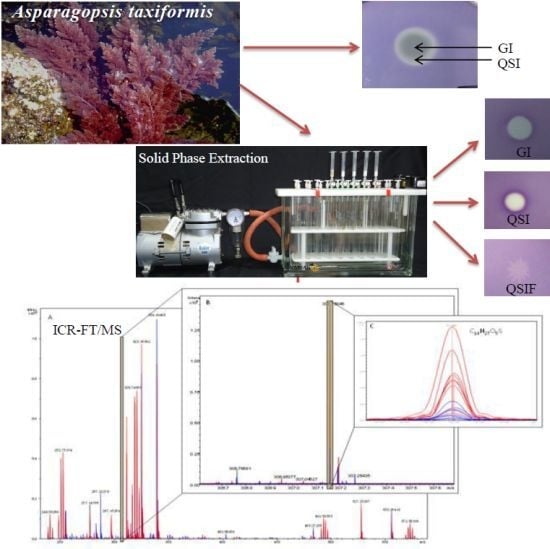
Quorum Sensing Inhibition by Asparagopsis taxiformis, a Marine Macro Alga: Separation of the Compound that Interrupts Bacterial Communication
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of Quorum Sensing (QS) Inhibition Activity from Seaweed Extracts
| Serial No. | Name of Algae | Division | Quorum sensing inhibition |
|---|---|---|---|
| 1. | Padina gymnospora | Phaeophyta | Negative |
| 2. | Sargassum wightii | Phaeophyta | Negative |
| 3. | Pocockiella variegate | Phaeophyta | Negative |
| 4. | Turbinaria ornate | Phaeophyta | Negative |
| 5. | Stoechospermum marginatum | Phaeophyta | Negative |
| 6. | Cystoseria trinodis | Phaeophyta | Negative |
| 7. | Sargassum myriocystum | Phaeophyta | Negative |
| 8. | Sargassum ploiophyllum | Phaeophyta | Negative |
| 9. | Asparagopsis taxiformis | Rhodophyta | Positive |
| 10. | Chondrococcus harnemanii | Rhodophyta | Negative |
| 11. | Gracilaria edulis | Rhodophyta | Negative |
| 12. | Hypnea pannosa | Rhodophyta | Negative |
| 13. | Jania adhaerens | Rhodophyta | Negative |
| 14. | Hypnea valentiae | Rhodophyta | Negative |
| 15. | Pterocladia heteroplatos | Rhodophyta | Negative |
| 16. | Galaxaura obtuse | Rhodophyta | Negative |
| 17. | Halicrysis tchivye | Rhodophyta | Negative |
| 18. | Acanthophora spicifera | Rhodophyta | Negative |
| 19. | Champia parvula | Rhodophyta | Negative |
| 20. | Hypnea flagelliformis | Rhodophyta | Negative |
| 21. | Chondracanthus acicularis | Rhodophyta | Negative |
| 22. | Porphyra kanyakumariensis | Rhodophyta | Negative |
| 23. | Polysiphonia tuticoriensis | Rhodophyta | Negative |
| 24. | Laurencia papillosa | Rhodophyta | Negative |
| 25. | Sarcodia ceylanica | Rhodophyta | Negative |
| 26. | Chaetomorpha antennina | Chlorophyta | Negative |
| 27. | Caulerpa veravalensis | Chlorophyta | Negative |
| 28. | Cladophora indica | Chlorophyta | Negative |
| 29. | Enteromorpha spp. | Chlorophyta | Negative |
| 30. | Ulva fasciata | Chlorophyta | Negative |
