New Casbane Diterpenoids from a South China Sea Soft Coral, Sinularia sp.
Abstract
:1. Introduction
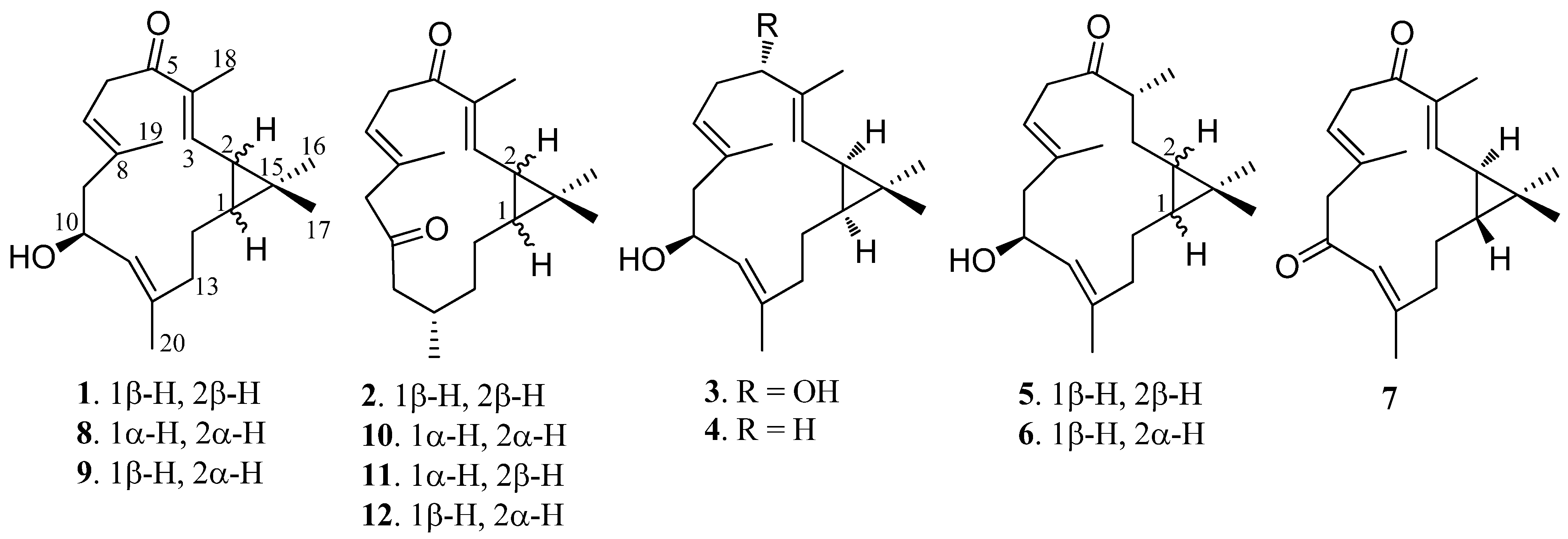
2. Results and Discussion
| No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1 | 34.6 (CH) | 34.8 (CH) | 31.5 (CH) | 30.7 (CH) | 24.4 (CH) | 28.1 (CH) |
| 2 | 27.3 (CH) | 27.6 (CH) | 25.7 (CH) | 25.9 (CH) | 24.6 (CH) | 24.8 (CH) |
| 3 | 142.6 (CH) | 144.0 (CH) | 125.5 (CH) | 121.2 (CH) | 28.5 (CH2) | 30.2 (CH2) |
| 4 | 137.2 (C) | 135.8 (C) | 136.9 (C) | 135.8 (C) | 43.9 (CH) | 46.5 (CH) |
| 5 | 199.8 (C) | 199.5 (C) | 79.3 (CH) | 39.4 (CH2) | 214.8 (C) | 211.9 (C) |
| 6 | 39.1 (CH2) | 40.3 (CH2) | 33.2 (CH2) | 25.0 (CH2) | 42.8 (CH2) | 40.5 (CH2) |
| 7 | 122.0 (CH) | 123.6 (CH) | 121.9 (CH) | 127.0 (CH) | 120.3 (CH) | 120.0 (CH) |
| 8 | 134.1 (C) | 129.9 (C) | 132.5 (C) | 130.8 (C) | 136.6 (C) | 134.5 (C) |
| 9 | 47.5 (CH2) | 52.2 (CH2) | 47.8 (CH2) | 48.0 (CH2) | 47.5 (CH2) | 47.2 (CH2) |
| 10 | 66.2 (CH) | 206.8 (C) | 66.5 (CH) | 66.9 (CH) | 66.5 (CH) | 67.4 (CH) |
| 11 | 127.1 (CH) | 47.4 (CH2) | 127.7 (CH) | 127.0 (CH) | 126.8 (CH) | 128.5 (CH) |
| 12 | 139.8 (C) | 26.7 (CH) | 140.1 (C) | 140.1 (C) | 140.2 (C) | 138.6 (C) |
| 13 | 39.9 (CH2) | 33.9 (CH2) | 40.0 (CH2) | 39.5 (CH2) | 38.0 (CH2) | 40.6 (CH2) |
| 14 | 25.4 (CH2) | 19.7 (CH2) | 24.3 (CH2) | 23.9 (CH2) | 21.1 (CH2) | 24.9 (CH2) |
| 15 | 25.8 (C) | 25.1 (C) | 20.7 (C) | 20.0 (C) | 17.1 (C) | 19.6 (C) |
