Antibacterial Activity of Long-Chain Polyunsaturated Fatty Acids against Propionibacterium acnes and Staphylococcus aureus
Abstract
:1. Introduction
2. Results
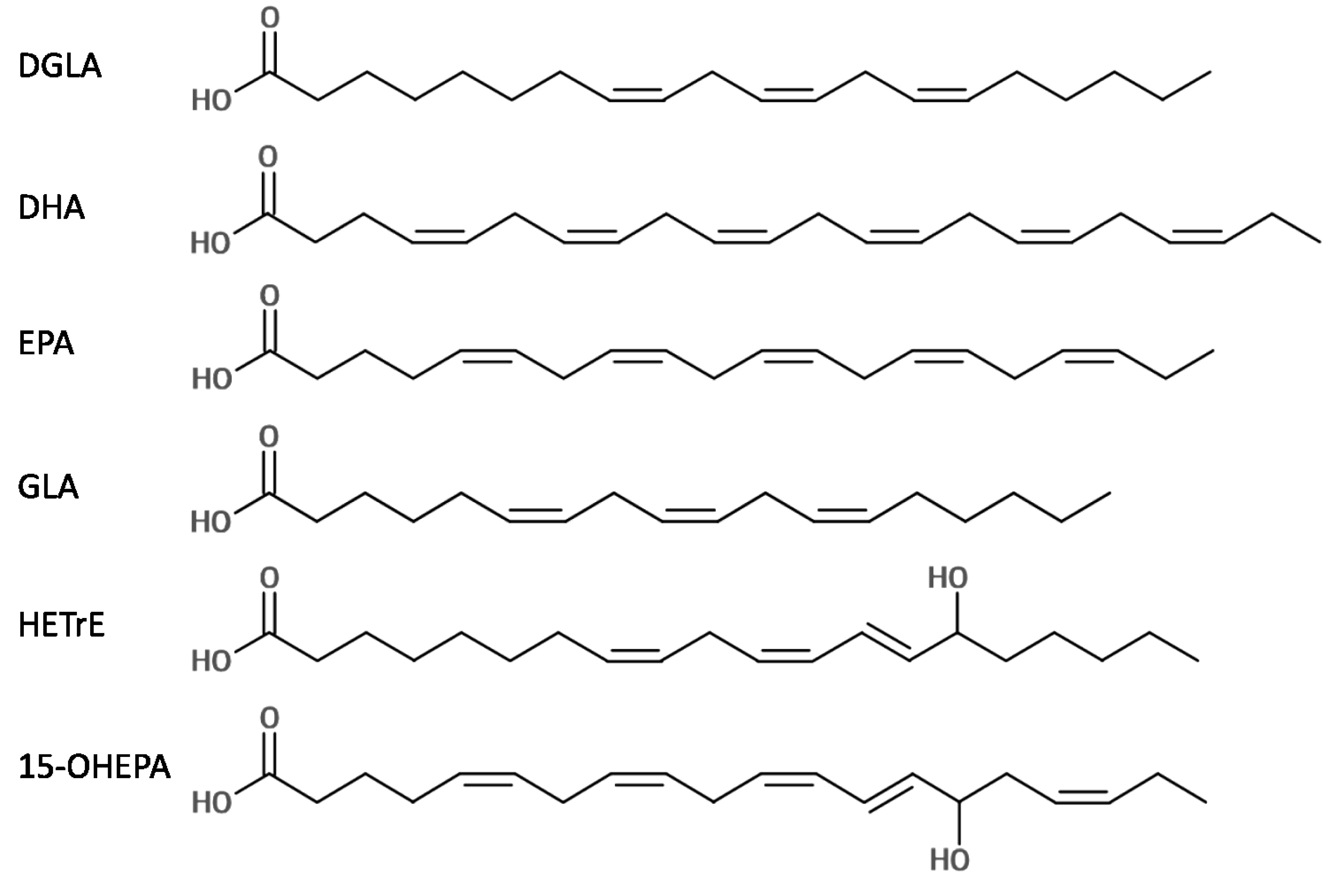
2.1. Antibacterial Activity of Six LC-PUFAs against P. acnes and S. aureus
| Compound | P. acnes | S. aureus | |||
|---|---|---|---|---|---|
| MIC (mg/L) | MBC (mg/L) | MIC (mg/L) | MBC (mg/L) | ||
| DGLA | 128 | >4096 | 1024 | 1024 | |
| DHA | 32 | >4096 | 128 | 128 | |
| EPA | 128 | >4096 | 128 | 256 | |
| GLA | 64 | >4096 | 512 | 512 | |
| HETrE | 32 | >4096 | 256 | 512 | |
| 15-OHEPA | 128 | >4096 | 512 | 1024 | |
| Compound | S. aureus | |
|---|---|---|
| MIC50 (mg/L) | MBC50 (mg/L) | |
| DGLA | 1024 | 2048 |
| DHA | 128 | 256 |
| EPA | 256 | 256 |
| GLA | 512 | 512 |
| HETrE | 512 | 1024 |
| 15-OHEPA | 1024 | 2048 |
2.2. Killing Kinetics of Six LC-PUFAs against S. aureus
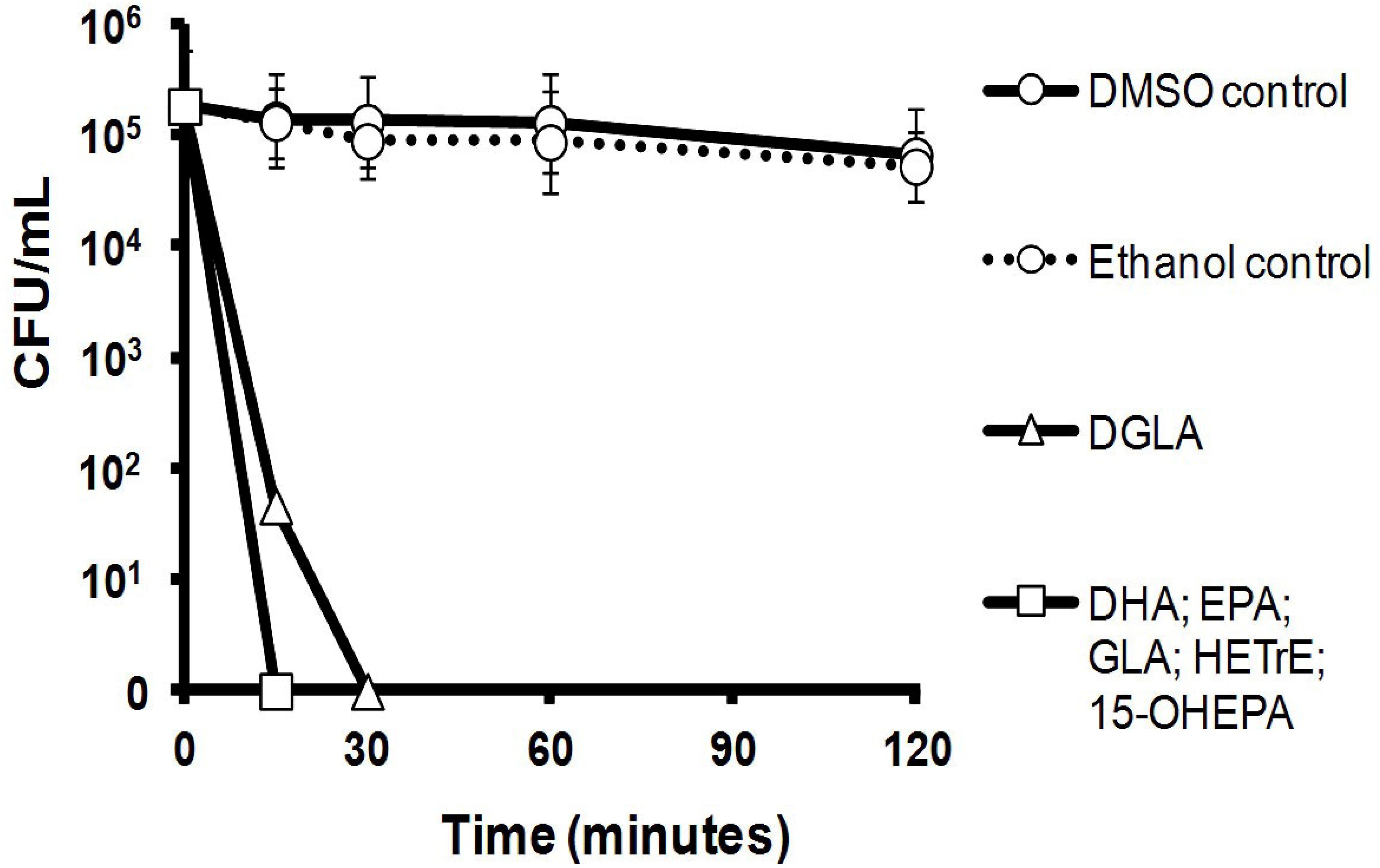
2.3. Efficacy of Six LC-PUFAs in Combination with Clinical Antimicrobial Agents
| Compound | P. acnes | S. aureus | |||
|---|---|---|---|---|---|
| MIC (mg/L) | MBC (mg/L) | MIC (mg/L) | MBC (mg/L) | ||
| BPO | 64 | >4096 | 512 | 512 | |
| FUS | n/d | n/d | 0.25 | 8 | |
| MUP | n/d | n/d | 0.25 | 16 | |
| NEO | n/d | n/d | 32 | 128 | |
| POL | n/d | n/d | 16 | 128 | |
| SA | 64 | >4096 | 1024 | 2048 | |
| P. acnes | DGLA | DHA | EPA | GLA | HETrE | 15-OHEPA |
|---|---|---|---|---|---|---|
| BPO | 1.50 ± 0.71 | 1.50 ± 0.71 | 1.50 ± 0.71 | 1.50 ± 0.71 | 0.75 ± 0.00 | 0.88 ± 0.18 |
| SA | 2.00 ± 0.00 | 1.13 ± 0.53 | 0.78 ± 0.31 | 1.50 ± 0.71 | 1.75 ± 0.35 | 2.00 ± 0.00 |
| S. aureus | DGLA | DHA | EPA | GLA | HETrE | 15-OHEPA |
|---|---|---|---|---|---|---|
| BPO | 0.44 ± 0.09 | 2.00 ± 0.00 | 1.00 ± 0.00 | 0.56 ± 0.09 | 0.50 ± 0.00 | 0.44 ± 0.09 |
| SA | 2.00 ± 0.00 | 1.25 ± 0.35 | 0.88 ± 0.18 | 0.88 ± 0.18 | 0.88 ± 0.18 | 1.00 ± 0.00 |
| FUS | 0.81 ± 0.27 | 2.00 ± 0.00 | 1.13 ± 0.18 | 1.50 ± 0.71 | 1.50 ± 0.71 | 2.00 ± 0.00 |
| MUP | 1.50 ± 0.71 | 1.50 ± 0.00 | 2.00 ± 0.00 | 2.25 ± 0.35 | 2.00 ± 0.00 | 1.38 ± 0.88 |
| NEO | 0.28 ± 0.13 | 0.56 ± 0.09 | 0.38 ± 0.00 | 0.19 ± 0.00 | 0.38 ± 0.00 | 0.34 ± 0.04 |
