Isolation and Purification of a Peptide from Bullacta exarata and Its Impaction of Apoptosis on Prostate Cancer Cell
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Purification of Peptide

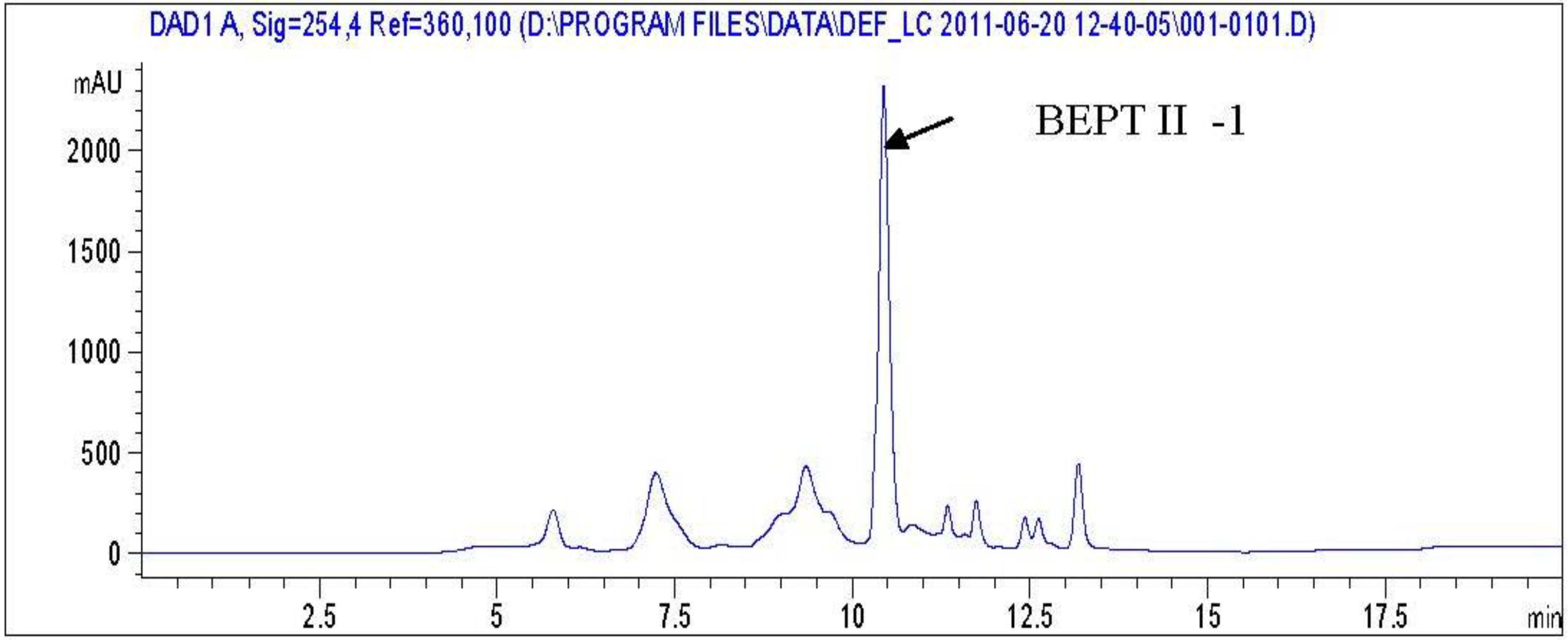
2.2. BEPT II and BEPT II-1 Inhibits Cell Proliferation of PC-3 Cell Lines
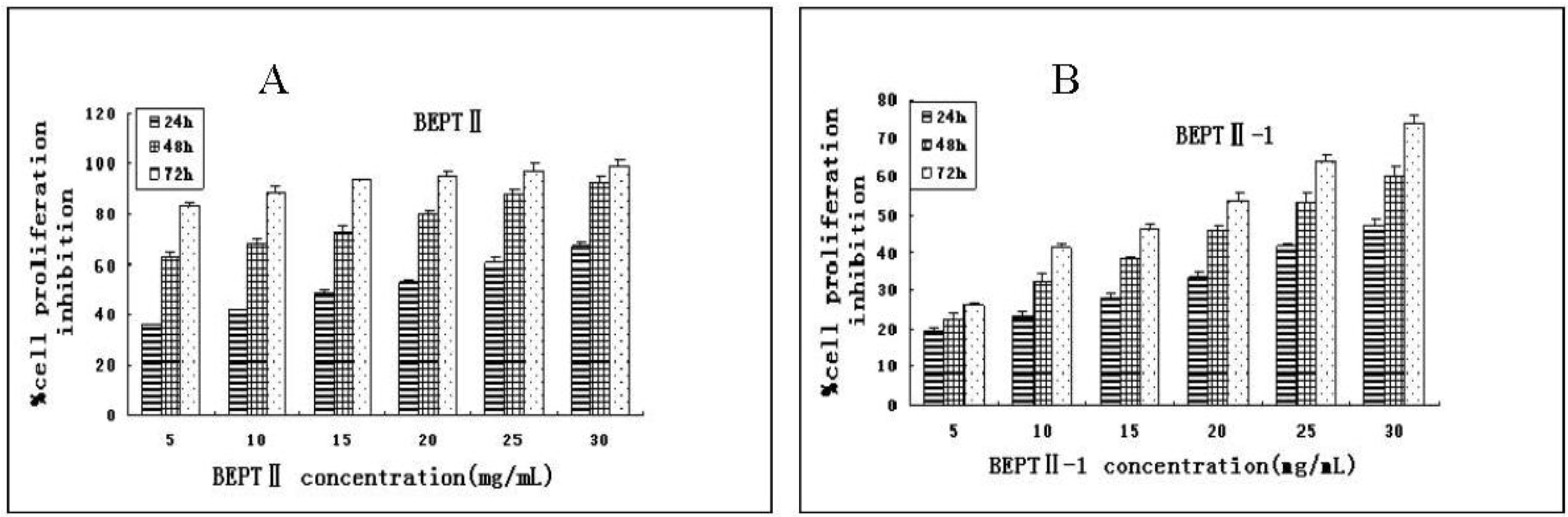
| IC50 (mg/mL) | |||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| BEPT II | 14.06 ± 0.41 | 3.68 ± 0.12 | 1.16 ± 0.11 |
| BEPT II-1 | 43.97 ± 0.34 | 22.19 ± 0.22 | 14.62 ± 0.23 |
2.3. Morphologic Observation by Acridine Orange and Ethidium Bromide (AO/EB) Staining
 ) indicates viable cells; (
) indicates viable cells; (  ) indicates early apoptotic cells; (
) indicates early apoptotic cells; (  ) indicates late apoptotic cells. Each experiment was performed in triplicate (n = 3) and generated similar morphologic features. Original magnification 400×.
) indicates late apoptotic cells. Each experiment was performed in triplicate (n = 3) and generated similar morphologic features. Original magnification 400×.
 ) indicates viable cells; (
) indicates viable cells; (  ) indicates early apoptotic cells; (
) indicates early apoptotic cells; (  ) indicates late apoptotic cells. Each experiment was performed in triplicate (n = 3) and generated similar morphologic features. Original magnification 400×.