| 16 | 15.9 (CH3) | 16.0 (CH3) | 28.8 (CH3) | 28.9 (CH3) | 14.9 (CH3) | 21.5 (CH3) |
| 17 | 29.0 (CH3) | 29.1 (CH3) | 15.5 (CH3) | 15.6 (CH3) | 29.2 (CH3) | 22.1 (CH3) |
| 18 | 11.7 (CH3) | 11.3 (CH3) | 10.2 (CH3) | 16.0 (CH3) | 17.3 (CH3) | 13.4 (CH3) |
| 19 | 16.6 (CH3) | 18.6 (CH3) | 17.2 (CH3) | 17.1 (CH3) | 16.9 (CH3) | 17.8 (CH3) |
| 20 | 15.0 (CH3) | 20.2 (CH3) | 18.5 (CH3) | 18.6 (CH3) | 17.2 (CH3) | 16.4 (CH3) |
| No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1 | 1.14 m | 1.02 m | 0.73 m | 0.68 m | 0.19 m | 0.06 m |
| 2 | 1.51 t (9.0) | 1.46 dd (8.0, 10.0) | 1.27 t (9.0) | 1.24 t (8.5) | 0.17 m | 0.16 m |
| 3 | 6.30 d (10.0) | 6.28 d (10.0) | 5.06 d (9.5) | 4.86 d (9.0) | 0.95 m 1.80 m | 1.60 m 1.98 m |
| 4 | - | - | - | - | 3.05 m | 2.70 m |
| 5 | - | - | 4.03 dd (4.0, 11.0) | 2.09 m | - | - |
| 2.21 m | ||||||
| 6 | 2.90 br d (14.0) | 3.00 br d (15.0) | 2.27 m | 2.07 m | 3.03 dd (7.5, 15.5) | 2.76 dd (6.5, 14.5) |
| 3.62 dd (9.0, 14.0) | 3.66 dd (8.0, 15.0) | 2.38 m | 2.20 m | 3.19 dd (7.5, 15.5) | 3.34 dd (8.5, 14.5) | |
| 7 | 5.09 br d (9.0) | 5.29 br d (8.0) | 4.74 t (6.5) | 4.89 t (6.0) | 5.32 t (7.5) | 5.15 t (7.0) |
| 9 | 2.12 m | 2.93 d (18.0) | 2.10 dd (11.0, 13.5) | 2.10 m | 2.36 dd (7.5, 13.0) | 2.10 m |
| 2.46 br d (12.0) | 2.95 d (18.0) | 2.35 m | 2.37 m | 2.47 dd (4.0, 13.0) | 2.50 br d (14.0) | |
| 10 | 4.53 dt (5.0, 9.5) | - | 4.52 dt (2.5, 9.0) | 4.55 dt (3.5, 9.0) | 4.66 dt (4.0, 8.5) | 4.69 dt (3.5, 9.0) |