| POL | 1.78 ± 1.72 | 2.00 ± 0.00 | 2.00 ± 0.00 | 1.06 ± 0.62 | 0.75 ± 0.00 | 0.94 ± 0.27 |
3. Discussion
4. Experimental Section
4.1. Reagents and Bacteria
4.2. MIC and MBC Determinations
4.3. Kill Kinetics
4.4. Checkerboard Tests
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bojar, R.A.; Holland, K.T. Acne and Propionibacterium acnes. Clin. Dermatol. 2004, 22, 375–379. [Google Scholar] [CrossRef]
- Harper, J.C. An update on the pathogenesis and management of acne vulgaris. J. Am. Acad. Dermatol. 2004, 51, S36–S38. [Google Scholar] [CrossRef]
- Motswaledi, M.H. Superficial skin infections and the use of topical and systemic antibiotics in general practice. S. Afr. Fam. Pract. 2011, 53, 139–142. [Google Scholar]
- Joint Formulary Committee. British National Formulary, 61st ed.; British Medical Association and Royal Pharmaceutical Society of Great Britain: London, UK, 2011. [Google Scholar]
- Upton, A.; Lang, S.; Heffernan, H. Mupirocin and Staphylococcus aureus: A paradigm of emerging antibiotic resistance. J. Antimicrob. Chemother. 2003, 51, 613–617. [Google Scholar] [CrossRef]
- El-Zimaity, D.; Kearns, A.M.; Dawson, S.J.; Price, S.; Harrison, G.A.J. Survey, characterisation and susceptibility to fusidic acid of Staphylococcus aureus in the Carmarthen area. J. Antimicrob. Chemother. 2004, 54, 441–446. [Google Scholar] [CrossRef]
- Moon, S.H.; Roh, H.S.; Kim, Y.H.; Kim, J.E.; Ko, J.Y.; Ro, Y.S. Antibiotic resistance of microbial strains isolated from Korean acne patients. J. Dermatol. 2012, 39, 833–837. [Google Scholar] [CrossRef]
- Simonart, T.; Dramaix, M. Treatment of acne with topical antibiotics: Lessons from clinical studies. Br. J. Dermatol. 2005, 153, 395–403. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [Green Version]
- Desbois, A.P. Potential applications of antimicrobial fatty acids in medicine, agriculture and other industries. Recent Pat. Antiinfect. Drug Discov. 2012, 7, 111–122. [Google Scholar] [CrossRef]
- Berge, J.P.; Barnathan, G. Fatty acids from lipids of marine organisms: molecular biodiversity, roles as biomarkers, biologically active compounds, and economical aspects. Adv. Biochem. Eng. Biotechnol. 2005, 96, 49–125. [Google Scholar]
