Lithothamnion muelleri Controls Inflammatory Responses, Target Organ Injury and Lethality Associated with Graft-versus-Host Disease in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. LM Treatment Reduced Intestinal and Hepatic Injury in Mice Subjected to GVHD

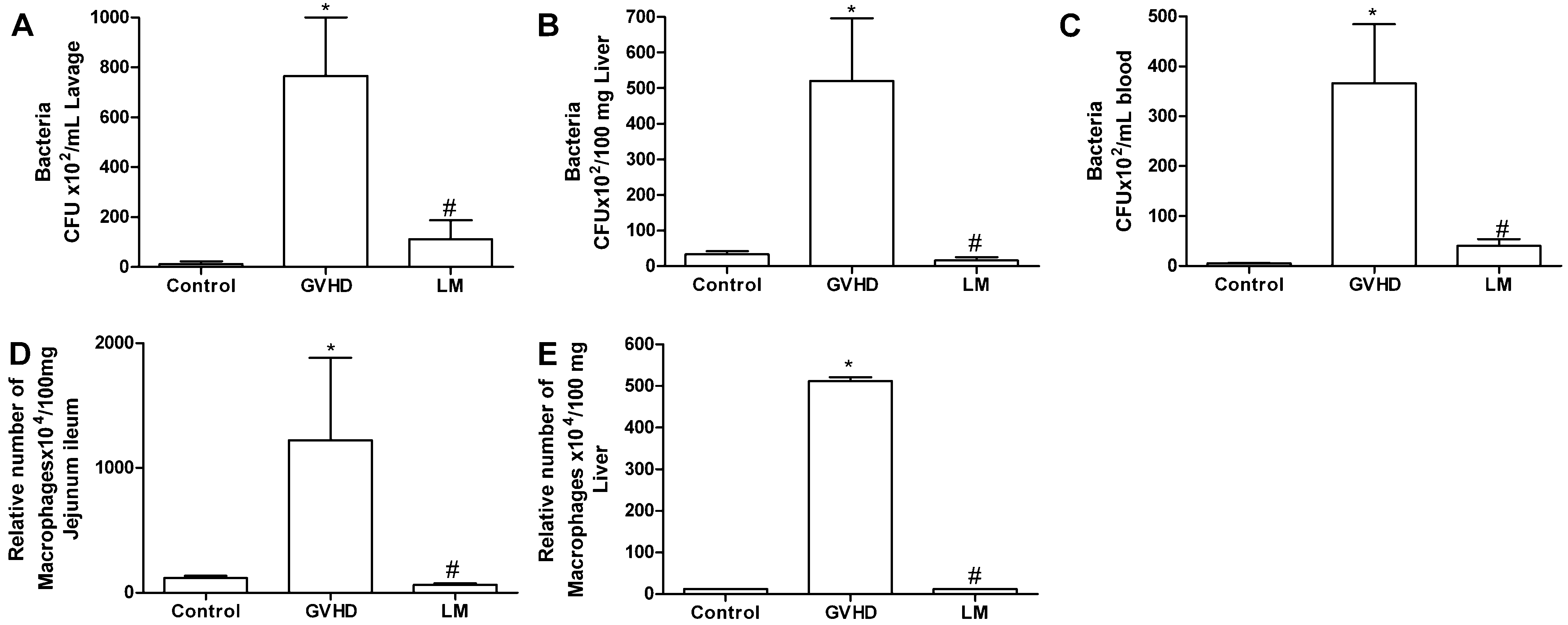
2.2. LM Treatment Reduced Bacterial Translocation and Macrophage Accumulation in Mice Subjected to GVHD
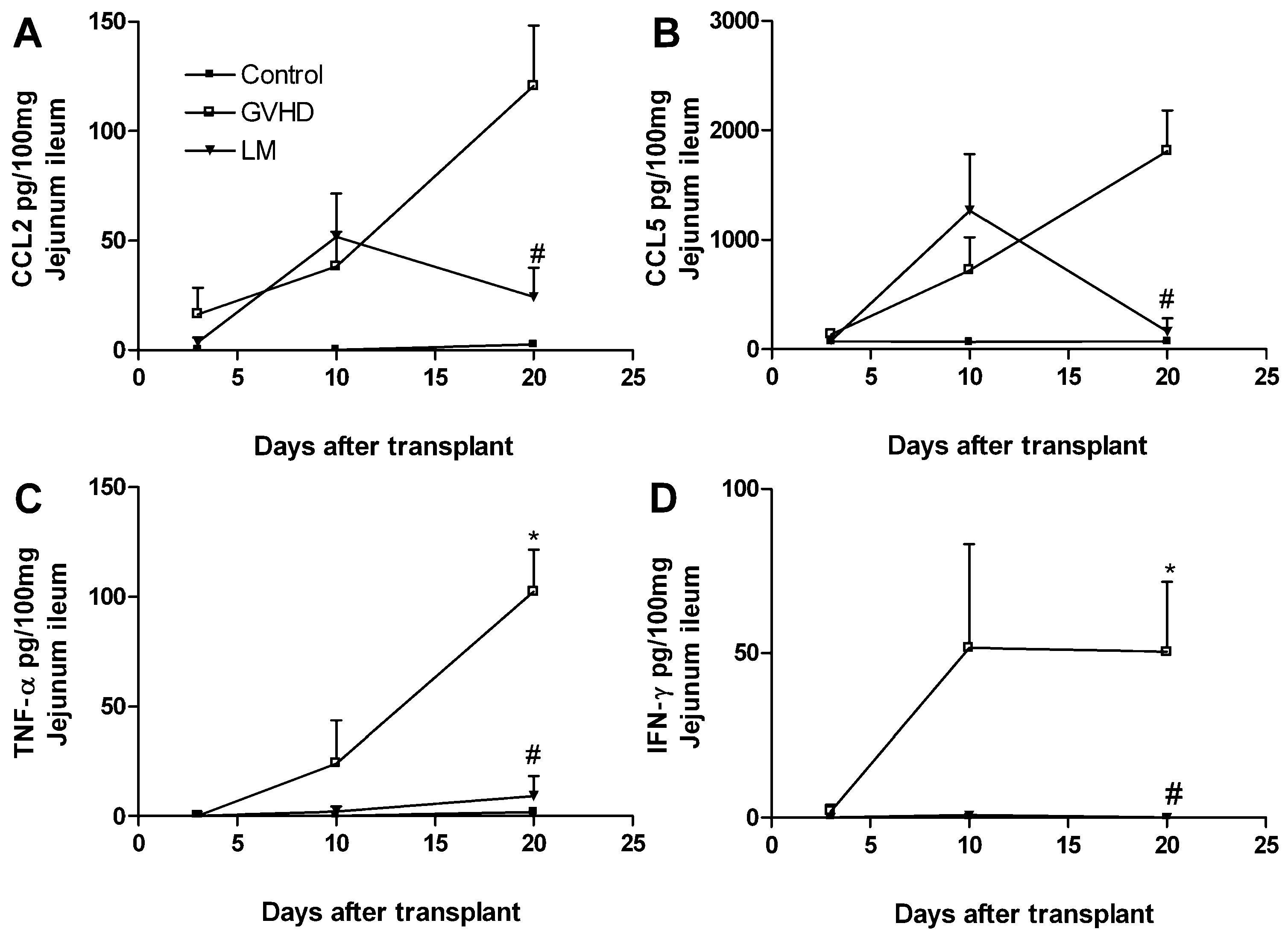
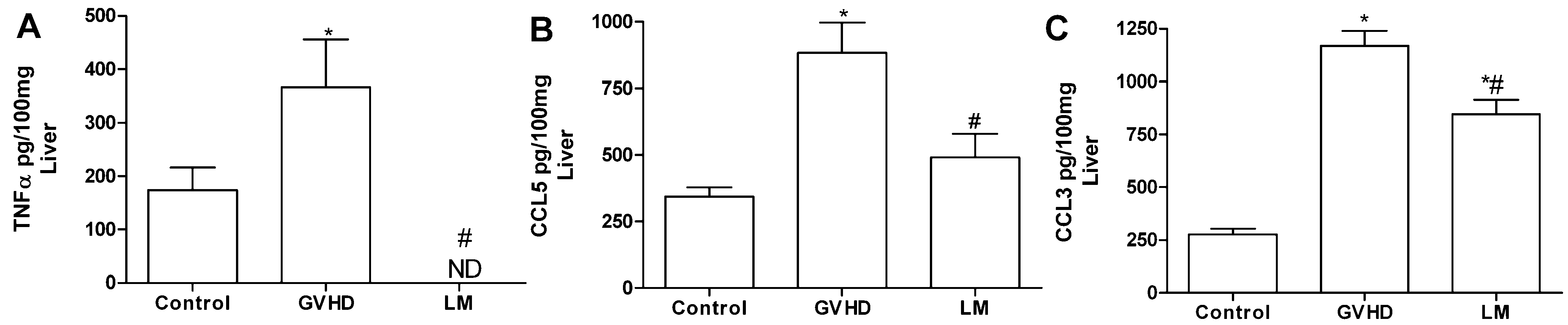
2.3. LM Treatment Reduced the Production of Chemokines and Proinflammatory Cytokines in the Intestines and Livers of GVHD Mice
2.4. Polysaccharide-Rich Fraction of L. muelleri Inhibited Leukocyte-Endothelial Cell Interactions in the Intestinal Microvasculature of GVHD Mice
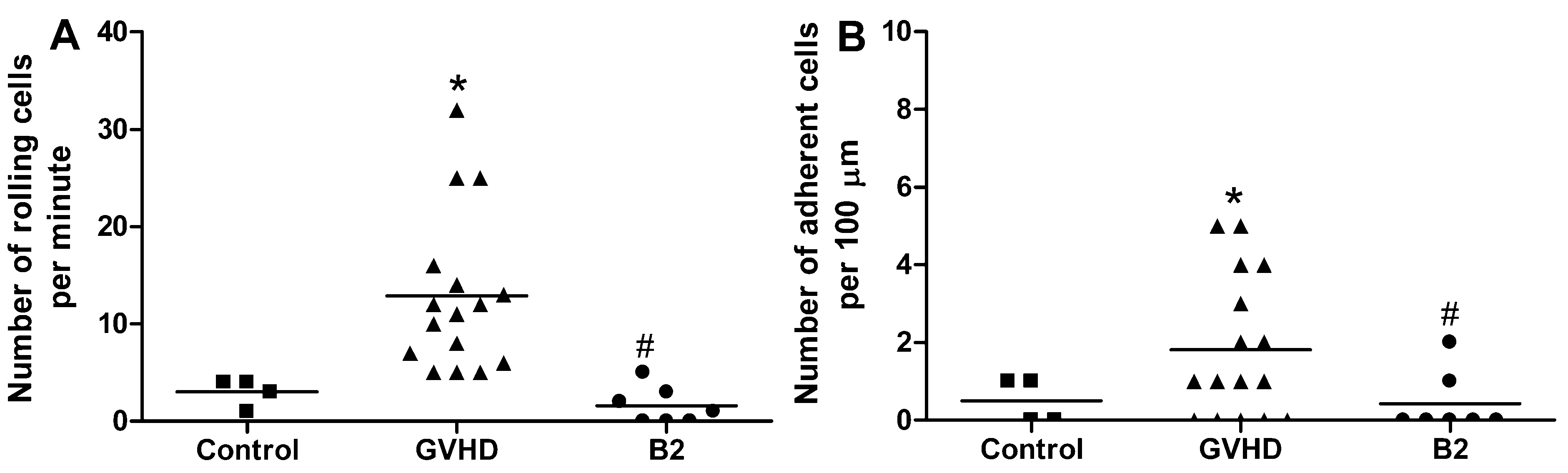
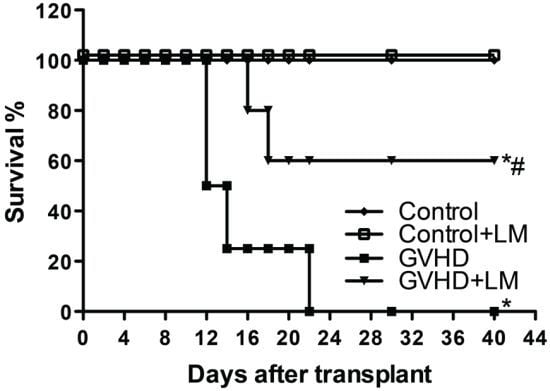
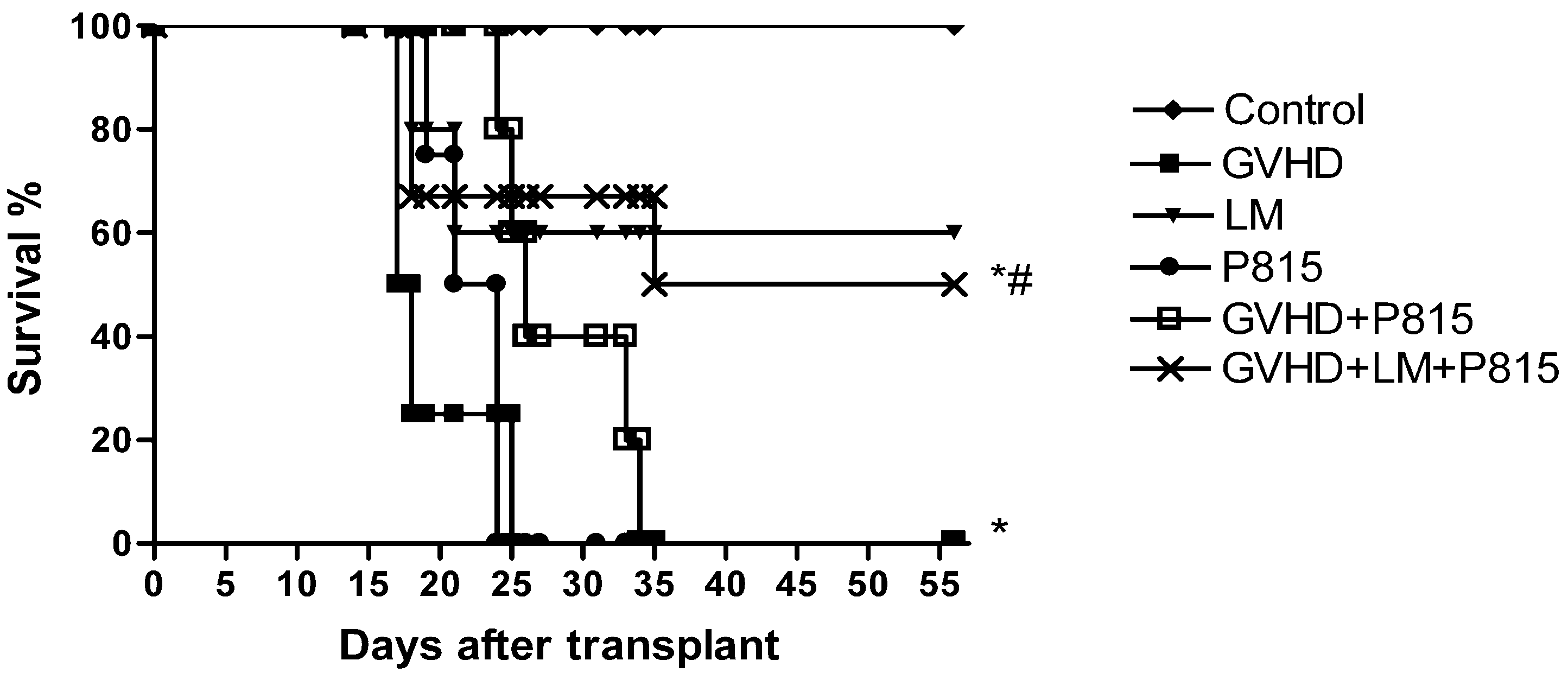
2.5. LM Treatment Reduced Clinical Disease and Mortality in Mice Subjected to GVHD

2.6. Treatment with LM Decreased GVHD but Did Not Interfere with GVL Response
3. Experimental Section
3.1. Mice
3.2. Algal Material
3.3. Polysaccharide-Rich Fraction
3.4. Induction of GVHD
3.4.1. Partial Body Irradiation
3.4.2. Total Body Irradiation
3.4.3. Cell Preparation
3.4.4. Experimental Groups
3.5. Therapies
3.6. Mortality Rate and Assessment of GVHD Clinical Score
3.7. Histopathology
3.8. Periodic Acid-Schiff (PAS) Staining
3.9. Quantification of Macrophage Infiltration
3.10. Bacterial Translocation
3.11. Quantification of Cytokines and Chemokines
3.12. Intravital Microscopy
3.13. GVL Induction
3.14. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Ferrara, J.L.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef]
- Pasquini, M.C.; Wang, Z.; Horowitz, M.M.; Gale, R.P. 2010 report from the Center for International Blood and Marrow Transplant Research (CIBMTR): Current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin. Transpl. 2010, 2010, 87–105. [Google Scholar]
