Secondary Metabolites of a Mangrove Endophytic Fungus Aspergillus terreus (No. GX7-3B) from the South China Sea
Abstract
:1. Introduction
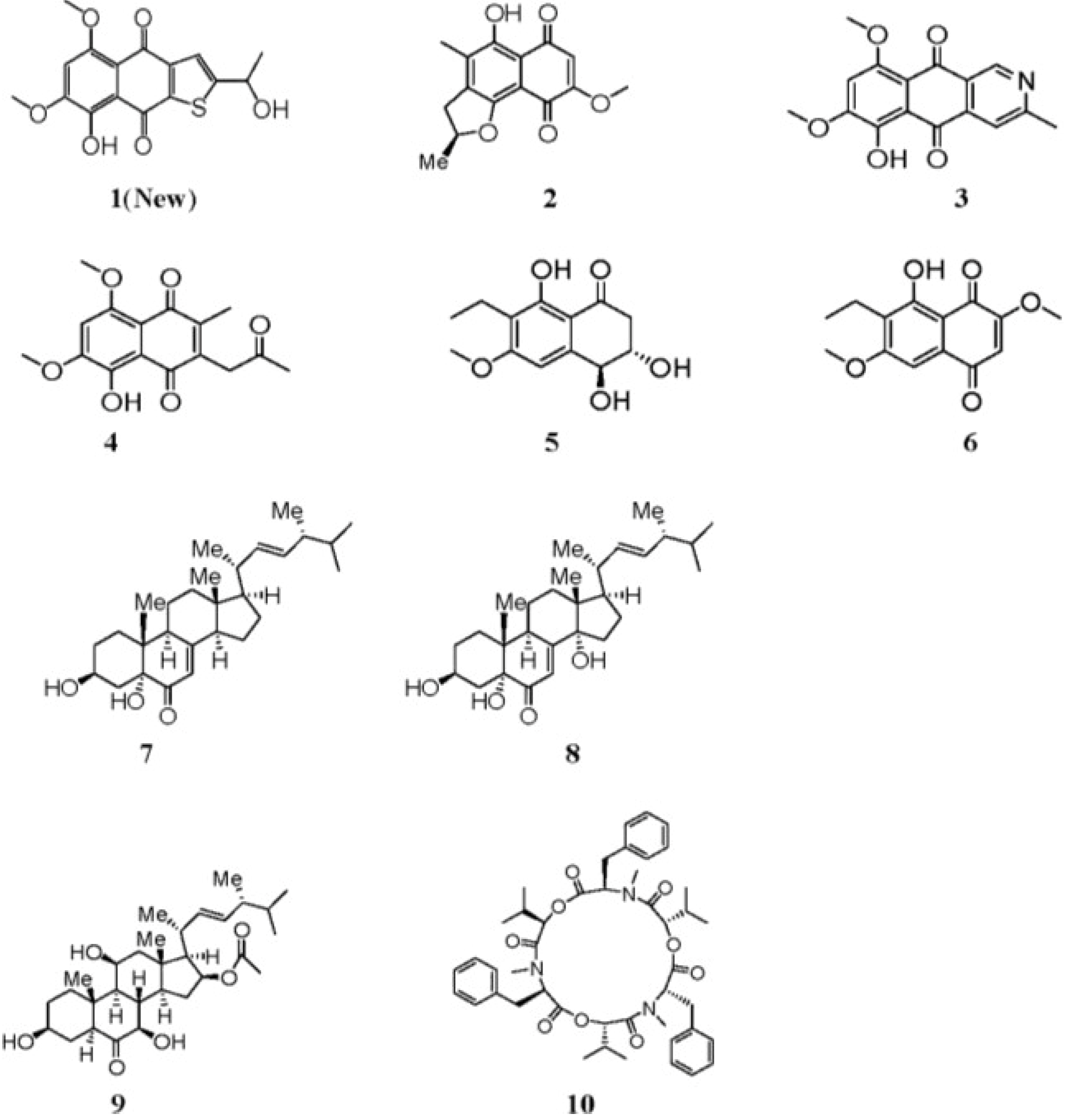
2. Results and Discussion
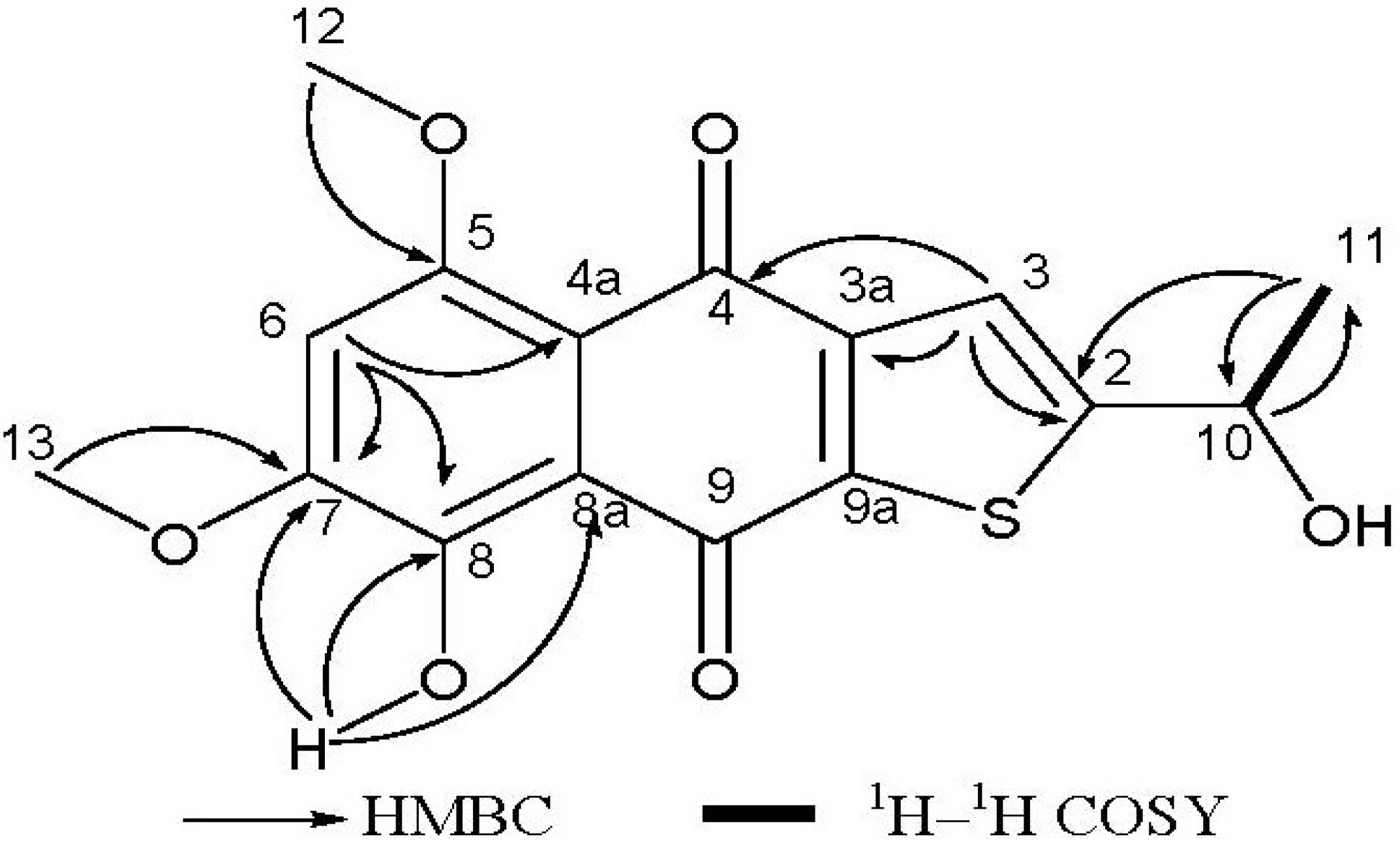
| No. | δC | δH | 1H–1H Cosy | HMBC (H to C) |
|---|---|---|---|---|
| 2 | 162.7 | |||
| 3 | 128.2 | 8.09 (1H, s) | C-2, C-3a, C-4 | |
| 3a | 129.4 | |||
| 4 | 177.5 | |||
| 4a | 112.0 | |||
| 5 | 156.5 | |||
| 6 | 104.2 | 6.84 (1H, s) | C-4a, C-7, C-8 | |
| 7 | 155.6 | |||
| 8 | 149.6 | |||
| 8a | 117.2 | |||
| 9 | 187.3 | |||
| 9a | 140.2 | |||
| 10 | 65.6 | 5.65 (1H, q, J = 6.4) | H-11 | C-11 |
| 11 | 23.3 | 1.71 (3H, d, J = 6.4) | C-2, C-10 | |
| 12 | 57.4 | 4.03 (3H, s) | C-5 | |
| 13 | 56.6 | 4.04 (3H, s) | C-7 | |