| 11 | 4.98 d (9.5) | 2.17 dd (3.0, 18.0) | 4.98 d (9.0) | 5.00 d (9.0) | 5.37 d (8.5) | 4.93 d (9.0) |
| 2.59 dd (10.0, 18.0) | ||||||
| 12 | - | 2.33 m | - | - | - | - |
| 13 | 1.80 m | 1.28 m | 1.85 m | 1.83 m | 2.08 m | 1.79 m |
| 2.23 m | 1.40 m | 2.29 m | 2.27 m | 2.12 m | 2.11 m | |
| 14 | 0.77 m | 0.97 m | 1.05 m | 1.15 m | 1.23 m | 1.03 m |
| 2.10 m | 1.43 m | 1.74 m | 1.73 m | 1.72 m | 1.48 m | |
| 16 | 1.06 s | 1.10 s | 1.08 s | 1.09 s | 0.87 s | 0.97 s |
| 17 | 1.17 s | 1.15 s | 0.97 s | 0.98 s | 0.96 s | 1.02 s |
| 18 | 1.88 s | 1.81 s | 1.65 s | 1.64 s | 1.07 d (7.0) | 1.04 d (7.0) |
| 19 | 1.58 s | 1.82 s | 1.63 s | 1.61 s | 1.69 s | 1.74 s |
| 20 | 1.68 s | 0.96 d (7.0) | 1.68 s | 1.68 s | 1.78 s | 1.73 s |
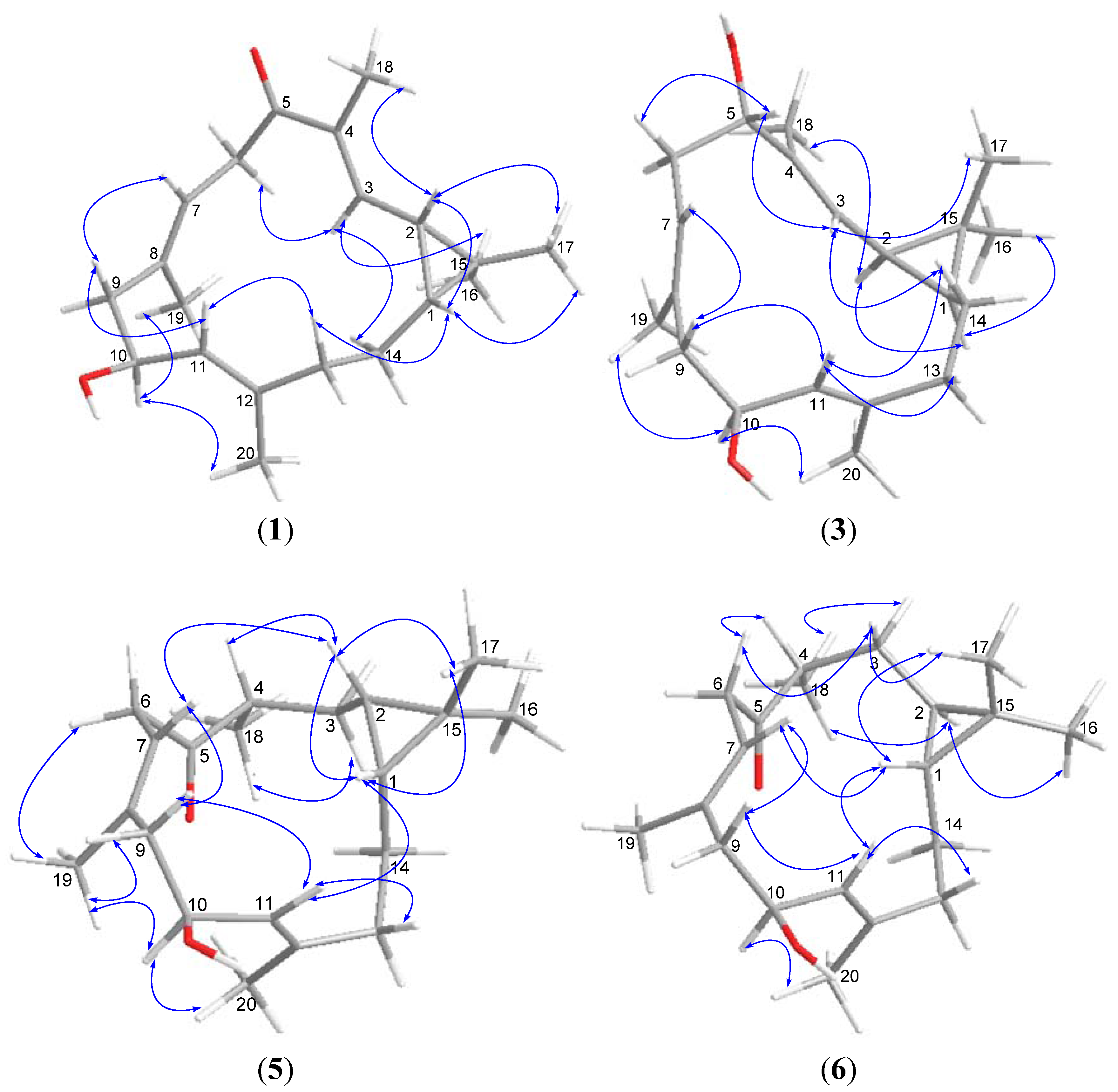
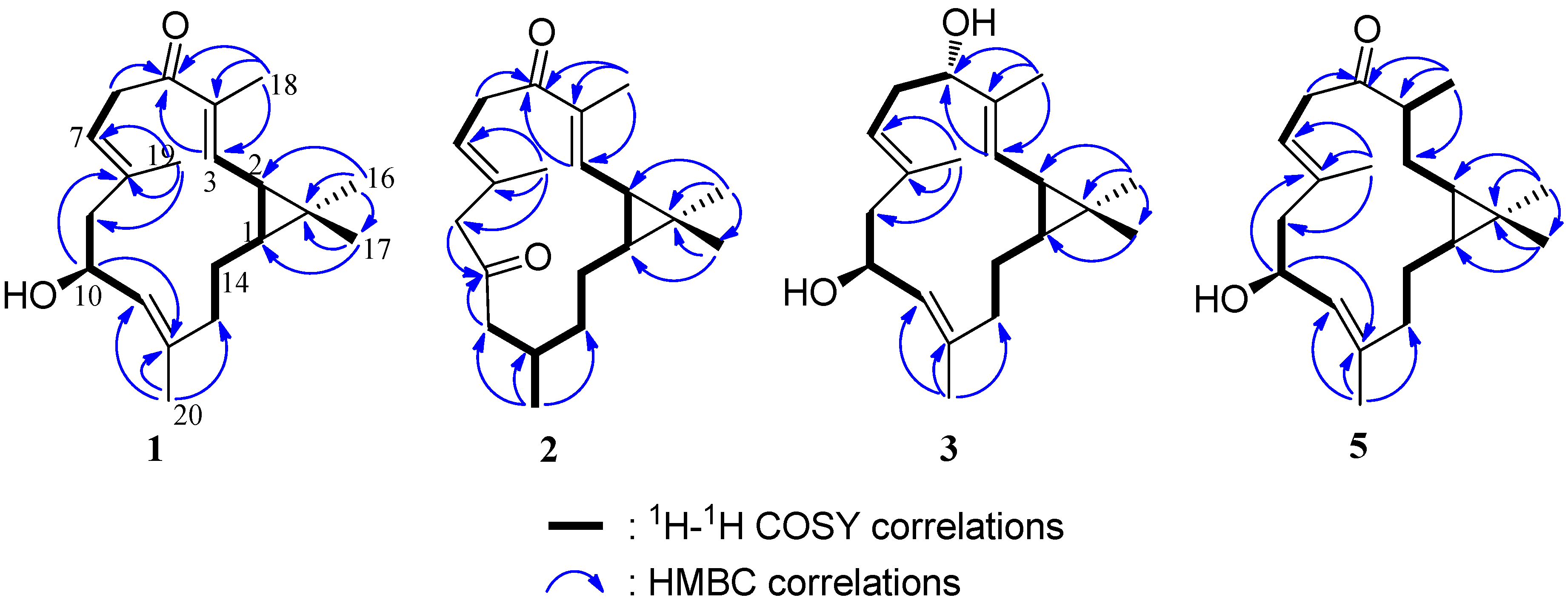
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Cytotoxicity Assay
3.5. Assay for Inhibition of Nitric Oxide (NO) Production and Cytotoxicity against Mouse Peritoneal Macrophages (PEMΦ)
4. Conclusions
Supplementary Files
Acknowledgments
References
- Robinson, D.R.; West, C.A. Biosynthesis of cyclic diterpenes in extracts from seedlings of Ricinus communis L. I. Identification of diterpene hydrocarbons formed from mevalonate. Biochemistry 1970, 9, 70–79. [Google Scholar]