- Goker, H.; Hanznedaroglu, I.C.; Chao, N.J. Acute graft-versus-host disease: Pathobiology and management. Exp. Hematol. 2001, 29, 259–277. [Google Scholar] [CrossRef]
- Socié, G.; Blazar, B.R. Acute graft-versus-host disease: From the bench to the bedside. Blood 2009, 114, 4327–4336. [Google Scholar] [CrossRef]
- Westin, J.R.; Saliba, R.M.; de Lima, M.; Alousi, A.; Hosing, C.; Qazilbash, M.H.; Khouri, I.F.; Shpall, E.J.; Anderlini, P.; Rondon, G.; et al. Steroid-refractory acute GVHD: Predictors and outcomes. Adv. Hematol. 2011, 2011, 1–8. [Google Scholar]
- Martin, P.J.J.; Rizzo, D.; Wingard, J.R.; Ballen, K.; Curtin, P.T.; Cutler, C.; Litzow, M.R.; Nieto, Y.; Savani, B.N.; Schriber, J.R.; et al. First- and second-line systemic treatment of acute graft-versus-host disease: Recommendations of the american society of blood and marrow transplantation. Biol. Blood Marrow Transpl. 2012, 18, 1150–1163. [Google Scholar] [CrossRef]
- Wolf, D.; von Lilienfeld-Toal, M.; Wolf, A.M.; Schleuning, M.; von Bergwelt-Baildon, M.; Held, S.A.; Brossart, P. Novel treatment concepts for graft-versus-host disease. Blood 2012, 119, 16–25. [Google Scholar] [CrossRef]
- Blazar, B.R.; Murphy, W.J.; Abedi, M. Advances in graft-versus-host disease biology and therapy. Nat. Rev. Immunol. 2012, 12, 443–458. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Rodríguez, A.D.; Berlinck, R.G.S.; Fusetani, N. Marine pharmacology in 2007–2008: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C 2011, 153, 191–222. [Google Scholar]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2000, 17, 1–57. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcotec, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2006, 23, 26–78. [Google Scholar] [CrossRef]
- Soares, C.M.; Malagoli, B.G.; Menezes, G.B.; Pinho, V.; Souza, D.G.; Teixeira, M.M.; Braga, F.C. Antiadhesive activity of polysaccharide-rich fractions from Lithothamnion muelleri. Z. Naturforsch.C 2012, 67, 391–397. [Google Scholar] [CrossRef]
