Design and Synthesis of Pro-Apoptotic Compounds Inspired by Diatom Oxylipins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Inhibition of Cell Division by Natural Oxylipins
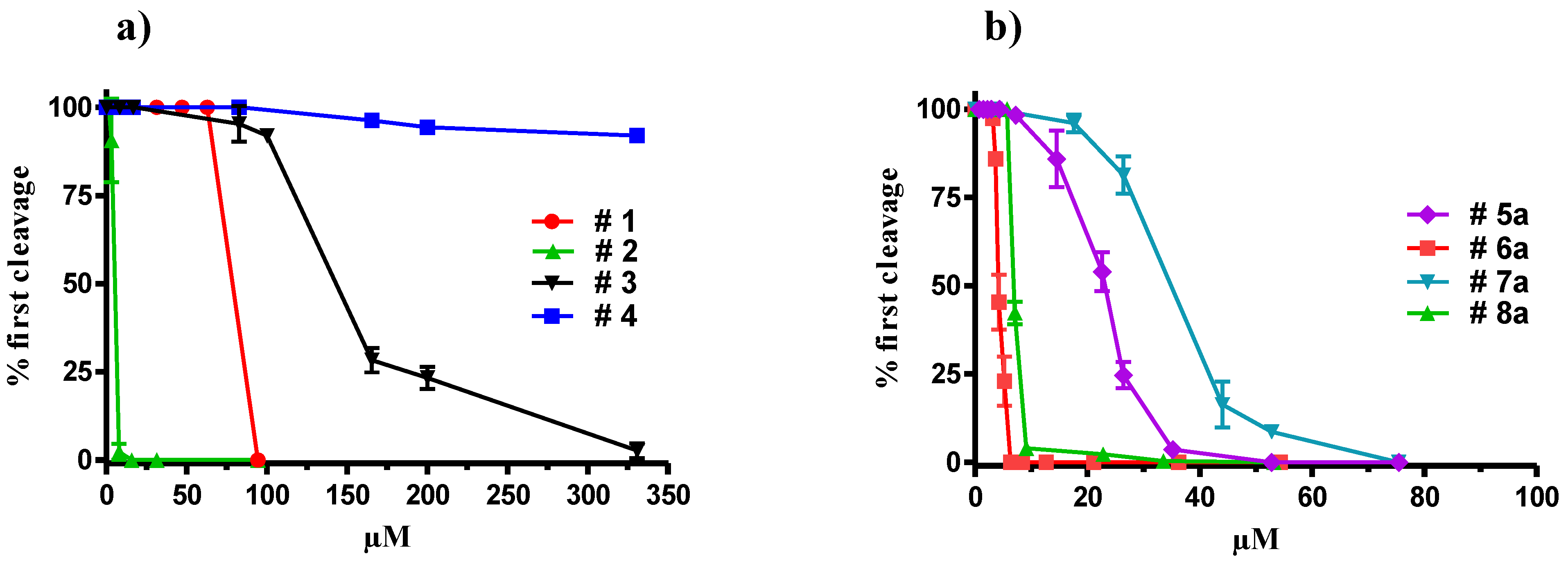
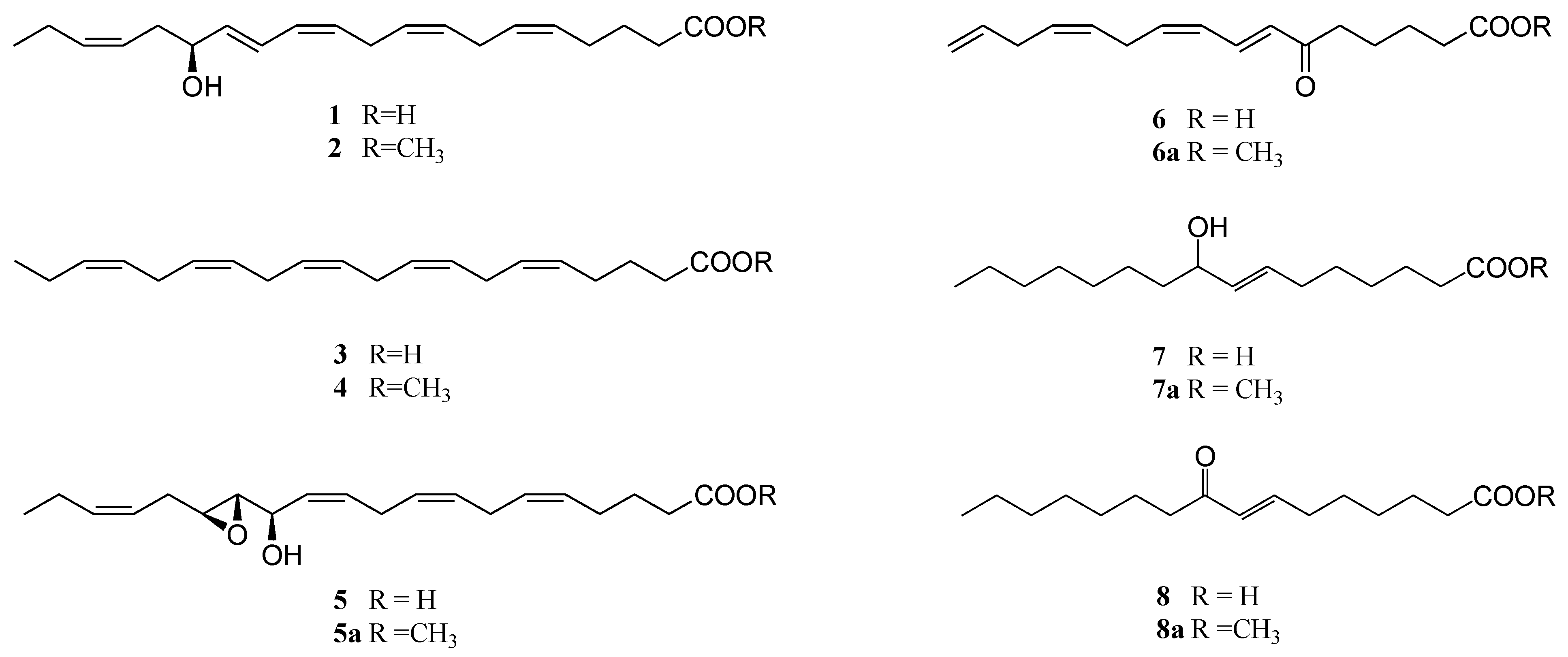
2.2. Synthesis of Oxylipin Analogues and Evaluation of Inhibitory Activity on Development of Sea-Urchin Embryos

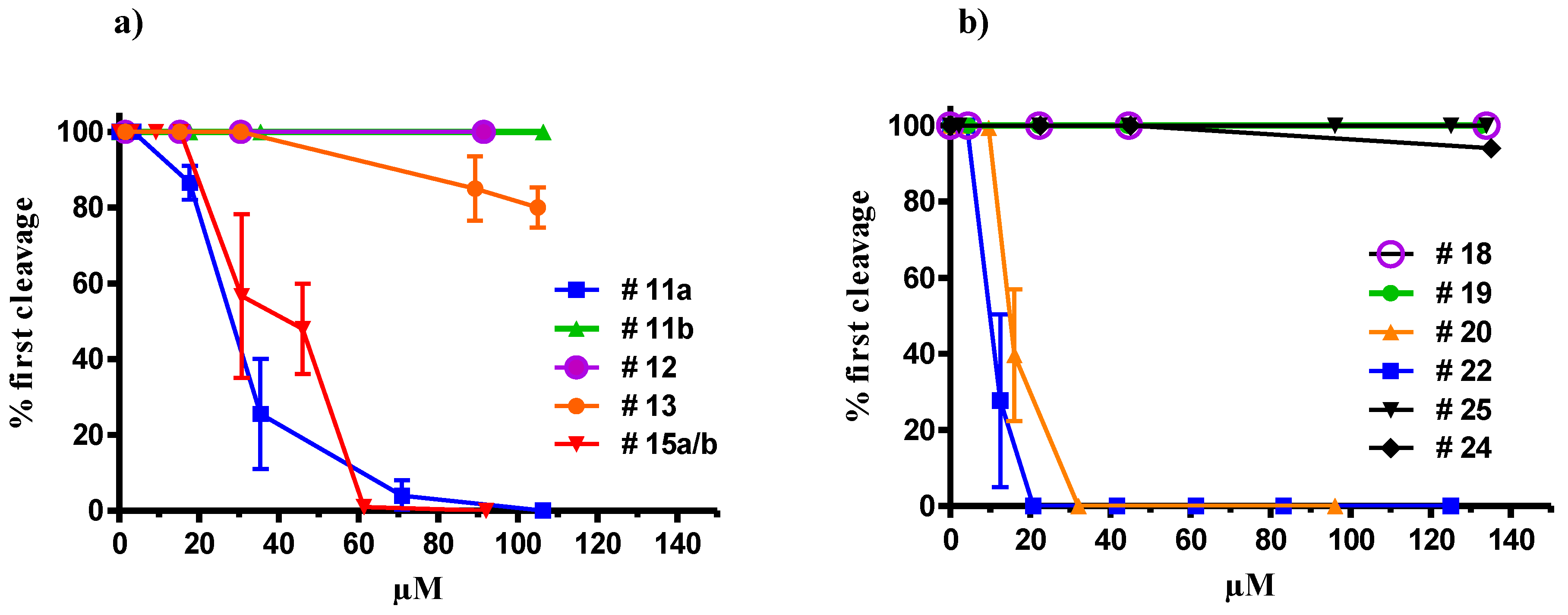
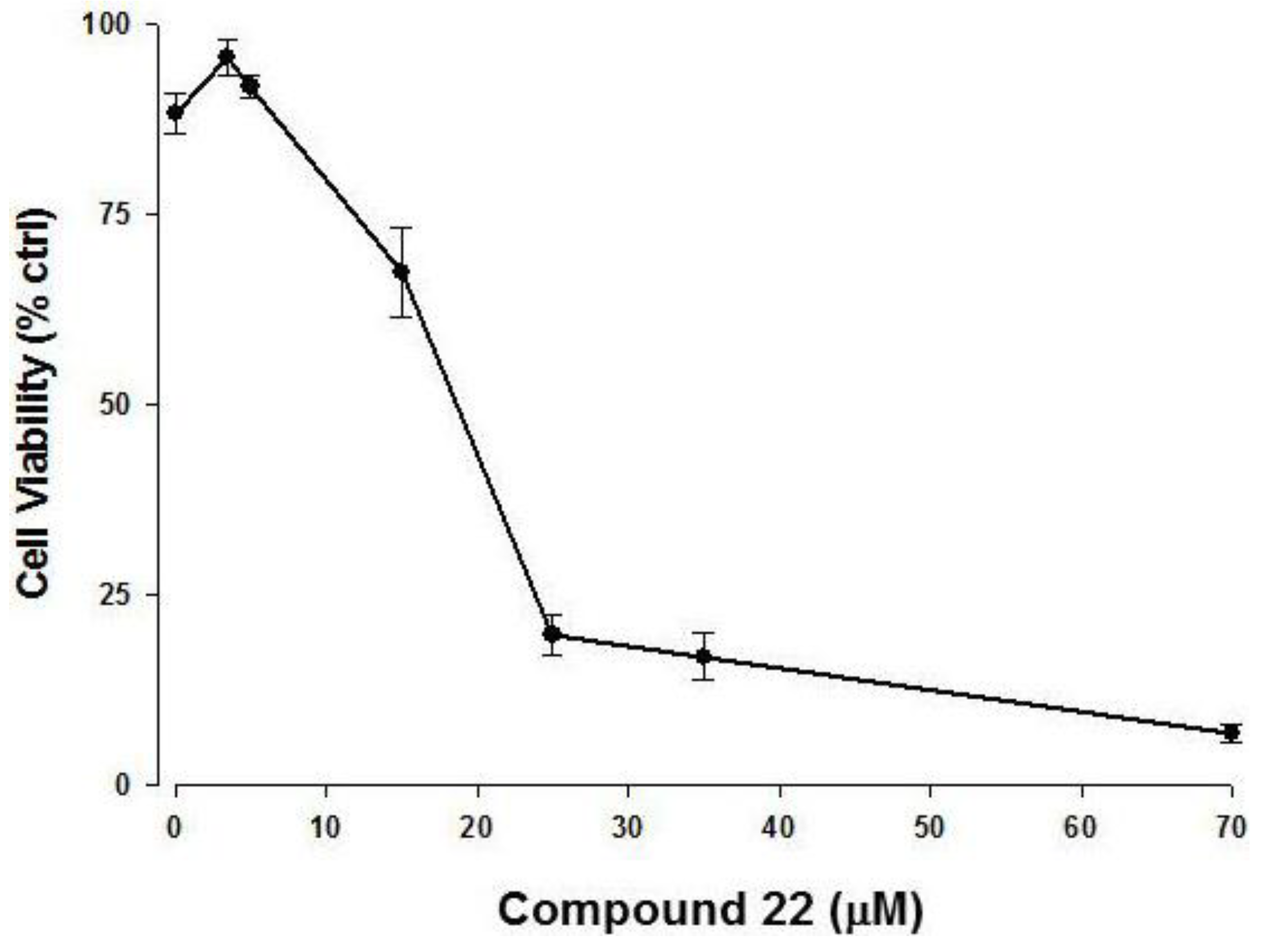
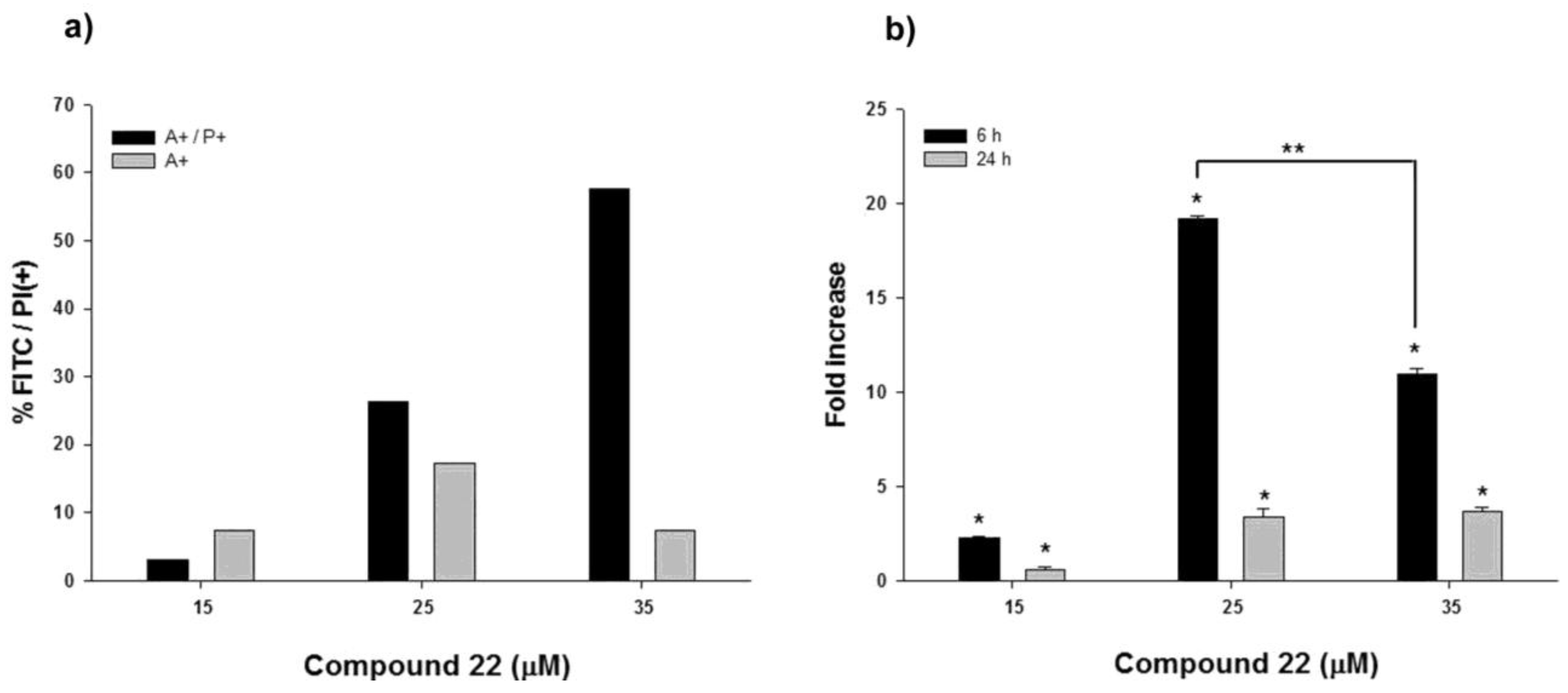
2.3. Apoptotic Activity of Compound 22
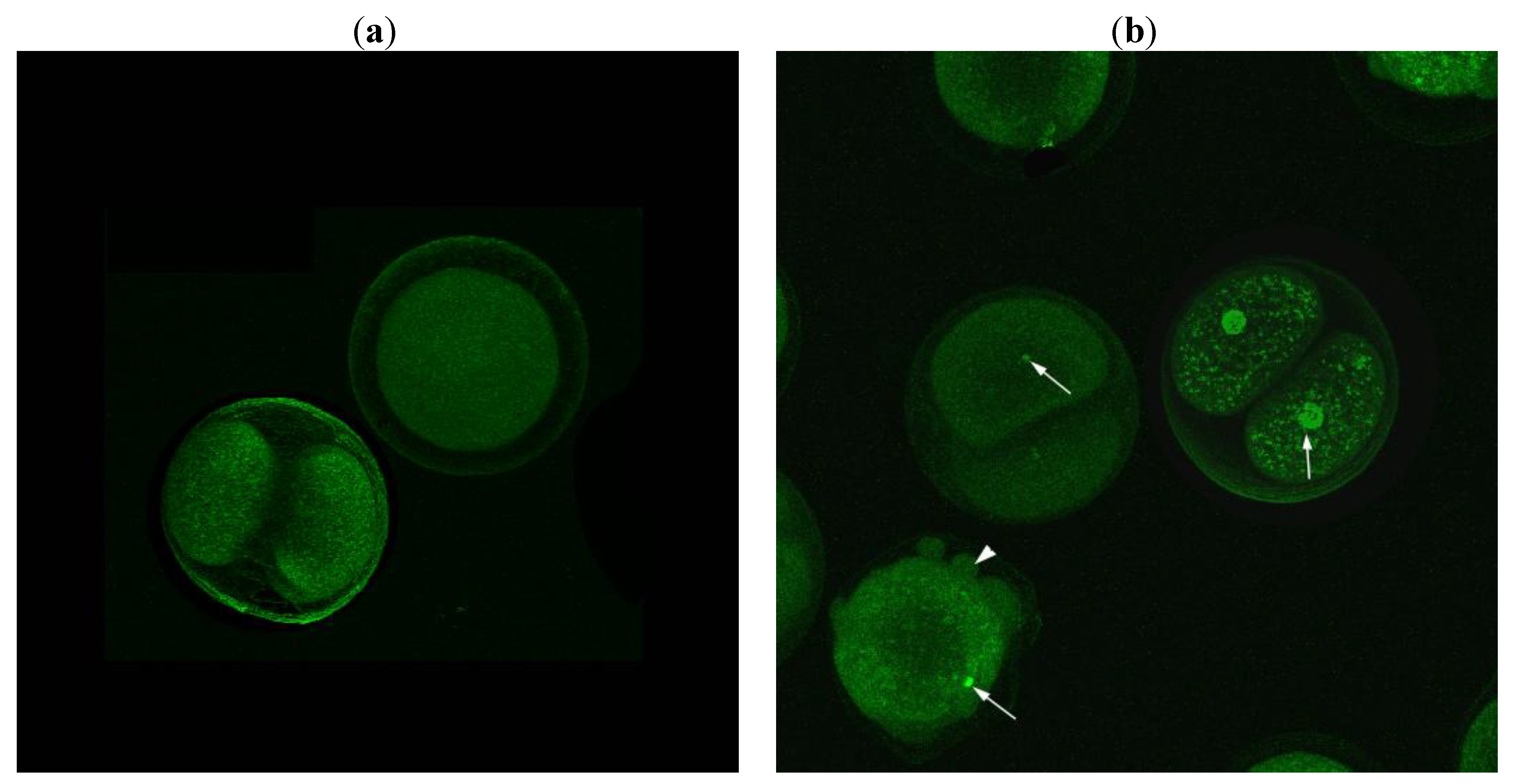
3. Experimental Section
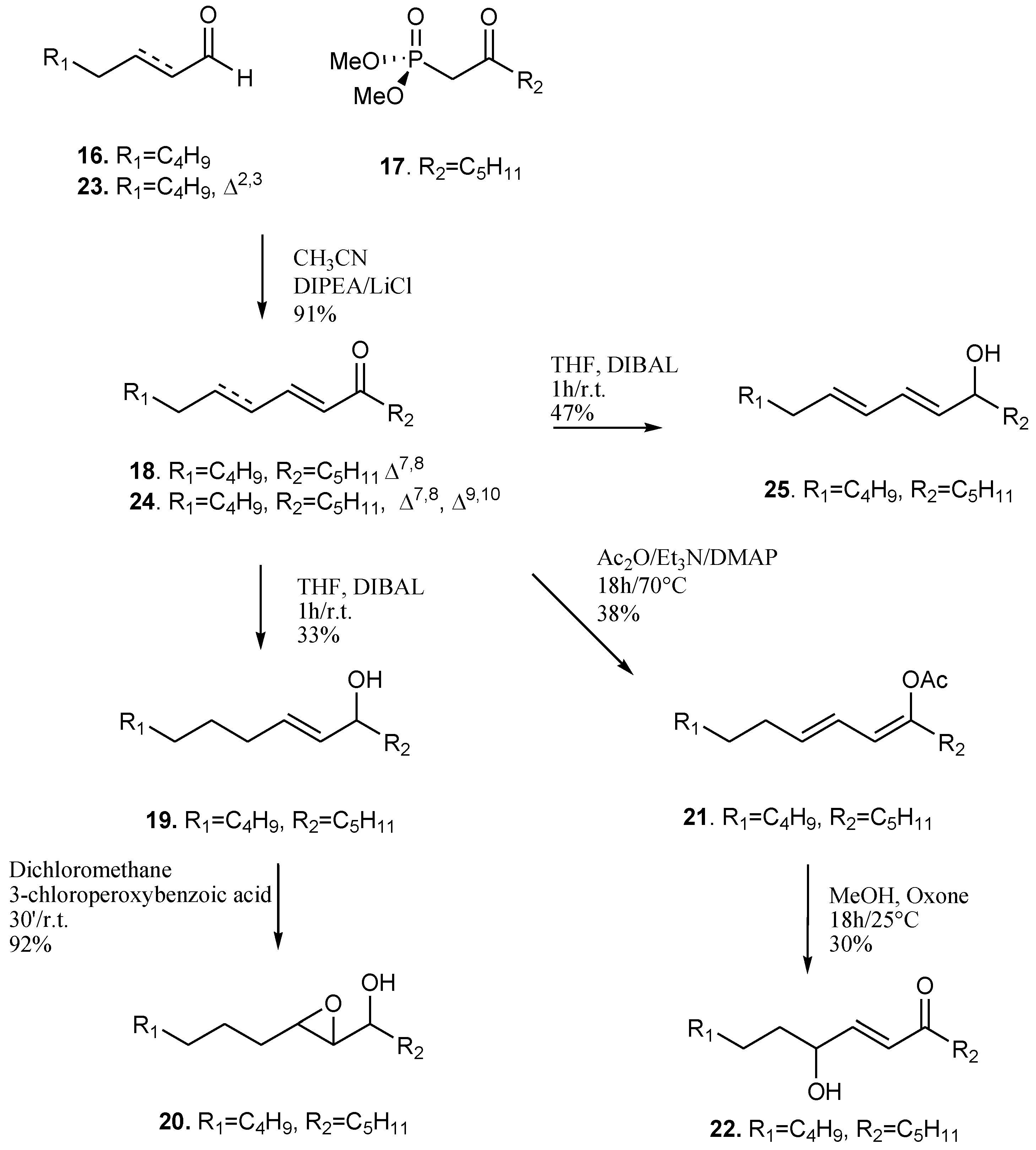
3.1. Chemical Synthesis of Oxylipin Analogues
3.2. Sea Urchin Gamete Collection
3.3. Antimitotic Assay on Sea Urchin Embryos
3.4. Tunel Fluorescence Labeling (TUNEL)