- Desbois, A.P.; Mearns-Spragg, A.; Smith, V.J. A fatty acid from the diatom Phaeodactylum tricornutum is antibacterial against diverse bacteria including multi-resistant Staphylococcus aureus (MRSA). Mar. Biotechnol. 2009, 11, 45–52. [Google Scholar] [CrossRef]
- Desbois, A.P. Antimicrobial properties of eicosapentaenoic acid (C20: 5n-3). In Marine Microbiology: Bioactive Compounds and Biotechnological Applications; Kim, S.-K., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 351–367. [Google Scholar]
- Coonrod, J.D. Rôle of surfactant free fatty acids in antimicrobial defences. Eur. J. Respir. Dis. 1987, 153, 209–214. [Google Scholar]
- Feldlaufer, M.F.; Knox, D.A.; Lusby, W.R.; Shimanuki, H. Antimicrobial activity of fatty acids against Bacillus larvae, the causative agent of American foulbrood disease. Apidologie 1993, 24, 95–99. [Google Scholar] [CrossRef]
- Maia, M.R.G.; Chaudhary, L.C.; Figueres, L.; Wallace, R.J. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Van Leeuwenhoek 2007, 91, 303–314. [Google Scholar] [CrossRef]
- Huang, C.B.; Ebersole, J.L. A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Mol. Oral Microbiol. 2010, 25, 75–80. [Google Scholar] [CrossRef]
- Butcher, G.W.; King, G.; Dyke, K.G.H. Sensitivity of Staphylococcus aureus to unsaturated fatty acids. J. Gen. Microbiol. 1976, 94, 290–296. [Google Scholar] [CrossRef]
- Asthana, R.K.; Srivastava, A.; Kayastha, A.M.; Nath, G.; Singh, S.P. Antibacterial potential of γ-linolenic acid from Fischerella sp. colonising Neem tree bark. World J. Microbiol. Biotechnol. 2006, 22, 443–448. [Google Scholar] [CrossRef]
- Huang, C.B.; George, B.; Ebersole, J.L. Antimicrobial activity of n-6, n-7 and n-9 fatty acids and their esters for oral microorganisms. Arch. Oral Biol. 2010, 55, 555–560. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Peng, L.; Dong, X.; Wu, D.; Wu, V.C.; Feng, F. Quantitative structure-activity relationships of antimicrobial fatty acids and derivatives against Staphylococcus aureus. J. Zhejiang Univ. Sci. B 2012, 13, 83–93. [Google Scholar]