- Aslam, M.N.; Paruchuri, T.; Bhagavathula, N.; Varani, J. A mineral-rich red algae extract inhibits polyp formation and inflammation in the gastrointestinal tract of mice on a high-fat diet. Integr. Cancer Theory 2010, 9, 93–99. [Google Scholar]
- Aslam, M.N.; Bergin, I.; Naik, M.; Hampton, A.; Allen, R.; Kunkel, S.L.; Rush, H.; Varani, J. A multi-mineral natural product inhibits liver tumor formation in C57BL/6 mice. Biol. Trace Elem. Res. 2012, 147, 267–274. [Google Scholar] [CrossRef]
- Derrien, M.; van Passel, M.W.J.; van de Bovenkamp, J.H.B.; Schipper, R.G.; de Vos, W.M.; Dekker, J. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microb. 2010, 1, 254–268. [Google Scholar] [CrossRef]
- Johansson, M.E.; Ambort, D.; Pelaseved, T.; Schütte, A.; Gustafsson, J.K.; Ermund, A.; Subramani, D.B.; Holmén-Larsson, J.M.; Thomsson, K.A.; Bergström, J.H.; et al. Composition and functional role of the mucus layers in the intestine. Cell. Mol. Life Sci. 2011, 68, 3635–3641. [Google Scholar] [CrossRef]
- Barnett, A.M.; Roy, N.C.; Mc Nabb, W.C.; Cookson, A.L. The interactions between endogenous bacteria, dietary components and the mucus layer of the large bowel. Food Funct. 2012, 3, 690–699. [Google Scholar] [CrossRef]
- Hong, I.H.; Ji, H.; Hwa, S.Y.; Jeong, W.I.; Jeong, D.H.; Do, S.H.; Kim, J.M.; Ki, M.R.; Park, J.K.; Goo, M.J.; et al. The protective effect of ENA actimineral resource A on CCl 4-induced liver injury in rats. Mar. Biotechnol. 2011, 13, 462–473. [Google Scholar] [CrossRef]
- Robb, R.J.; Hill, G.R. The interferon-dependent orchestration of innate and adaptive immunity after transplantation. Blood 2012, 119, 5351–5358. [Google Scholar] [CrossRef]
- Tuncer, H.H.; Rana, N.; Milani, C.; Darko, A.; Al-Homsi, S.A. Gastrointestinal and hepatic complications of hematopoietic stem cell transplantation. World J. Gastroenterol. 2012, 18, 1851–1860. [Google Scholar]
