Okadaic Acid Toxin at Sublethal Dose Produced Cell Proliferation in Gastric and Colon Epithelial Cell Lines
Abstract
:1. Introduction
2. Results and Discussion
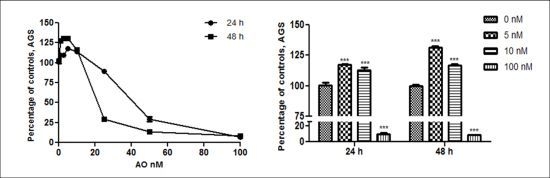
2.1. OA Generate Cellular Proliferation at Sublethal Doses in Gastric and Colon Epithelium


2.2. Overexpression of Cyclin B as an OA Effect

2.3. Activation of Akt and Erk Pathways as an OA Effect

3. Experimental Section
3.1. Reagents and Chemicals
3.2. Cell Culture
3.3. Cell Viability Assay
3.3.1. MTT Assay and AlamarBlue Assay
3.3.2. Trypan Blue Assay
3.4. Cell Extracts
3.5. Western Blotting
3.6. Statistical Analyses
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Alexander, J.; Audunsson, G.A.; Benford, D.; Cockburn, A.; Cradevi, J.P.; Dogliotti, E.; Domenico, A.D.; Fernandez-Cruz, M.L.; Fink-Gremmels, J.; Furst, P.; et al. Marine biotoxins in shellfish—okadaic acid and analogues—Scientific Opinion of the Panel on Contaminants in the Food chain. EFSA J. 2008, 589, 1–62. [Google Scholar]
- Aune, T.; Stabell, O.B.; Nordstoga, K.; Tjotta, K. Oral toxicity in mice of algal toxins from the diarrheic shellfish toxin (DST) complex and associated toxins. J. Nat. Toxins 1998, 7, 141–158. [Google Scholar]
- Tripuraneni, J.; Koutsouris, A.; Pestic, L.; De Lanerolle, P.; Hecht, G. The toxin of diarrheic shellfish poisoning, okadaic acid, increases intestinal epithelial paracellular permeability. Gastroenterology 1997, 112, 100–108. [Google Scholar] [CrossRef]
- Fujiki, H.; Suganuma, M. Carcinogenic aspects of protein phosphatase 1 and 2A inhibitors. Prog. Mol. Subcell Biol. 2009, 46, 221–254. [Google Scholar] [CrossRef]
- Manerio, E.; Rodas, V.L.; Costas, E.; Hernandez, J.M. Shellfish consumption: A major risk factor for colorectal cancer. Med. Hypotheses 2008, 70, 409–412. [Google Scholar] [CrossRef]
- Bialojan, C.; Takai, A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Biochem. J. 1988, 256, 283–290. [Google Scholar]
- Westermarck, J.; Hahn, W.C. Multiple pathways regulated by the tumor suppressor PP2A in transformation. Trends Mol. Med. 2008, 14, 152–160. [Google Scholar] [CrossRef]
- Fujiki, H.; Suganuma, M. Tumor promoters—microcystin-LR, nodularin and TNF-α and human cancer development. Anticancer Agents Med. Chem. 2011, 11, 4–18. [Google Scholar] [CrossRef]
- Baharians, Z.; Schonthal, A. Reduction of Ha-ras-induced cellular transformation by elevated expression of protein phosphatase type 2A. Mol. Carcinog. 1999, 24, 246–254. [Google Scholar] [CrossRef]
- Guénin, S.; Schwartz, L.; Morvan, D.; Steyaert, J.M.; Poignet, A.; Madelmont, J.C.; Demidem, A. PP2A activity is controlled by methylation and regulates oncoprotein expression in melanoma cells: A mechanism which participates in growth inhibition induced by chloroethylnitrosourea treatment. Int. J. Oncol. 2008, 32, 49–57. [Google Scholar]
- Matias, W.G.; Traore, A.; Creppy, E.E. Variations in the distribution of okadaic acid in organs and biological fluids of mice related to diarrhoeic syndrome. Hum. Exp. Toxicol. 1999, 18, 345–350. [Google Scholar] [CrossRef]