| 8-OH | 13.53 (s) | C-7, C-8, C-8a | ||
| 10-OH | 3.49 (s) |
| Compounds | Inhibition of AChE | Cytotoxicity | ||||
|---|---|---|---|---|---|---|
| IC50 (μM) | IC50 (μM) | |||||
| Hela | A549 | MCF-7 | KB | |||
| 1 | - | - | - | - | - | |
| 2 | 2.01 | - | - | - | - | |
| 3 | 6.71 | - | - | - | - | |
| 4 | - | - | - | - | - | |
| 5 | - | - | - | - | - | |
| 6 | - | - | - | - | - | |
| 7 | - | 4.98 | 1.95 | 0.68 | 1.50 | |
| 8 | - | 25.4 | 27.1 | 24.4 | 19.4 | |
| 9 | 1.89 | - | - | - | - | |
| 10 | 3.09 | 2.02 | 0.82 | 1.14 | 1.10 | |
| Huperzine A a | 0.003 | |||||
| Epirubicin a | 1.07 | 0.79 | 0.42 | 0.05 | ||
3. Experimental Section
3.1. General
3.2. Strain Isolation, Taxonomic Classification and Endophyte Fermentation
3.3. Extraction and Separation of Metabolites
 = +271.80 (c = 0.883 mg/mL, MeOH). 1H NMR (400 MHz, CDCl3): δ 1.71 (d, J = 6.4 Hz, 3H, H-11), δ 4.03 (s, 3H, H-12), δ 4.04 (s, 3H, H-13), δ 5.65 (q, J = 6.4 Hz, 1H, H-10), δ 6.84 (s, 1H, H-6), δ 8.09 (s, 1H, H-3), δ 3.49 (s, OH-10), δ 13.53 (s, OH-8). 13C NMR (101 MHz, CDCl3): δ 23.3 (C-11), 56.6 (C-13), 57.4 (C-12), 65.6 (C-10), 104.2 (C-6), 112.0 (C-4a), 117.2 (C-8a), 128.2 (C-3), 129.4 (C-3a), 140.2 (C-9a), 149.6 (C-8), 155.6 (C-7), 156.5 (C-5), 162.7 (C-2), 177.5 (C-4), 187.3 (C-9). EI-MS at m/z = 334, ESI-MS at m/z = 335 [M + H]+; HR-EI-MS m/z: 334.0501 [M]+, (calcd. for C16H14O6S, 334.0506). UV (MeOH): λmax (log ε) = 259 (0.32) nm. IR (KBr): νmax = 3428, 2921, 2852, 1652, 1623, 1544, 1515, 1508, 1460, 1434, 1371, 1316, 1216, 1028, 896, 758, 581, 469 cm−1.