- Hill, G.R.; Ferrara, J.L. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: Rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood 2000, 95, 2754–2759. [Google Scholar]
- Jones, J.M.; Wilson, R.; Bealmear, P.M. Mortality and gross pathology of secondary disease in germfree mouse radiation chimeras. Radiat. Res. 1971, 45, 577–588. [Google Scholar] [CrossRef]
- Beelen, D.W.; Haralambie, E.; Brandt, H.; Linzenmeier, G.; Muller, K.D.; Quabeck, K.; Sayer, H.G.; Graeven, U.; Mahmoud, H.K.; Schaefer, U.W. Evidence that sustained growth suppression of intestinal anaerobic bacteria reduces the risk of acute graft-versus-host disease after sibling marrow transplantation. Blood 1992, 80, 2668–2676. [Google Scholar]
- Beelen, D.W.; Elmaagacli, A.; Muller, K.D.; Hirche, H.; Schaefer, U.W. Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease after marrow transplantation in patients with hematologic malignancies: Final results and long-term follow-up of an open-label prospective randomized trial. Blood 1999, 93, 3267–3275. [Google Scholar]
- Irani, J.L.; Cutler, C.S.; Whang, E.E.; Clancy, T.E.; Russel, S.; Swanson, R.S.; Ashley, S.W.; Zinner, M.J.; Raut, C.P. Severe acute gastro-intestinal graft-vs.-host disease: An emerging surgical dilemma in contemporary cancer care. Arch. Surg. 2008, 143, 1041–1045. [Google Scholar] [CrossRef]
- Cooke, K.R.; Gerbitz, A.; Crawford, J.M.; Teshima, T.; Hill, G.R.; Tesolin, A.; Rossignol, D.P.; Ferrara, J.L. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J. Clin. Invest. 2001, 107, 1581–1589. [Google Scholar] [CrossRef]
- Lang, T.; Hansson, G.C.; Samuelsson, T. Gel forming mucins appeared early in metazoan evolution. Proc. Natl. Acad. Sci. USA 2007, 104, 16209–16214. [Google Scholar] [CrossRef]
- Fieren, M.W. The local inflammatory responses to infection of the peritoneal cavity in humans: Their regulation by cytokines, macrophages, and other leukocytes. Mediat. Inflamm. 2012, 2012, 1450–1462. [Google Scholar] [CrossRef]