- Kristmundsdóttir, T.; Skúlason, S. Lipids as active ingredients in pharmaceuticals, cosmetics and health foods. In Lipids and Essential Oils as Antimicrobial Agents; Thormar, H., Ed.; John Wiley & Sons, Ltd.: Philadelphia, PA, USA, 2011; pp. 151–177. [Google Scholar]
- Mullen, A.; Loscher, C.E.; Roche, H.M. Anti-inflammatory effects of EPA and DHA are dependent upon time and dose-response elements associated with LPS stimulation in THP-1-derived macrophages. J. Nutr. Biochem. 2010, 21, 444–450. [Google Scholar] [CrossRef]
- Kalan, L.; Wright, G.D. Antibiotic adjuvants: multicomponent anti-infective strategies. Expert Rev. Mol. Med. 2011, 13, 1–17. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Ko, H.L.; Heczko, P.B.; Pulverer, G. Differential susceptibility of Propionibacterium acnes, P. granulosum and P. avidum to free fatty acids. J. Invest. Dermatol. 1978, 71, 363–365. [Google Scholar]
- Nakatsuji, T.; Kao, M.C.; Fang, J.-Y.; Zouboulis, C.C.; Zhang, L.; Gallo, R.L.; Huang, C.M. Antimicrobial property of lauric acid against Propionibacterium acnes: its therapeutic potential for inflammatory acne vulgaris. J. Invest. Dermatol. 2009, 129, 2480–2488. [Google Scholar] [CrossRef]
- Decker, L.C.; Deuel, D.M.; Sedlock, D.M. Role of lipids in augmenting the antibacterialk activity of benzoyl peroxide against Propionibacterium acnes. Antimicrob. Agents Chemother. 1989, 33, 326–330. [Google Scholar] [CrossRef]
- Pannu, J.; McCarthy, A.; Martin, A.; Hamouda, T.; Ciotti, S.; Ma, L.; Sutcliffe, J.; Baker, J.R., Jr. In vitro antibacterial activity of NB-003 against Propionibacterium acnes. Antimicrob. Agents Chemother. 2011, 55, 4211–4217. [Google Scholar] [CrossRef]
- Blondeau, J.M. New concepts in antimicrobial susceptibility testing: the mutant prevention concentration and mutant selection window approach. Vet. Dermatol. 2009, 20, 383–396. [Google Scholar] [CrossRef]
- Shin, S.Y.; Bajpai, V.K.; Kim, H.R.; Kang, S.C. Antibacterial activity of bioconverted eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) against foodborne pathogenic bacteria. Int. J. Food Microbiol. 2007, 113, 233–236. [Google Scholar] [CrossRef]