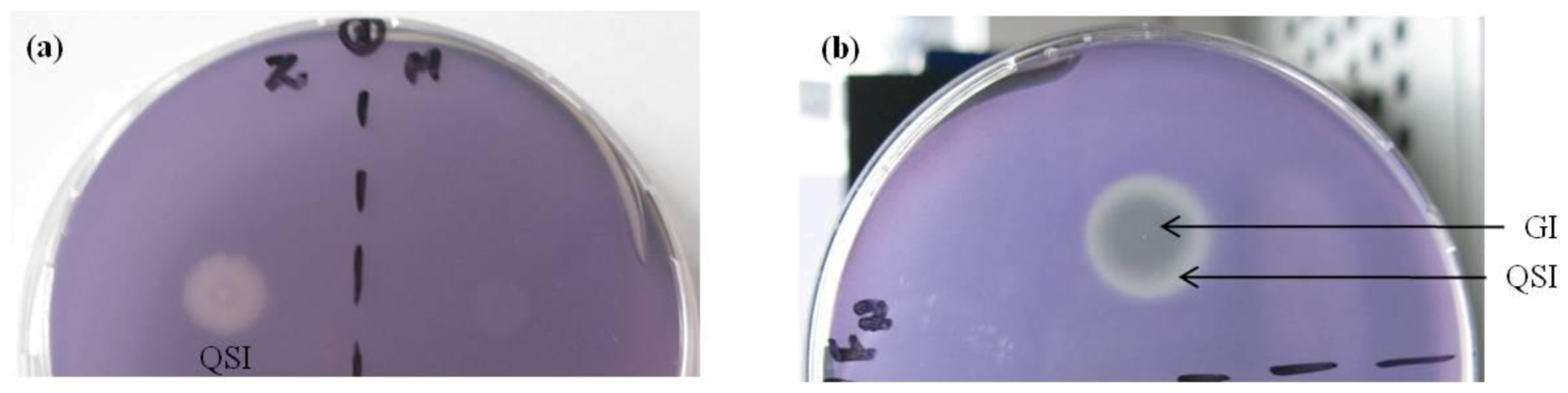
2.2. Evaluation of A. taxiformis Extract Fractions Obtained from SPE Cartridges
| Bioassay results of fractions of Asparagopsis taxiformis extract | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bond Elut SPE cartridges | Effects on plate based bioassay | Methanol (v/v) | |||||||||
| 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% | ||
| C2 | QS inhibition | (+) | − | − | (+) | − | − | (+) | − | − | + |
| Zone of clearance | − | − | − | − | ++ | +++ | − | − | − | − | |
| C18 | QS inhibition | + | − | − | − | − | + | − | − | +fing | (+)fing |
| Zone of clearance | − | − | − | − | − | − | ++ | +++ | − | − | |
| CN-E | QS inhibition | − | − | − | − | − | − | − | − | − | − |
| Zone of clearance | − | − | ++ | ++ | ++ | − | − | − | − | − | |
| PH | QS inhibition | − | − | − | − | ++ | − | − | ++ | (+)fing | +fing |
| Zone of clearance | − | − | − | − | − | ++ | ++ | − | − | − | |
| PPL | QS inhibition | +fing | − | − | − | − | − | − | (+)fing | +fing | ++fing |
| Zone of clearance | − | − | − | − | − | − | − | − | − | − | |
| Bond Elut SPE-Cartridges | Type of Material | Properties | Primary Retention Mechanism | Typical Sample Types |
|---|---|---|---|---|
| C2 | Silica based, ethyl bonded, endcapped | Alternative sorbent, if analytes are retained too strongly on C8 or C18 phases | Weakly nonpolar | Plasma, urine, aqueous samples |
| C18 | Silica based, trifunctional octadecyl bonded, endcapped | Extreme retentive nature for nonpolar compounds, applicable for desalting aqueous matrices | Strongly nonpolar | Water, aqueous biological fluids |
| CN-E | Silica based, cyanopropyl bonded, endcapped | Ideal sorbent for extracting extremely nonpolar compounds | Moderately nonpolar (aqueous matrix) or polar (nonpolar organic matrix) | Aqueous samples (nonpolar), organic samples (polar) |
| PH | Silica based, phenyl bonded, endcapped | Different selectivity to alkyl and aliphatic functionalized phases due to electron density of the aromatic ring | Moderately nonpolar | Water, biological fluids |