- Serody, J.S.; Burkett, S.E.; Panoskaltsis-Mortari, A.; Ng-Cashin, J.; McMahon, E.; Matsushima, G.R.; Lira, S.A.; Cook, D.N.; Blazar, B.R. T-Lymphocyte production of macrophage inflammatory protein-1 is critical to recruitment of CD8T cells to the liver, lung, and spleen during graft-versus-host disease. Blood 2000, 96, 2973–2980. [Google Scholar]
- Wysocki, C.A.; Panoskalsis-Mortari, A.; Blazar, B.R.; Serody, J.S. Leukocyte migration and graft-versus-host disease. Blood 2005, 105, 4191–4199. [Google Scholar] [CrossRef]
- Jaksch, M.; Mattsson, J. The pathophysiology of graft-versus-host disease. Scand. J. Immunol. 2005, 61, 398–409. [Google Scholar] [CrossRef]
- Choi, S.W.; Hildebrandt, G.C.; Olkiewicz, K.M.; Hanauer, D.A.; Chaudhary, M.N.; Silva, I.A.; Rogers, C.E.; Deurloo, D.T.; Fisher, J.M.; Liu, C.; et al. CCR1:CCL5 (RANTES) receptor ligand interactions modulates allogeneic T cell responses and reduces graft-versus-host disease following stem cell transplantation. Blood 2007, 110, 3447–3455. [Google Scholar] [CrossRef]
- Bouazzaoui, A.; Spacenko, E.; Muller, G.; Miklos, S.; Huber, E.; Holler, E.; Andreesen, R.; Hildebrandt, G.C. Chemokine and chemokine receptor expression analysis in target organs of acute graft-versus-host disease. Genes Immun. 2009, 10, 687–701. [Google Scholar] [CrossRef]
- Castor, M.G.; Rezende, B.; Resende, C.B.; Alessandri, A.L.; Fagundes, C.T.; Sousa, L.P.; Arantes, R.M.; Souza, D.G.; Silva, T.A.; Proudfoot, A.E.; et al. The CCL3/macrophage inflammatory protein-1-binding protein evasin-1 protects from graft-versus-host disease but does not modify graft-versus-leukemia in mice. J. Immunol. 2010, 184, 2646–2654. [Google Scholar] [CrossRef]
- Castor, M.G.M.; Rezende, B.; Bernardes, P.T.T.; Vieira, T.A.; Arantes, R.M.E.; Souza, D.G.; Silva, T.A.; Teixeira, M.M.; Pinho, V. PI3K controls leukocyte recruitment, tissue injury and lethality in a model of graft versus host disease in mice. J. Leukoc. Biol. 2011, 89, 955–964. [Google Scholar] [CrossRef]