3.5. Cell Culture and Cell Viability Assay
3.6. Annexin V Assay
3.7. Caspase Assay
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- D’Ippolito, G.; Cutignano, A.; Briante, R.; Febbraio, F.; Cimino, G.; Fontana, A. New C16 Fatty Acid-Based Oxylipin Pathway in the Marine Diatom Thalassiosira rotula. Org. Biomol. Chem. 2005, 3, 4065–4070. [Google Scholar] [CrossRef]
- Fontana, A.; d’Ippolito, G.; Cutignano, A.; Romano, G.; Lamari, N.; Gallucci, A.M.; Cimino, G.; Miralto, A.; Ianora, A. LOX-induced peroxidation mechanism responsible for the detrimental effect of marine diatoms on zooplankton grazers. ChemBioChem 2007, 8, 1810–1818. [Google Scholar] [CrossRef]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef]
- Kuhn, H.; Walther, M.; Kuban, R.J. Mammalian arachidonate 15-LOXs. Structure, function, and biological implications. Prostaglandins Other Lipid Mediat. 2002, 68–69, 263–290. [Google Scholar] [CrossRef]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef]
- Miralto, A.; Barone, G.; Romano, G.; Poulet, S.A.; Ianora, A.; Russo, G.L.; Buttino, I.; Mazzarella, G.; Laabir, M.; Cabrini, M.; et al. The insidious effect of diatoms on copepod reproduction. Nature 1999, 402, 173–176. [Google Scholar] [CrossRef]
- Ianora, A.; Miralto, A. Toxigenic effects of diatoms on grazers, phytoplankton and other microbes: A review. Ecotoxicology 2010, 19, 493–511. [Google Scholar] [CrossRef]
- d’Ippolito, G.; Lamari, N.; Montresor, M.; Romano, G.; Cutignano, A.; Gerecht, A.; Cimino, G.; Fontana, A. 15S-Lipoxygenase metabolism in the marine diatom Pseudo-nitzschia delicatissima. New Phytol. 2009, 183, 1064–1071. [Google Scholar] [CrossRef]