= +271.80 (c = 0.883 mg/mL, MeOH). 1H NMR (400 MHz, CDCl3): δ 1.71 (d, J = 6.4 Hz, 3H, H-11), δ 4.03 (s, 3H, H-12), δ 4.04 (s, 3H, H-13), δ 5.65 (q, J = 6.4 Hz, 1H, H-10), δ 6.84 (s, 1H, H-6), δ 8.09 (s, 1H, H-3), δ 3.49 (s, OH-10), δ 13.53 (s, OH-8). 13C NMR (101 MHz, CDCl3): δ 23.3 (C-11), 56.6 (C-13), 57.4 (C-12), 65.6 (C-10), 104.2 (C-6), 112.0 (C-4a), 117.2 (C-8a), 128.2 (C-3), 129.4 (C-3a), 140.2 (C-9a), 149.6 (C-8), 155.6 (C-7), 156.5 (C-5), 162.7 (C-2), 177.5 (C-4), 187.3 (C-9). EI-MS at m/z = 334, ESI-MS at m/z = 335 [M + H]+; HR-EI-MS m/z: 334.0501 [M]+, (calcd. for C16H14O6S, 334.0506). UV (MeOH): λmax (log ε) = 259 (0.32) nm. IR (KBr): νmax = 3428, 2921, 2852, 1652, 1623, 1544, 1515, 1508, 1460, 1434, 1371, 1316, 1216, 1028, 896, 758, 581, 469 cm−1.3.4. Method of α-Acetylcholinesterase Inhibitory Activities
3.5. Cytotoxicity Assays
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Huang, C.H.; Pan, J.H.; Chen, B.; Yu, M.; Huang, H.B.; Zhu, X.; Lu, Y.J.; She, Z.G.; Lin, Y.C. Three bianthraquinone derivatives from the mangrove endophytic fungus Alternaria sp. ZJ9-6B from the South China Sea. Mar. Drugs 2011, 9, 832–843. [Google Scholar] [CrossRef]
- Li, J.; Zhang, D.M.; Zhu, X.; He, Z.J.; Liu, S.; Li, M.F.; Pang, J.Y.; Lin, Y.C. Studies on synthesis and structure-activity relationship (SAR) of derivatives of a new natural product from marine fungi as inhibitors of influenza virus neuraminidase. Mar. Drugs 2011, 9, 1887–1901. [Google Scholar] [CrossRef]
- Chen, G.Y.; Lin, Y.C.; Wen, L.; Vrijmoed, L.L.P.; Jones, E.B.G. Two new metabolites of a marine endophytic fungus (No. 1893) from an estuarine mangrove on the South China Sea Coast. Tetrahedron 2003, 59, 4907–4909. [Google Scholar] [CrossRef]
- Huang, H.B.; Feng, X.J.; Liu, L.; Chen, B.; Lu, Y.J.; Ma, L.; She, Z.G.; Lin, Y.C. Three dimeric naphtho-y-pyrones from the mangrove endophytic fungus Aspergillus tubingensis isolated from pongamia pinnata. Planta Med. 2010, 76, 1–4. [Google Scholar] [CrossRef]
- Huang, H.; She, Z.; Lin, Y.; Vrijmoed, L.L.P.; Lin, W. Cyclic peptides from an endophytic fungus obtained from a mangrove leaf (Kandelia candel). J. Nat. Prod. 2007, 70, 1696–1699. [Google Scholar] [CrossRef]
- Xia, X.K.; Li, Q.; Li, J.; Shao, C.L.; Zhang, J.Y.; Zhang, Y.G.; Liu, X.; Lin, Y.C.; Liu, C.H.; She, Z.G. Two new derivatives of griseofulvin from the mangrove endophytic fungus nigrospora sp. (No. 1403) from Kandelia Candel (L.) Druce. Planta Med. 2011, 77, 1735–1738. [Google Scholar] [CrossRef]
- Xia, X.K.; Huang, H.R.; She, Z.G.; Shao, C.L.; Liu, F.; Cai, X.L.; Vrijmoed, L.L.P.; Lin, Y.C. 1H and 13C NMR assignments for five anthraquinones from the mangrove endophytic fungus Halorosellinia sp. (No. 1403). Magn. Reson. Chem. 2007, 45, 1006–1009. [Google Scholar]
- Deng, C.M.; Huang, C.H.; Wu, Q.L.; Pang, J.Y.; Lin, Y.C. A new sesquiterpene from the mangrove endophytic fungus Aspergillus terreus (No. GX7-3B). Nat. Prod. Res. 2013. [Google Scholar] [CrossRef]
- Yasuo, K.; Atsumi, S.; Hiromitsu, N.; Hamasaki, T. Structures of naphthoquinones produced by the fungus, Fusarium sp., and their biological activity toward Pollen Germination. Agric. Biol. Chem. 1988, 52, 1253–1259. [Google Scholar] [CrossRef]