- Knapp, H.R.; Melly, M.A. Bactericidal effects of polyunsaturated fatty acids. J. Infect. Dis. 1986, 154, 84–94. [Google Scholar] [CrossRef]
- Nair, M.K.M.; Joy, J.; Vasudevan, P.; Hinckley, L.; Hoagland, T.A.; Venkitanarayanan, K.S. Antibacterial effect of caprylic acid and monocaprylin on major bacterial mastitis pathogens. J. Dairy Sci. 2005, 88, 3488–3495. [Google Scholar] [CrossRef]
- Bergsson, G.; Arnfinnsson, J.; Steingrímsson, Ó.; Thormar, H. Killing of Gram-positive cocci by fatty acids and monoglycerides. APMIS 2001, 109, 670–678. [Google Scholar]
- Wille, J.J.; Kydonieus, A. Palmitoleic acid isomer (C16:1Δ6) in human skin sebum is effective against Gram-positive bacteria. Skin Pharmacol. Appl. Skin Physiol. 2003, 16, 176–187. [Google Scholar] [CrossRef]
- Clarke, S.R.; Mohamed, R.; Bian, L.; Routh, A.F.; Kokai-Kun, J.F.; Mond, J.J.; Tarkowski, A.; Foster, S.J. The Staphylococcus aureus surface protein isdA mediates resistance to innate defences of human skin. Cell Host Microb. 2007, 1, 1–14. [Google Scholar] [CrossRef]
- Chamberlain, N.R.; Mehrtens, B.G.; Xiong, Z.; Kapral, F.A.; Boardman, J.L.; Rearick, J.I. Correlation of carotenoid production, decreased membrane fluidity, and resistance to oleic acid killing in Staphylococcus aureus 18Z. Infect. Immun. 1991, 59, 4332–4337. [Google Scholar]
- Shin, S.Y.; Bajpai, V.K.; Kim, H.R.; Kang, S.C. Antibacterial activity of eicosapentaenoic acid (EPA) against foodborne and food spoilage microorganisms. LWT 2007, 40, 1515–1519. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Grecka, P.; Dionyssiou-Asteriou, A.; Giamarellou, H. Impact of n-6 polyunsaturated fatty acids on growth of multidrug-resistant Pseudomonas aeruginosa: Interactions with amikacin and ceftazidime. Antimicrob. Agents Chemother. 2000, 44, 2187–2189. [Google Scholar] [CrossRef]
- Kitahara, T.; Aoyama, Y.; Hirakata, Y.; Kamihira, S.; Kohno, S.; Ichikawa, N.; Nakashima, M.; Sasaki, H.; Higuchi, S. In vitro activity of lauric acid or myristylamine in combination with six antimicrobial agents against methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Antimicrob. Agents 2006, 27, 51–57. [Google Scholar] [CrossRef]