| PPL | Styrene-divinylbenzene (SDVB) polymer with a proprietary derivitized nonpolar surface | Extreme hydrophobicity and surface area, achieves high recovery levels and fast extraction speeds | Highly polar | Waste water (phenols) |
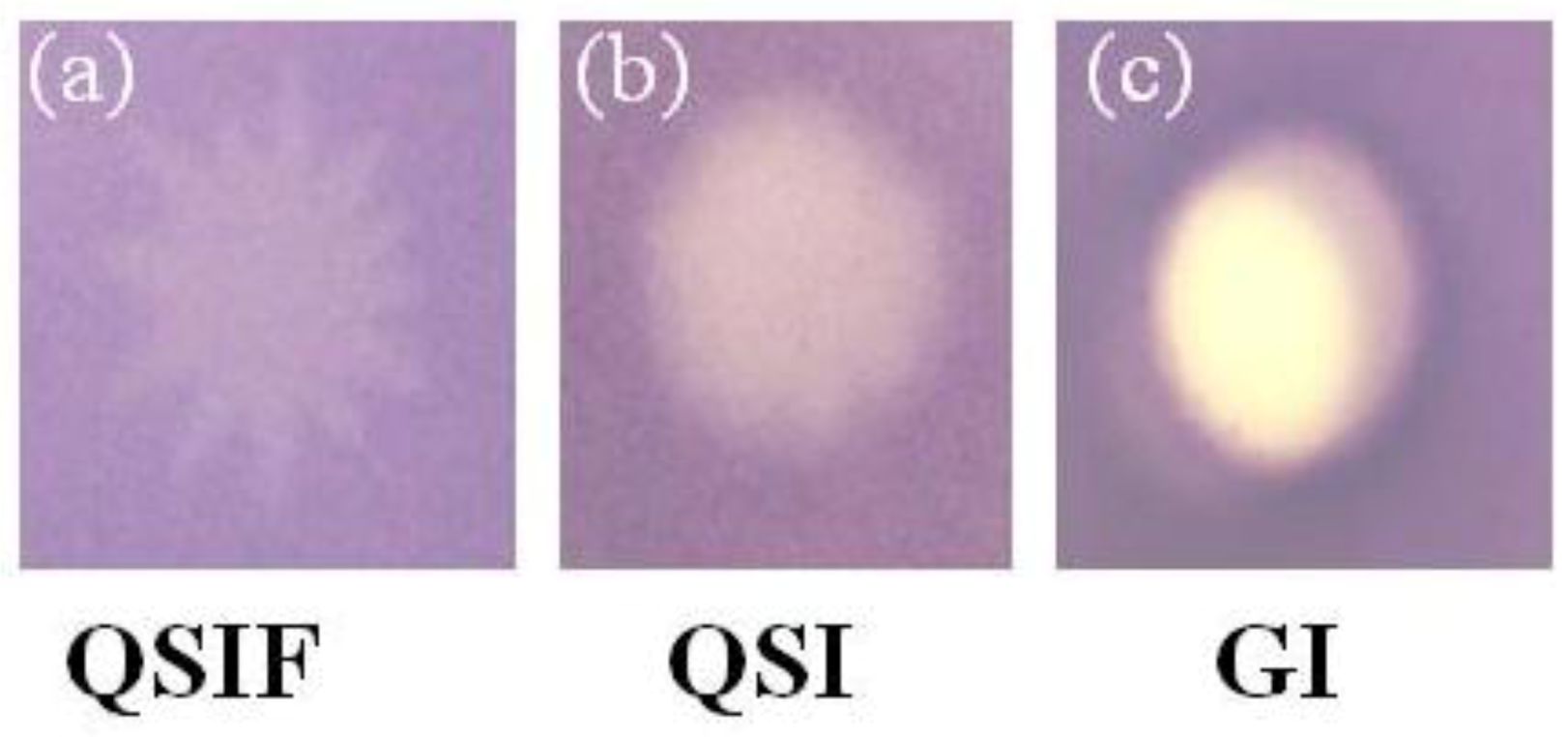
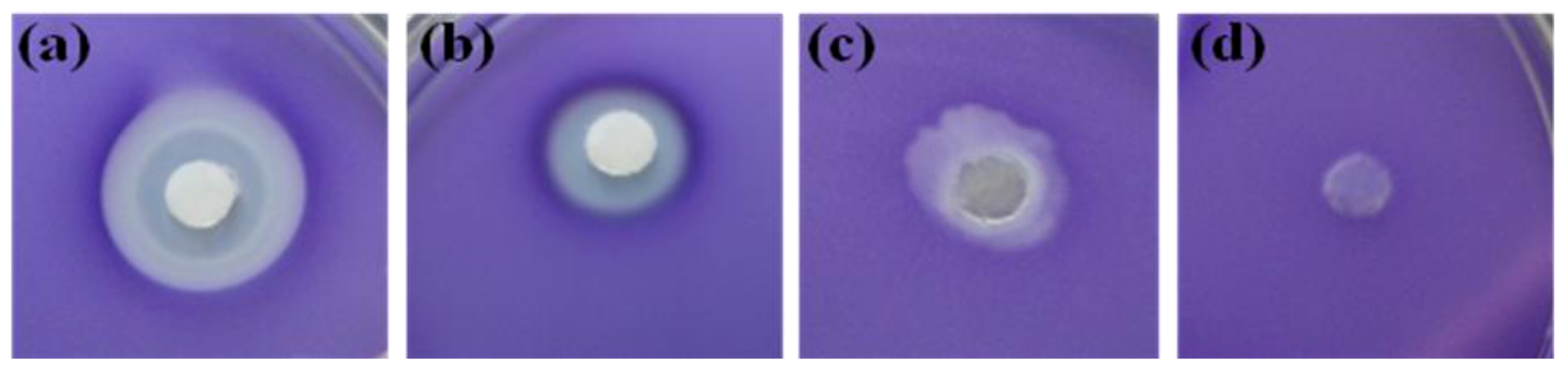
2.3. Evaluation of Bioassay Using Serratia liquefaciens MG44

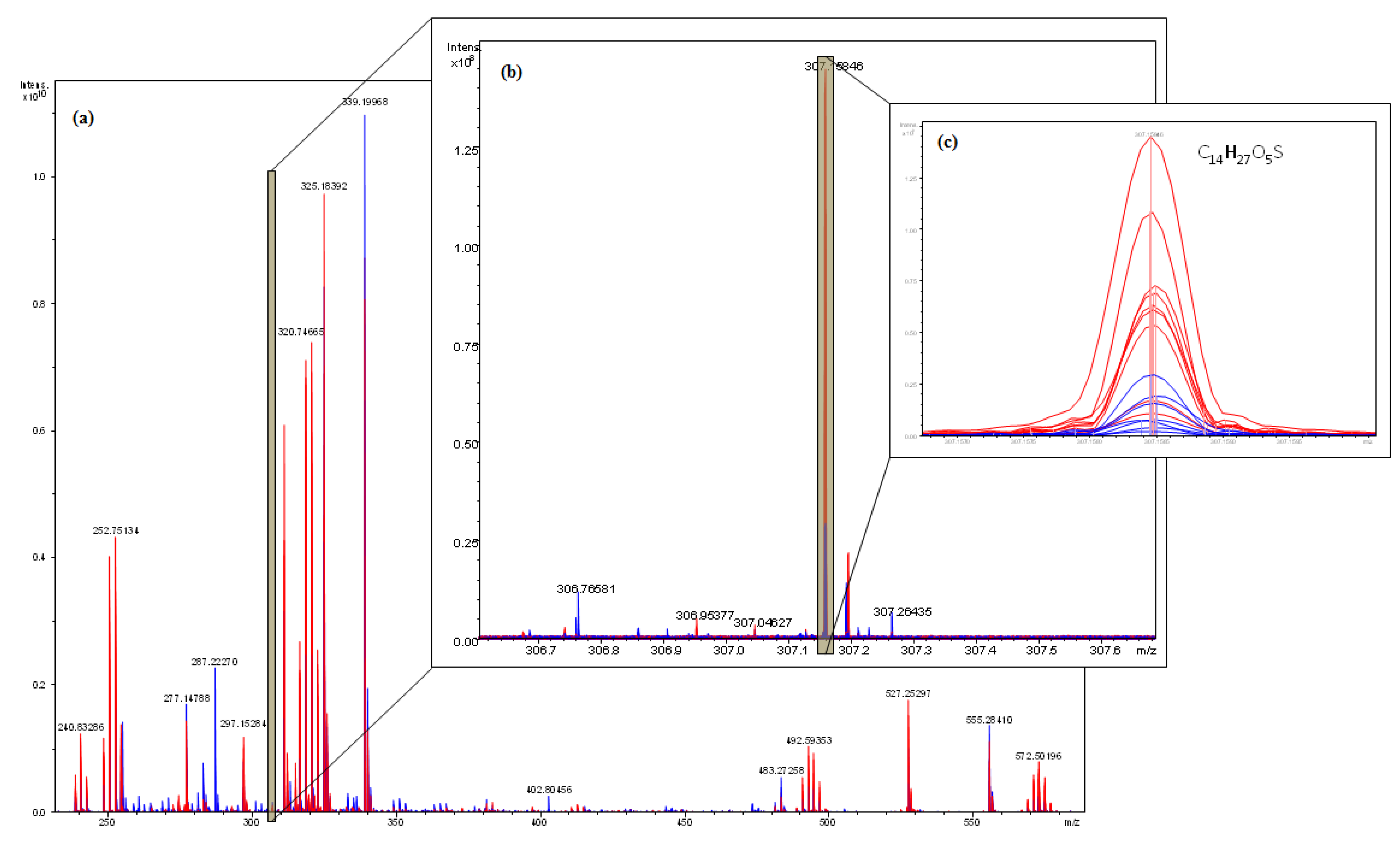
2.4. Interpretation of ICR-FT/MS Analysis
3. Experimental Section
3.1. Collection and Extract Preparation of Algal Samples
3.2. Bioassay for Quorum Sensing Inhibition (QSI)
3.3. Fractionation of Extract
3.4. Agar-Based Bioassay of Fractions
3.5. Serratia liquefaciens MG44 Bioassay
3.6. ICR-FT/MS Analysis
4. Conclusions
Acknowledgments
References
- Dickschat, J. Quorum sensing and bacterial biofilms. Nat. Prod. Rep. 2010, 27, 343–369. [Google Scholar]
- Antunes, L.; Ferreira, R.; Buckner, M.; Finlay, B. Quorum sensing in bacterial virulence. J. Med. Microbiol. 2010, 156, 2271–2282. [Google Scholar]
- Dong, Y.; Wang, L.; Xu, J.; Zhang, H.; Zhang, X.; Zhang, L. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 2001, 411, 813–817. [Google Scholar] [CrossRef]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.; Coenye, T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef]
- Fux, C.; Costerton, J.; Stewart, P.; Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef]
- Singh, R.; Paul, D.; Jain, R. Biofilms: Implications in bioremediation. Trends Microbiol. 2006, 14, 389–397. [Google Scholar] [CrossRef]
- Bhadury, P.; Wright, P.C. Exploitation of marine algae: Biogenic compoundsfor potential antifouling applications. Planta 2004, 219, 561–578. [Google Scholar]
- Xu, Y.; He, H.; Schulz, S.; Liu, X.; Fusetani, N.; Xiong, H.; Xiao, X.; Qian, P. Potent antifouling compounds produced by marine Streptomyces. Bioresour. Technol. 2010, 101, 1331–1336. [Google Scholar]