- Aune, T.; Espenes, A.; Aasen, J.A.; Quilliam, M.A.; Hess, P.; Larsen, S. Study of possible combined toxic effects of azaspiracid-1 and okadaic acid in mice via the oral route. Toxicon 2012, 60, 895–906. [Google Scholar] [CrossRef]
- Ito, E.; Yasumoto, T.; Takai, A.; Imanishi, S.; Harada, K. Investigation of the distribution and excretion of okadaic acid in mice using immunostaining method. Toxicon 2002, 40, 159–165. [Google Scholar] [CrossRef]
- Fessard, V.; Grosse, Y.; Pfohl-Leszkowicz, A.; Puiseux-Dao, S. Okadaic acid treatment induces DNA adduct formation in BHK21 C13 fibroblasts and HESV keratinocytes. Mutat. Res. 1996, 36, 133–141. [Google Scholar]
- Jayaraj, R.; Gupta, N.; Rao, P.V. Multiple signal transduction pathways in okadaic acid induced apoptosis in HeLa cells. Toxicology 2009, 256, 118–127. [Google Scholar]
- Kitazumi, I.; Maseki, Y.; Nomura, Y.; Shimanuki, A.; Sugita, Y.; Tsukahara, M. Okadaic acid induces DNA fragmentation via caspase-3-dependent and caspase-3-independent pathways in Chinese hamster ovary (CHO)-K1 cells. FEBS J. 2010, 277, 404–412. [Google Scholar] [CrossRef]
- Ravindran, J.; Gupta, N.; Agrawal, M.; Bala Bhaskar, A.S.; Lakshmana Rao, P.V. Modulation of ROS/MAPK signaling pathways by okadaic acid leads to cell death via, mitochondrial mediated caspase-dependent mechanism. Apoptosis 2011, 16, 145–161. [Google Scholar] [CrossRef]
- Fisher, D.; Krasinska, L.; Coudreuse, D.; Novák, B. Phosphorylation network dynamics in the control of cell cycle transitions. J. Cell Sci. 2012, 125, 4703–4711. [Google Scholar] [CrossRef]
- Rodgers, J.T.; Vogel, R.O.; Puigserver, P. Clk2 and B56β mediate insulin-regulated assembly of the PP2A phosphatase holoenzyme complex on Akt. Mol. Cell 2011, 41, 471–479. [Google Scholar] [CrossRef]
- Letourneux, C.; Rocher, G.; Porteu, F. B56-containing PP2A dephosphorylate ERK and their activity is controlled by the early gene IEX-1 and ERK. EMBO J. 2006, 25, 727–738. [Google Scholar] [CrossRef]
- Souid-Mensi, G.; Moukha, S.; Mobio, T.A.; Maaroufi, K.; Creppy, E.E. The cytotoxicity and genotoxicity of okadaic acid are cell-line dependent. Toxicon 2008, 51, 1338–1344. [Google Scholar] [CrossRef]
- Parkman, H.P.; Jones, M.P. Tests of gastric neuromuscular function. Gastroenterology 2009, 136, 1526–1543. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Del Campo, M.; Toledo, H.; Lagos, N. Okadaic Acid Toxin at Sublethal Dose Produced Cell Proliferation in Gastric and Colon Epithelial Cell Lines. Mar. Drugs 2013, 11, 4751-4760. https://doi.org/10.3390/md11124751
Del Campo M, Toledo H, Lagos N. Okadaic Acid Toxin at Sublethal Dose Produced Cell Proliferation in Gastric and Colon Epithelial Cell Lines. Marine Drugs. 2013; 11(12):4751-4760. https://doi.org/10.3390/md11124751
Chicago/Turabian StyleDel Campo, Miguel, Héctor Toledo, and Néstor Lagos. 2013. "Okadaic Acid Toxin at Sublethal Dose Produced Cell Proliferation in Gastric and Colon Epithelial Cell Lines" Marine Drugs 11, no. 12: 4751-4760. https://doi.org/10.3390/md11124751