- Kravchenko, I.A.; Golovenko, N.Y.; Larionov, V.B.; Aleksandrova, A.I.; Ovcharenko, N.V. Effect of lauric acid on transdermal penetration of phenazepam in vivo. Bull. Exp. Biol. Med. 2003, 6, 579–581. [Google Scholar]
- Saraiva, R.A.; Matias, E.F.F.; Coutinho, H.D.M.; Costa, J.G.M.; Souza, H.H.F.; Fernandes, C.N.; Rocha, J.B.T.; Menezes, I.R.A. Synergistic action between Caryocar coriaceum Wittm. fixed oil with aminoglycosides in vitro. Eur. J. Lipid Sci. Technol. 2011, 113, 967–972. [Google Scholar] [CrossRef]
- Chen, C.-H.; Wang, Y.; Nakatsuji, T.; Liu, Y.-T.; Zouboulis, C.C.; Gallo, R.L.; Zhang, L.; Hsieh, M.F.; Huang, C.M. An innate bactericidal oleic acid affective against skin infection of methicillin-resistant Staphylococcus aureus: A therapy concordant with evolutionary medicine. J. Microbiol. Biotechnol. 2011, 21, 391–399. [Google Scholar]
- Huang, C.-M.; Chen, C.-H.; Pornpattananangkul, D.; Zhang, L.; Chan, M.; Hsieh, M.-F.; Zhang, L. Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials 2011, 32, 214–221. [Google Scholar] [CrossRef]
- Hart, R.; Bell-Syer, S.E.M.; Crawford, F.; Torgerson, D.J.; Young, P.; Russell, I. Systematic review of topical treatments for fungal infections of the skin and nails of the feet. BMJ 1999, 319, 79–82. [Google Scholar] [CrossRef]
- Georgel, P.; Crozat, K.; Lauth, X.; Makrantonaki, E.; Seltmann, H.; Sovath, S.; Hoebe, K.; Du, X.; Rutschmann, S.; Jiang, Z.; Bigby, T.; Nizet, V.; Zouboulis, C.C.; Beutler, B. A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with Gram-positive bacteria. Infect. Immun. 2005, 73, 4512–4521. [Google Scholar] [CrossRef]
- Drake, D.R.; Brogden, K.A.; Dawson, D.V.; Wertz, P.W. Antimicrobial lipids at the skin surface. J. Lipid Res. 2008, 49, 4–11. [Google Scholar] [CrossRef]
- Brogden, N.K.; Mehalick, L.; Fischer, C.L.; Wertz, P.W.; Brogden, K.A. The emerging role of peptides and lipids as antimicrobial epidermal barriers and modulators of local inflammation. Skin Pharmacol. Physiol. 2012, 25, 167–181. [Google Scholar] [CrossRef]