- Caldwell, G.S. The influence of bioactive oxylipins from marine diatoms on invertebrate reproduction and development. Mar. Drugs 2009, 7, 367–400. [Google Scholar] [CrossRef]
- Ianora, A.; Romano, G.; Carotenuto, Y.; Esposito, F.; Roncalli, V.; Buttino, I.; Miralto, A. Impact of the diatom oxylipin 15(S)-HEPE on the reproductive success of the copepod Temora stylifera. Hydrobiologia 2011, 666, 265–275. [Google Scholar] [CrossRef]
- Cutignano, A.; Lamari, N.; d’Ippolito, G.; Manzo, E.; Cimino, G.; Fontana, A. Lipoxygenase products in marine diatoms: A concise analytical method to explore the functional potential of oxylipins. J. Phycol. 2011, 47, 233–243. [Google Scholar] [CrossRef]
- Romano, G.; Russo, G.L.; Buttino, I.; Ianora, A.; Miralto, A. A marine diatom-derived aldehyde induces apoptosis in copepod and sea urchin embryos. J. Exp. Biol. 2003, 206, 3487–3494. [Google Scholar] [CrossRef]
- Romano, G.; Miralto, A.; Ianora, A. Teratogenic effects of diatom metabolites on sea urchin Paracentrotus lividus embryos. Mar. Drugs 2010, 8, 950–967. [Google Scholar] [CrossRef]
- Adolph, S.; Bach, S.; Blondel, M.; Cueff, A.; Moreau, M.; Pohnert, G.; Poulet, S.A.; Wichard, T.; Zuccaro, A. Cytotoxicity of diatom-derived oxylipins in organisms belonging to different phyla. J. Exp. Biol. 2004, 207, 2935–2946. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Sae Melo, M.L.; Campos Neves, A.S. Copper-catalysed allylic oxidation of Δ5-steroids by t-butyl hydroperoxide. Tetrahedron Lett. 1997, 38, 119–122. [Google Scholar] [CrossRef]
- Suryawanshi, S.N.; Fuchs, P.L. Synthesis of gamma-hydroxy enones via persulfate oxidation of dienyl ethers. Tetrahedron Lett. 1981, 22, 4201–4204. [Google Scholar] [CrossRef]