- Burke, B.A.; Chan, W.R.; Pascoe, K.O.; Biount, J.F.; Manchand, P.S. The structure of crotonitenone, a novel casbane diterpene from Croton nitens Sw. J. Chem. Soc. Perkin Trans. 1 1981, 10, 2666–2669. [Google Scholar]
- Commissiong, M.A.; Pascoe, K.O. The absolute stereochemistry of crotonitenone. Tetrahedron Lett. 1984, 25, 711–712. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, J.; Pezzuto, J.M.; Kinghorn, A.D.; Farnsworth, N.R. Agrostistachin, a novel cytotoxic macrocyclic diterpene from Agrostistachys hookeri. Tetrahedron Lett. 1986, 27, 5795–5798. [Google Scholar] [CrossRef]
- Choi, Y.H.; Pezzuto, J.M.; Kinghorn, A.D.; Farnsworth, N.R. Plant anticancer agents, XLVI. Cytotoxic casbane-type constituents of Agrostistachys hookeri. J. Nat. Prod. 1988, 51, 110–116. [Google Scholar] [CrossRef]
- Moura, V.L.A.; Monte, F.J.O.; Braz-Filho, R. A new casbane-type diterpenoid from Croton nepetaefolius. J. Nat. Prod. 1990, 53, 1566–1571. [Google Scholar] [CrossRef]
- Kashman, Y.; Bernart, M.W.; Tischler, M.; Cardellina, J.H., II; Boyd, M.R. Koumbalones A and B, new casbane diterpenes from Maprounea africana. J. Nat. Prod. 1994, 57, 426–430. [Google Scholar] [CrossRef]
- Hao, C.; Zhong, J.J. Diterpenes and sesquiterpene from two Euphorbia species-structure elucidation of Euphorhylonal A and Euphoranin E. Indian J. Chem. 1996, 35, 1308–1310. [Google Scholar]
- Xu, Z.H.; Sun, J.; Xu, R.S.; Qin, G.W. Casbane diterpenoids from Euphorbia ebracteolata. Phytochemistry 1998, 49, 149–151. [Google Scholar]