- Castor, M.G.M.; Rezende, B.M.; Resende, C.B.; Bernardes, P.T.; Cisalpino, D.; Vieira, A.T.; Souza, D.G.; Silva, T.A.; Teixeira, M.M.; Pinho, V. Platelet-activating factor receptor plays a role in the pathogenesis of graft-versus-host disease by regulating leukocyte recruitment, tissue injury, and lethality. J. Leukoc. Biol. 2012, 89, 629–639. [Google Scholar]
- Castor, M.G.M.; Pinho, V.; Teixeira, M.M. The role of chemokines in mediating graft versus host disease: Opportunities for novel therapeutics. Front. Pharmacol. 2012, 3, 1–13. [Google Scholar]
- Terwey, T.H.; Kim, T.D.; Kochman, A.A.; Hubbard, V.M.; Lu, S.; Zakrzewski, J.L. CCR2 is required for CD8-induced graft-versus-host disease. Blood 2005, 106, 3322–3330. [Google Scholar]
- Piguet, P.F.; Grau, G.E.; Allet, B.; Vassalli, P. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-versus-host disease. Exp. Med. 1987, 166, 1280–1289. [Google Scholar] [CrossRef]
- Backer, M.B.; Altman, N.H.; Podack, E.R.; Levy, R.B. The role of cell-mediated cytotoxicity in acute GVHD after MHC-matched allogeneic bone marrow transplantation in mice. J. Exp. Med. 1996, 183, 2645–2656. [Google Scholar] [CrossRef]
- Koide, S.; McVay, L.D.; Frankel, W.L.; Betling, C.A.; Zhou, E.D.; Shimada, T.; Zhang, W.; Rombeau, J.L. Increased expression of tissue cytokines in graft-versus-host disease after small bowel transplantation in the rat. Transplantation 1997, 64, 518–524. [Google Scholar] [CrossRef]
- Schroeder, M.A.; DiPersio, J.F. Mouse models of graft-versus-host disease: Advances and limitations. Dis. Model. Mech. 2011, 4, 318–333. [Google Scholar] [CrossRef]
- Dias, C.T.M. Granulados bioclásticos—Algas calcárias. Rev. Bras. Geof. 2000, 8, 307–318. [Google Scholar]
- Dame, M.K.; Veerapaneni, I.; Bhagavathula, N.; Naik, M.; Varani, J. Human colon tissue in organ culture: Calcium and multi-mineral-induced mucosal differentiation. InVitro Cell. Dev. Biol. Anim. 2011, 47, 32–38. [Google Scholar] [CrossRef]