- Qian, P.; Xu, Y.; Fusetani, N. Natural products as antifouling compounds: Recent progress and future perspectives. Biofouling 2010, 26, 223–234. [Google Scholar] [CrossRef]
- Hellio, C.; Bremer, G.; Pons, A.; le Gal, Y.; Bourgougnon, N. Inhibition of the development of microorganisms (bacteria and fungi) by extracts of marine algae from Brittany, France. Appl. Microbiol. Biotechnol. 2000, 54, 543–549. [Google Scholar] [CrossRef]
- Hellio, C.; de la Broise, D.; Dufosse, L.; le Gal, Y.; Bourgougnon, N. Inhibition of marine bacteria by extracts of macroalgae: Potential use for environmentally friendly antifouling paints. Mar. Environ. Res. 2001, 52, 231–247. [Google Scholar] [CrossRef]
- Culioli, G.; Ortalo-Magne, A.; Valls, R.; Hellio, C.; Clare, A.; Piovetti, L. Antifouling activity of meroditerpenoids from the marine brown alga Halidrys siliquosa. J. Nat. Prod. 2008, 71, 1121–1126. [Google Scholar] [CrossRef]
- Plouguerne, E.; Hellio, C.; Deslandes, E.; Veron, B.; Stiger-Pouvreau, V. Anti-Microfouling activities in extracts of two invasive algae: Grateloupia turuturu and Sargassum muticum. Bot. Mar. 2008, 51, 202–208. [Google Scholar]
- Plouguerne, E.; Ioannou, E.; Georgantea, P.; Vagias, C.; Roussis, V.; Hellio, C.; Kraffe, E.; Stiger-Pouvreau, V. Anti-Microfouling activity of lipidic metabolites from the invasive brown alga Sargassum muticum (Yendo) fensholt. Mar. Biotechnol. 2010, 12, 52–61. [Google Scholar] [CrossRef]
- Steinberg, P.D.; Rice, S.A.; Campbell, A.H.; McDougald, D.; Harder, T. Interfaces between bacterial and eukaryotic “neuroecology”. Integr. Comp. Biol. 2011, 5, 794–806. [Google Scholar]
- Wahl, M.; Goecke, F.; Labes, A.; Dobretsov, S.; Weinberger, F. The second skin: Ecological role of epibiotic biofilms on marine organisms. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Givskov, M.; DeNys, R.; Manefield, M.; Gram, L.; Maximilien, R.; Eberl, L.; Molin, S.; Steinberg, P.; Kjelleberg, S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signaling. J. Bacteriol. 1996, 178, 6618–6622. [Google Scholar]