) indicates late apoptotic cells. Each experiment was performed in triplicate (n = 3) and generated similar morphologic features. Original magnification 400×.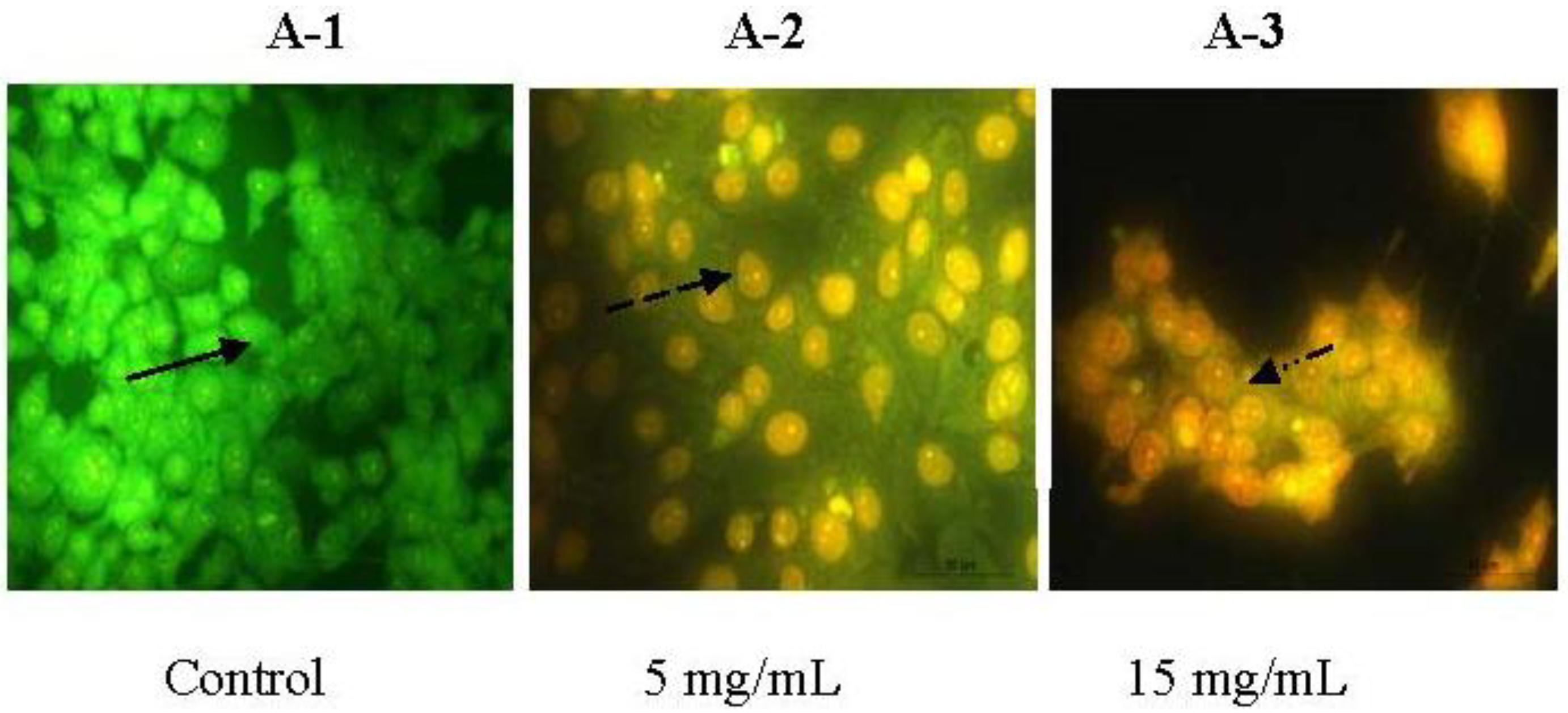
2.4. BEPT II-1 Induces Apoptosis in PC-3 Cells Based on Flow Cytometry Analysis
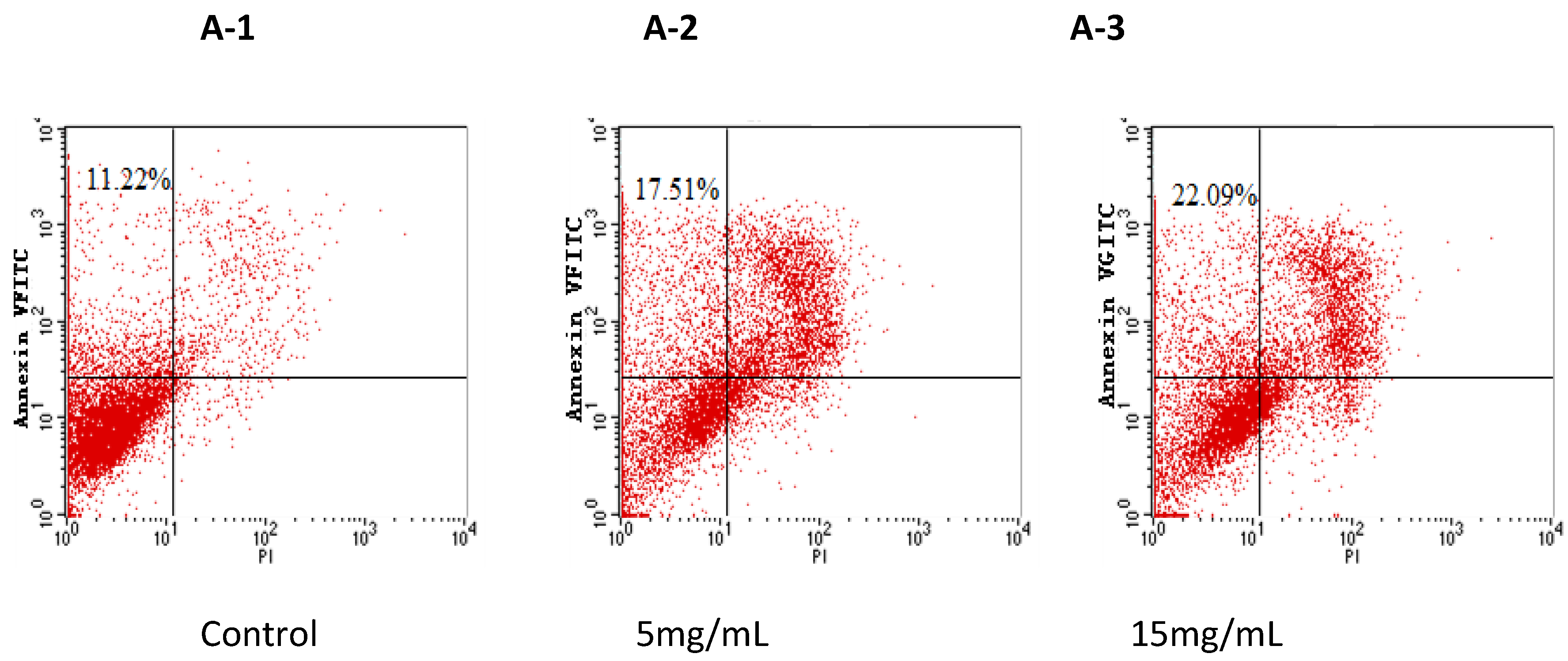
3. Experimental Section
3.1. Materials
3.2. Enzymatic Hydrolysis
3.3. Isolation and Purification of Anticancer Peptide
3.3.1. Ultrafiltration
3.3.2. Sephadex G-25 Gel Chromatography
3.3.3. High Performance Liquid Chromatography (HPLC)
3.4. PC-3 Cells Culture
3.5. Anti-Proliferative Activity Using MTT Assay
3.6. Morphologic Study with Fluorescence Microscope
3.7. Cell Apoptosis Analysis
4. Conclusions
Acknowledgments
References
- Hong, H.; Zhang, Y.; Sun, J.; Cai, W. Positron emission tomography imaging of prostate cancer. Amino Acids 2009, 39, 11–27. [Google Scholar]
- Braun, K.; Ehemann, V.; Wiessler, M.; Pipkorn, R.; Didinger, B.; Mueller, G.; Waldeck, W. High-resolution flow cytometry: A suitable tool for monitoring aneuploid prostate cancer cells after TMZ and TMZ-BioShuttle treatment. Int. J. Med. Sci. 2009, 6, 338–347. [Google Scholar]