- Russo, M.; Nigro, P.; Rosiello, R.; D’Arienzo, R.; Russo, G.L. Quercetin enhances CD95- and TRAIL-induced apoptosis in leukemia cell lines. Leukemia 2007, 21, 1130–1133. [Google Scholar]
- Caldwell, G.S.; Olive, P.J.W.; Bentley, M.G. Inhibition of embryonic development and fertilization in broadcast spawning marine invertebrates by water soluble diatom extracts and the diatom toxin 2-trans,4-trans decadienal. Aquat. Toxicol. 2002, 60, 123–137. [Google Scholar] [CrossRef]
- Poulet, S.A.; Richer de Forge, M.; Cueff, A.; Lennon, J.F. Double labelling methods used to diagnose apoptotic and necrotic cell degradations in copepod nauplii. Mar. Biol. 2003, 143, 889–895. [Google Scholar] [CrossRef]
- Ianora, A.; Miralto, A.; Poulet, S.A.; Carotenuto, Y.; Buttino, I.; Romano, G.; Casotti, R.; Pohnert, G.; Wichard, T.; Colucci-D’Amato, L.; et al. Aldehyde suppression of copepod recruitment in blooms of a ubiquitous planktonic diatom. Nature 2004, 429, 403–407. [Google Scholar] [CrossRef]
- Blanchette, M.A.; Choy, W.; Davis, J.T.; Essenfeld, A.P.; Masamune, S.; Roush, W.R.; Sakai, T. Horner-wadsworth-emmons reaction: Use of lithium chloride and an amine for base-sensitive compounds. Tetrahedron Lett. 1984, 25, 2183–2186. [Google Scholar]
- Russo, M.; Palumbo, R.; Mupo, A.; Tosto, M.; Iacomino, G.; Scognamiglio, A.; Tedesco, I.; Galano, G.; Russo, G.L. Flavonoid quercetin sensitizes a CD95-resistant cell line to apoptosis by activating protein kinase Cα. Oncogene 2003, 22, 3330–3342. [Google Scholar] [CrossRef]
Abbreviations
| DIBAL | diisobutylaluminum hydride |
| THF | tetrahydrofuran |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Romano, G.; Manzo, E.; Russo, G.L.; D'Ippolito, G.; Cutignano, A.; Russo, M.; Fontana, A. Design and Synthesis of Pro-Apoptotic Compounds Inspired by Diatom Oxylipins. Mar. Drugs 2013, 11, 4527-4543. https://doi.org/10.3390/md11114527
Romano G, Manzo E, Russo GL, D'Ippolito G, Cutignano A, Russo M, Fontana A. Design and Synthesis of Pro-Apoptotic Compounds Inspired by Diatom Oxylipins. Marine Drugs. 2013; 11(11):4527-4543. https://doi.org/10.3390/md11114527
Chicago/Turabian StyleRomano, Giovanna, Emiliano Manzo, Gian Luigi Russo, Giuliana D'Ippolito, Adele Cutignano, Maria Russo, and Angelo Fontana. 2013. "Design and Synthesis of Pro-Apoptotic Compounds Inspired by Diatom Oxylipins" Marine Drugs 11, no. 11: 4527-4543. https://doi.org/10.3390/md11114527