- Kjelleberg, S.; Steinberg, P.; Givskov, M.; Gram, L.; Manefield, M.; deNys, R. Do marine natural products interfere with prokaryotic AHL regulatory systems? Aquat. Microbl. Ecol. 1997, 13, 85–93. [Google Scholar] [CrossRef]
- Manefield, M.; Rasmussen, T.; Henzter, M.; Andersen, J.; Steinberg, P.; Kjelleberg, S.; Givskov, M. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology (Engl.) 2002, 148, 1119–1127. [Google Scholar]
- Dobretsov, S.; Teplitski, M.; Paul, V. Mini-Review: Quorum sensing in the marine environment and its relationship to biofouling. Biofouling 2009, 25, 413–427. [Google Scholar] [CrossRef]
- Chang, C.Y.; Koh, C.L.; Sam, C.K.; Chan, X.Y.; Yin, W.F.; Chan, K.G. Unusual long-chain N-acyl homoserine lactone production by and presence of quorum quenching activity in bacterial isolates from diseased tilapia fish. PLoSOne 2012, 7, e44034. [Google Scholar]
- Zhu, H.; Liu, W.; Wang, S.X.; Tian, B.Z.; Zhang, S.S. Evaluation of anti-quorum-sensing activity of fermentation metabolites from different strains of a medicinal mushroom, Phellinus igniarius. Chemotherapy 2012, 58, 195–199. [Google Scholar] [CrossRef]
- Tan, L.Y.; Yin, W.F.; Chan, K.G. Silencing Quorum sensing through extracts of Melicope lunuankenda. Sensors 2012, 12, 4339–4351. [Google Scholar] [CrossRef]
- Hunt, L.R.; Smith, S.M.; Downum, K.R.; Mydlarz, L.D. Microbial regulation in gorgonian corals. Mar. Drugs 2012, 10, 1225–1243. [Google Scholar] [CrossRef]
- Choo, J.H.; Rukayadi, Y.; Hwang, J.K. Inhibition of bacterial quorum sensing by vanilla extract. Lett. Appl. Microbiol. 2006, 42, 637–641. [Google Scholar]
- Niu, C.; Afre, S.; Gilbert, E.S. Subinhibitory concentrations of cinnamaldehyde interfere withquorum sensing. Lett. Appl. Microbiol. 2006, 43, 489–494. [Google Scholar] [CrossRef]
- Brackman, G.; Defoirdt, T.; Miyamoto, C.; Bossier, P.; Calenbergh, S.V.; Nelis, H.; Coenye, T. Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 2008, 8, 149–162. [Google Scholar] [CrossRef]