- Matsui, M.S.; Muizzuddin, N.; Arad, S.; Marenus, K. Sulfated polysaccharides from red microalgae have antiinflammatory properties in vitro and in vivo. Appl. Biochem. Biotechnol. 2003, 104, 13–22. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Hill, G.R.; Crawford, J.M.; Cooke, K.R.; Brinson, Y.S.; Pan, L.; Ferrara, J.L. Total body irradiation and acute graft-versus-host disease: The role of gastrointestinal damage and inflammatory cytokines. Blood 1997, 90, 3204–3213. [Google Scholar]
- Cooke, K.R.; Hill, G.R.; Crawford, J.M.; Bungard, D.; Brinson, Y.S.; Delmonte, J.J.; Ferrara, J.L. Tumor necrosis factor-α production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J. Clin. Invest. 1998, 102, 1882–1891. [Google Scholar] [CrossRef]
- Howard, J.G.; Woodruff, M.F.A. Effect of the graft-versus-host reaction on the immunological responsiveness of the mouse. Proc. R. Soc. Lond. 1961, 154, 532–539. [Google Scholar] [CrossRef]
- Horowitz, M.M.; Gale, R.P.; Sondel, P.M.; Goldman, J.M.; Kersey, J.; Kolb, H.J.; Rimm, A.A.; Ringden, O.; Rozaman, C.; Speck, B.; et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood 1990, 75, 555–561. [Google Scholar]
- Johnson, B.D.; Hanke, C.A.; Truitt, R.L. The graft-versus-leukemia effect of post-transplant donor leukocyte infusion. Leuk. Lymphoma 1996, 23, 1–9. [Google Scholar] [CrossRef]
- Matejková, E.; Ocadlíková, D.; Smejkalová, J.; Muzíkova, J.; Raida, L.; Tousovská, K.; Pacasová, R.; Nenicková, M.; Tesarová, E.; Sterba, J.; et al. Selective depletion of alloreactive T cells and study of anti-tumor activity of specific T cell clones in patients with leukemia. Klin. Onkol. 2008, 21, 104–109. [Google Scholar]
- Li, J.M.; Giver, C.R.; Lu, Y.; Hossain, M.S.; Akhtari, M.; Waller, E.K. Separating graft-versus-leukemia from graft-versus-host disease in allogeneic hematopoietic stem cell transplantation. Immunotherapy 2009, 1, 599–621. [Google Scholar]