- Peyromaure, M.; Valéri, A.; Rebillard, X.; Beuzeboc, P.; Soulié, M.; Salomon, L. Characteristics of prostate cancer in men less than 50-year-old. Prog. Urol. 2009, 19, 803–809. [Google Scholar] [CrossRef]
- Ramakrishna, N.R.; de Weese, T.L. Prostate Cancer: Biology, Genetics, and the New Therapeutics; Chung, L.W.K., Isaacs, W.B., Simons, J.W., Eds.; Humana Press: Totowa, NJ, USA, 2001; pp. 387–413. [Google Scholar]
- Gilligan, T.; Kantoff, P.W. Chemotherapy for prostate cancer. Urology 2002, 60, 94–100. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta 2008, 1778, 357–375. [Google Scholar]
- Ma, J.Y.; Guo, X.M.; Hu, J.M.; Wang, X. New antimicrobial peptides purified directly from Bullacta exarata. Afr. J. Pharm. Pharmacol. 2011, 5, 1508–1512. [Google Scholar]
- Sivalokanathan, S.; Vijayababu, M.R.; Balasubramanian, M.P. Effects of Terminalia arjuna bark extract on apoptosis of human hepatoma cell line HepG2. World J. Gastroenterol. 2006, 12, 1018–1024. [Google Scholar]
- Martin, S.J.; Green, D.R. Apoptosis and cancer: The failure of controls on cell death and cell survival. Crit. Rev. Oncol. Hematol. 1995, 8, 137–153. [Google Scholar] [CrossRef]
- Pan, G.; O’Rourke, K.; Chinnaiyan, A.M.; Gentz, R.; Ebner, R.; Ni, J.; Dixit, V.M. The receptor for the cytotoxic ligand TRAIL. Science 1997, 276, 111–113. [Google Scholar] [CrossRef]