- Thormar, H.; Hilmarsson, H. The role of microbicidal lipids in host defense against pathogens and their potential as therapeutic agents. Chem. Phys. Lipids 2007, 150, 1–11. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Kao, J.; Jain, M.; Ahn, S.K.; Feingold, K.R.; Elias, P.M. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J. Invest. Dermatol. 2001, 117, 44–51. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Huang, C.-M.; Nakatsuji, T.; Thiboutot, D.; Kang, S.-A.; Monestier, M.; Gallo, R.L. Histone H4 is a major component of the antimicrobial action of human sebocytes. J. Invest. Dermatol. 2009, 129, 2489–2496. [Google Scholar] [CrossRef]
- Desbois, A.P.; Gemmell, C.G.; Coote, P.J. In vivo efficacy of the antimicrobial peptide ranalexin in combination with the endopeptidase lysostaphin against wound and systemic meticillin-resistant Staphylococcus aureus (MRSA) infections. Int. J. Antimicrob. Agents 2010, 35, 559–565. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard M07-A8; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard M11-A5; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2001. [Google Scholar]
- American Society for Microbiology. Synergism testing: Broth microdilution checkerboard and broth macrodilution methods. In Clinical Microbiology Procedures Handbook; Isenberg, H.D., Ed.; ASM Press: Washington, DC, USA, 1992. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Desbois, A.P.; Lawlor, K.C. Antibacterial Activity of Long-Chain Polyunsaturated Fatty Acids against Propionibacterium acnes and Staphylococcus aureus. Mar. Drugs 2013, 11, 4544-4557. https://doi.org/10.3390/md11114544
Desbois AP, Lawlor KC. Antibacterial Activity of Long-Chain Polyunsaturated Fatty Acids against Propionibacterium acnes and Staphylococcus aureus. Marine Drugs. 2013; 11(11):4544-4557. https://doi.org/10.3390/md11114544
Chicago/Turabian StyleDesbois, Andrew P., and Keelan C. Lawlor. 2013. "Antibacterial Activity of Long-Chain Polyunsaturated Fatty Acids against Propionibacterium acnes and Staphylococcus aureus" Marine Drugs 11, no. 11: 4544-4557. https://doi.org/10.3390/md11114544