- Kong, L.Y.; Li, Y.; Wu, X.L.; Min, Z.D. Cytotoxic diterpenoids from Euphorbia pekinensis. Planta Med. 2002, 68, 249–252. [Google Scholar] [CrossRef]
- Liang, Q.L.; Dai, C.C.; Jiang, J.H.; Tang, Y.P.; Duan, J.A. A new cytotoxic casbane diterpene from Euphorbia pekinensis. Fitoterapia 2009, 80, 514–516. [Google Scholar] [CrossRef]
- Silva-Filho, F.A.; Braz-Filho, R.; Silveira, E.R.; Lima, M.A.S. Structure elucidation of casbane diterpenes from Croton argyrophyllus. Magn. Reson. Chem. 2011, 49, 370–373. [Google Scholar] [CrossRef]
- Shao, F.G.; Bu, R.; Zhang, C.; Chen, C.J.; Huang, J.; Wang, J.H. Two new casbane diterpenoids from the roots of Euphorbia pekinensis. J. Asian Nat. Prod. Res. 2011, 13, 805–810. [Google Scholar] [CrossRef]
- Zhang, C.X.; Yan, S.J.; Zhang, G.W.; Lu, W.G.; Su, J.Y.; Zeng, L.M.; Gu, L.Q.; Yang, X.P.; Lian, Y.J. Cytotoxic diterpenoids from the soft coral Sinularia microclavata. J. Nat. Prod. 2005, 68, 1087–1089. [Google Scholar] [CrossRef]
- Li, Y.; Carbone, M.; Vitale, R.M.; Amodeo, P.; Castelluccio, F.; Sicilia, G.; Mollo, E.; Nappo, M.; Cimino, G.; Guo, Y.W.; Gavagnin, M. Rare casbane diterpenoids from the Hainan soft coral Sinularia depressa. J. Nat. Prod. 2010, 73, 133–138. [Google Scholar] [CrossRef]
- Lakshmi, V.; Kumar, R. Metabolites from Sinularia species. Nat. Prod. Res. 2009, 23, 801–850. [Google Scholar] [CrossRef]