- Dazzi, F.; Szydlo, R.M.; Goldman, J.M. Donor lymphocyte infusions for relapse of chronic myeloid leukemia after allogeneic stem cell transplant: Where we now stand. Exp. Hematol. 1999, 27, 1477–1486. [Google Scholar] [CrossRef]
- Porter, D.L.; Antin, J.H. The graft-vs.-leukemia effects of allogeneic cell therapy. Annu. Rev. Med. 1999, 50, 369–386. [Google Scholar] [CrossRef]
- Xia, G.; Truitt, R.L.; Johnson, B.D. Graft-versus-leukemia and graft-versus-host reactions after donor lymphocyte infusion are initiated by host-type antigen-presenting cells and regulated by regulatory T cells in early and long-term chimeras. Biol. Blood Marrow Transpl. 2006, 12, 397–407. [Google Scholar] [CrossRef]
- Barcelos, L.S.; Talvani, A.; Teixeira, A.S.; Cassali, G.D.; Andrade, S.P.; Teixeira, M.M. Production and in vivo effects of chemokines CXCL1-3/KC and CCL2/JE in a model of inflammatory angiogenesis in mice. Inflamm. Res. 2004, 53, 576–584. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rezende, B.M.; Bernardes, P.T.T.; Resende, C.B.; Arantes, R.M.E.; Souza, D.G.; Braga, F.C.; Castor, M.G.M.; Teixeira, M.M.; Pinho, V. Lithothamnion muelleri Controls Inflammatory Responses, Target Organ Injury and Lethality Associated with Graft-versus-Host Disease in Mice. Mar. Drugs 2013, 11, 2595-2615. https://doi.org/10.3390/md11072595
Rezende BM, Bernardes PTT, Resende CB, Arantes RME, Souza DG, Braga FC, Castor MGM, Teixeira MM, Pinho V. Lithothamnion muelleri Controls Inflammatory Responses, Target Organ Injury and Lethality Associated with Graft-versus-Host Disease in Mice. Marine Drugs. 2013; 11(7):2595-2615. https://doi.org/10.3390/md11072595
Chicago/Turabian StyleRezende, Barbara M., Priscila T. T. Bernardes, Carolina B. Resende, Rosa M. E. Arantes, Danielle G. Souza, Fernão C. Braga, Marina G. M. Castor, Mauro M. Teixeira, and Vanessa Pinho. 2013. "Lithothamnion muelleri Controls Inflammatory Responses, Target Organ Injury and Lethality Associated with Graft-versus-Host Disease in Mice" Marine Drugs 11, no. 7: 2595-2615. https://doi.org/10.3390/md11072595