- Da Gama, B.; Carvalho, A.; Weidner, K.; Soares, A.; Coutinho, R.; Fleury, B.; Teixeira, V.; Pereira, R. Antifouling activity of natural products from Brazilian seaweeds. Bot. Mar. 2008, 51, 191–201. [Google Scholar]
- Kwan, J.C.; Meickle, T.; Ladwa, D.; Teplitski, M.; Paul, V.; Luesch, H. Lyngbyoic acid, a “tagged” fatty acid from a marine cyanobacterium, disrupts quorum sensing in Pseudomonas aeruginos. Mol. BioSyst. 2011, 7, 1205–1216. [Google Scholar] [CrossRef]
- Burreson, B.J.; Moore, R.E.; Roller, P. Haloforms in the essential oil of the alga Asparagopsis taxiformis (Rhodophyta). TetrahedronLett. 1975, 7, 473–476. [Google Scholar]
- McConnell, O.; Fenical, W. Halogen chemistry of the red alga Asparagopsis. Phytochemistry 1977, 16, 367–374. [Google Scholar] [CrossRef]
- Bansemir, A.; Blume, M.; Schroder, S.; Lindequist, U. Screening of cultivated seaweeds for antibacterial activity against fish pathogenic bacteria. Aquaculture 2006, 252, 79–84. [Google Scholar] [CrossRef]
- Genovese, G.; Tedone, L.; Hamann, M.; Morabito, M. The mediterranean red alga Asparagopsis: A source of compounds against Leishmania. Mar. Drugs 2009, 7, 361–366. [Google Scholar] [CrossRef]
- Salvador, N.; Garreta, A.; Lavelli, L.; Ribera, M. Antimicrobial activity of Iberian macroalgae. Sci. Mar. 2007, 71, 101–113. [Google Scholar]
- Genovese, G.; Faggio, C.; Gugliandolo, C.; Torre, A.; Spano, A.; Morabito, M.; Maugeri, T. In vitro evaluation of antibacterial activity of Asparagopsis taxiformis from the Straits of Messina against pathogens relevant in aquaculture. Mar. Environ. Res. 2012, 73, 1–6. [Google Scholar] [CrossRef]
- Eberl, L.; Winson, M.; Sternberg, C.; Stewart, G.; Christiansen, G.; Chhabra, S.; Bycroft, B.; Williams, P.; Molin, S.; Givskov, M. Involvement of N-acyl-L-homoserine lactone autoinducers in controlling the multicellular behaviour of Serratia liquefaciens. Mol. Microbiol. 1996, 20, 127–136. [Google Scholar] [CrossRef]
- Koch, B.; Liljefors, T.; Persson, T.; Nielsen, J.; Kjelleberg, S.; Givskov, M. The LuxR receptor: The sites of interaction with quorum-sensing signals and inhibitors. Microbiology 2005, 151, 3589–3602. [Google Scholar] [CrossRef]