- Zapata, J.M.; Pawlowski, K.; Haas, E.; Ware, C.F.; Godzik, A.; Reed, J.C. A diverse family of proteins containing tumor necrosis factor receptor-associated factor domains. J. Biol. Chem. 2001, 276, 24242–24252. [Google Scholar]
- Ashe, P.C.; Berry, M.D. Apoptotic signaling cascades. Prog. Neuropsychopharmacol. Biol. Psychiatr. 2003, 27, 199–214. [Google Scholar] [CrossRef]
- Samples Availability: Available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ma, J.; Huang, F.; Lin, H.; Wang, X. Isolation and Purification of a Peptide from Bullacta exarata and Its Impaction of Apoptosis on Prostate Cancer Cell. Mar. Drugs 2013, 11, 266-273. https://doi.org/10.3390/md11010266
Ma J, Huang F, Lin H, Wang X. Isolation and Purification of a Peptide from Bullacta exarata and Its Impaction of Apoptosis on Prostate Cancer Cell. Marine Drugs. 2013; 11(1):266-273. https://doi.org/10.3390/md11010266
Chicago/Turabian StyleMa, Jianyin, Fangfang Huang, Huanle Lin, and Xian Wang. 2013. "Isolation and Purification of a Peptide from Bullacta exarata and Its Impaction of Apoptosis on Prostate Cancer Cell" Marine Drugs 11, no. 1: 266-273. https://doi.org/10.3390/md11010266