- Li, Y.; Pattenden, G. Novel macrocyclic and polycyclic norcembranoid diterpenes from Sinularia species of soft coral: Structural relationships and biosynthetic speculations. Nat. Prod. Rep. 2011, 28, 429–440. [Google Scholar] [CrossRef]
- Geran, R.I.; Greenberg, N.H.; MacDonald, M.M.; Schumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological syatems. Cancer Chemother. Rep. 1972, 3, 1–91. [Google Scholar]
- Hou, R.-S.; Duh, C.-Y; Chiang, M.Y.; Lin, C.-N. Sinugibberol, a new cytotoxic cembranoid diterpene from the soft coral Sinularia gibberosa. J. Nat. Prod. 1995, 58, 1126–1130. [Google Scholar] [CrossRef]
- Samples Availability: Available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yin, J.; Zhao, M.; Ma, M.; Xu, Y.; Xiang, Z.; Cai, Y.; Dong, J.; Lei, X.; Huang, K.; Yan, P. New Casbane Diterpenoids from a South China Sea Soft Coral, Sinularia sp. Mar. Drugs 2013, 11, 455-465. https://doi.org/10.3390/md11020455
Yin J, Zhao M, Ma M, Xu Y, Xiang Z, Cai Y, Dong J, Lei X, Huang K, Yan P. New Casbane Diterpenoids from a South China Sea Soft Coral, Sinularia sp. Marine Drugs. 2013; 11(2):455-465. https://doi.org/10.3390/md11020455
Chicago/Turabian StyleYin, Jian, Min Zhao, Minshan Ma, Yuping Xu, Zheng Xiang, Yuepiao Cai, Jianyong Dong, Xinxiang Lei, Kexin Huang, and Pengcheng Yan. 2013. "New Casbane Diterpenoids from a South China Sea Soft Coral, Sinularia sp." Marine Drugs 11, no. 2: 455-465. https://doi.org/10.3390/md11020455