- Kientz, B.; Thabard, M.; Cragg, S.; Pope, J.; Hellio, C. A new method for removing microflora from macroalgal surfaces: An important step for natural product discovery. Bot. Mar. 2011, 54, 457–469. [Google Scholar]
- Milton, D.L.; Hardman, A.; Camara, M.; Chhabra, S.R.; Bycroft, B.W.; Stewart, G.S.; Williams, P. Quorum sensing in Vibrio anguillarum: Characterization of the vanI/vanR locus and identification of the autoinducer N-(3-Oxodecanoyl)-L-Homoserine lactone. J. Bacteriol. 1997, 179, 3004–3012. [Google Scholar]
- SPE-AgilentTechnologies. Available online: http://www.chem.agilent.com/en-US/products/columns-supplies/samplepreparation/spe/pages/default.aspx (accessed on 8 July 2012).
- Veselova, M.; Kholmeckaya, M.; Klein, S.; Voronina, E.; Lipasova, V.; Metlitskaya, A.; Mayatskaya, A.; Lobanok, E.; Khmel, I.; Chernin, L. Production of N-acylhomoserine lactone signal molecules by gram-negative soil-borne and plant-associated bacteria. Folia Microbiol. 2003, 48, 794–798. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jha, B.; Kavita, K.; Westphal, J.; Hartmann, A.; Schmitt-Kopplin, P. Quorum Sensing Inhibition by Asparagopsis taxiformis, a Marine Macro Alga: Separation of the Compound that Interrupts Bacterial Communication. Mar. Drugs 2013, 11, 253-265. https://doi.org/10.3390/md11010253
Jha B, Kavita K, Westphal J, Hartmann A, Schmitt-Kopplin P. Quorum Sensing Inhibition by Asparagopsis taxiformis, a Marine Macro Alga: Separation of the Compound that Interrupts Bacterial Communication. Marine Drugs. 2013; 11(1):253-265. https://doi.org/10.3390/md11010253
Chicago/Turabian StyleJha, Bhavanath, Kumari Kavita, Jenny Westphal, Anton Hartmann, and Philippe Schmitt-Kopplin. 2013. "Quorum Sensing Inhibition by Asparagopsis taxiformis, a Marine Macro Alga: Separation of the Compound that Interrupts Bacterial Communication" Marine Drugs 11, no. 1: 253-265. https://doi.org/10.3390/md11010253