- Tatum, J.H.; Baker, R.A.; Berry, R.E. Metabolites of Fusarium solani. Phytochemistry 1988, 28, 283–284. [Google Scholar] [CrossRef]
- Cameron, D.W.; Deutscher, K.R.; Feutrill, G.I. Nucleophilic Alkenes. IX. Addition of 1,l-dimethoxyethene to azanaphthoquinones: synthesis of bostrycoidin and 8-O-methylbostrycoidin. Aust. J. Chem. 1982, 35, 1439–1450. [Google Scholar] [CrossRef]
- Kimura, Y.; Hamasaki, T.; Nakajima, H. Isolation, identification and biological activities of 8-O-methyljavanicin produced by Fusarium solani. Agric. Biol. Chem. 1981, 45, 2653–2654. [Google Scholar] [CrossRef]
- Xu, Y.H.; Lu, C.H.; Zheng, Z.H.; Shen, Y.M. New polyketides isolated from Botryosphaeria australis Strain ZJ12-1A. Helv. Chim. Acta 2011, 94, 897–902. [Google Scholar] [CrossRef]
- Caron, B.; Brassard, P. Regiospecific α-substitution of crotonic esters: Synthesis of naturally occurring derivatives of 6-ethyljuglone. Tetrahedron 1991, 47, 4287–4298. [Google Scholar] [CrossRef]
- Ishizuka, T.; Yaoita, Y.; Kikuchi, M. Sterol constituents from the fruit bodies of Grifola frondosa (Fr.) SF Gray. Chem. Pharm. Bull 1997, 45, 1756–1760. [Google Scholar] [CrossRef]
- Valisolalao, J.; Luu, B.; Ourisson, G. Chemical and biochemical study of chinese drugs. Part VIII. Cytotoxic steroids from Polyporus versicolor. Tetrahedron 1983, 39, 2779–2785. [Google Scholar] [CrossRef]
- Nozawa, Y.; Sakai, N.; Matsumoto, K.; Mizoue, K. A novel neuritogenic compound, NGA0187. J. Antibiot. 2002, 55, 629–634. [Google Scholar] [CrossRef]
- Hamill, R.L.; Higgens, C.E.; Boaz, H.E.; Gorman, M. The structure of beauvericin, a new depsipeptide antibiotic toxic to Artemia salina. Tetrahedron Lett. 1969, 49, 4255–4258. [Google Scholar]
- Li, C.Y.; Chen, S.; Zuo, C.W.; Kuang, R.B.; Yi, G.J. Identification of beauvericin, a novel mycotoxin from Fusarium oxysporum f. sp. Cubense. Acta Hortic. Sin. 2011, 38, 2092–2098. [Google Scholar]
- Yu, B.Z.; Song, C.Z.; Du, Z.Z.; Cai, X.H.; Huang, S.X.; Luo, X.D. Naphthaquinones from Fusarium sp. 1RGa-1b, an endophytic fungus associated with Trewia nudiflora (Euphorbiaceae). Nat. Prod. Res. Dev. 2009, 21, 574–576. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.J.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Tao, L.Y.; Liang, Y.J.; Yan, Y.Y.; Dai, C.L.; Xia, X.K.; She, Z.G.; Lin, Y.C.; Fu, L.W. Secalonic acid D induced leukemia cell apoptosis and cell cycle arrest of G(1) with involvement of GSK-3beta/beta-catenin/c-Myc pathway. Cell Cycle 2009, 8, 2444–2450. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Deng, C.-M.; Liu, S.-X.; Huang, C.-H.; Pang, J.-Y.; Lin, Y.-C. Secondary Metabolites of a Mangrove Endophytic Fungus Aspergillus terreus (No. GX7-3B) from the South China Sea. Mar. Drugs 2013, 11, 2616-2624. https://doi.org/10.3390/md11072616
Deng C-M, Liu S-X, Huang C-H, Pang J-Y, Lin Y-C. Secondary Metabolites of a Mangrove Endophytic Fungus Aspergillus terreus (No. GX7-3B) from the South China Sea. Marine Drugs. 2013; 11(7):2616-2624. https://doi.org/10.3390/md11072616
Chicago/Turabian StyleDeng, Chun-Mei, Shi-Xin Liu, Cai-Huan Huang, Ji-Yan Pang, and Yong-Cheng Lin. 2013. "Secondary Metabolites of a Mangrove Endophytic Fungus Aspergillus terreus (No. GX7-3B) from the South China Sea" Marine Drugs 11, no. 7: 2616-2624. https://doi.org/10.3390/md11072616





