Oxylipin Diversity in the Diatom Family Leptocylindraceae Reveals DHA Derivatives in Marine Diatoms
Abstract
:1. Introduction
2. Results and Discussion
2.1. Oxylipin Characterization
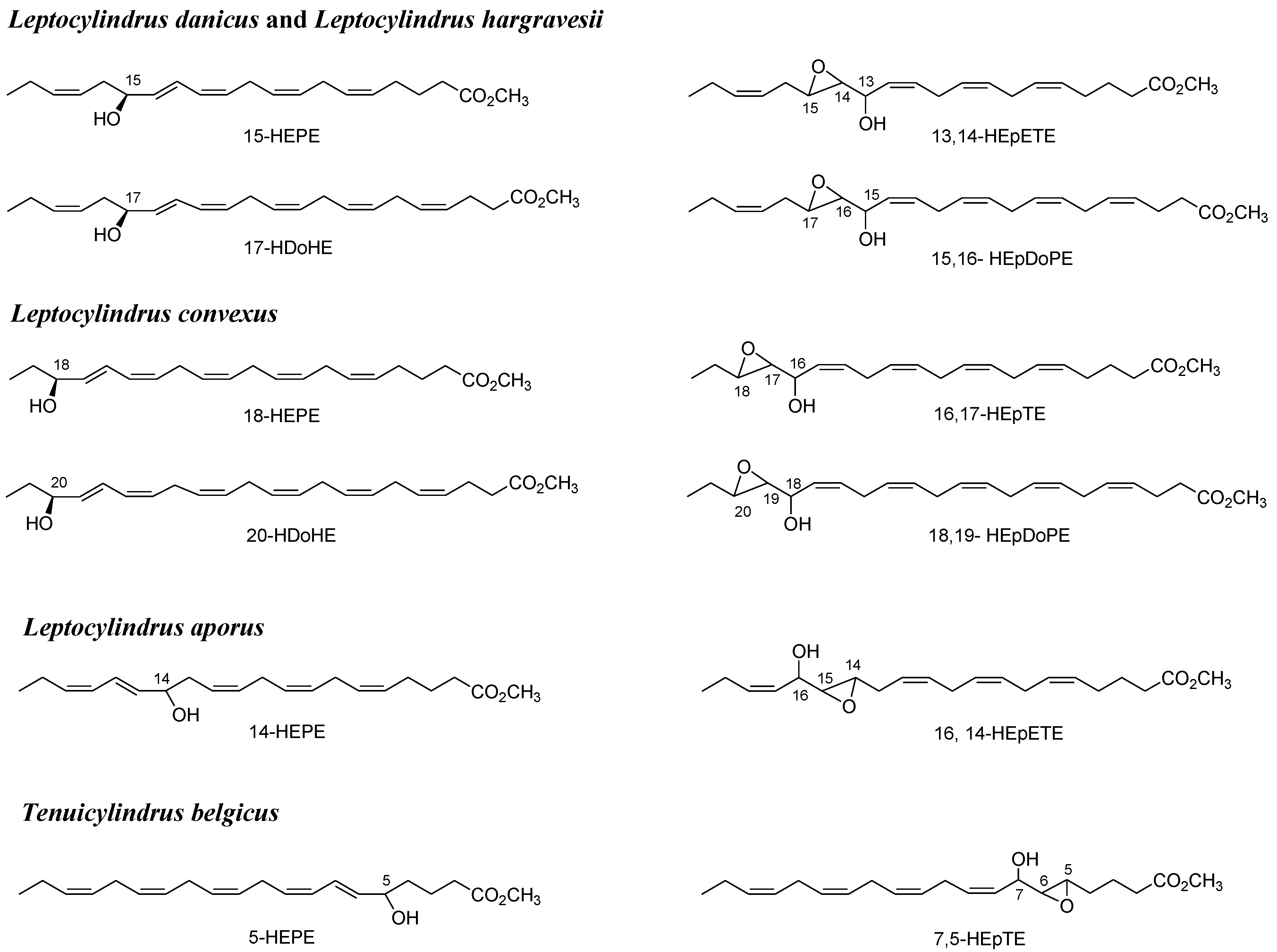
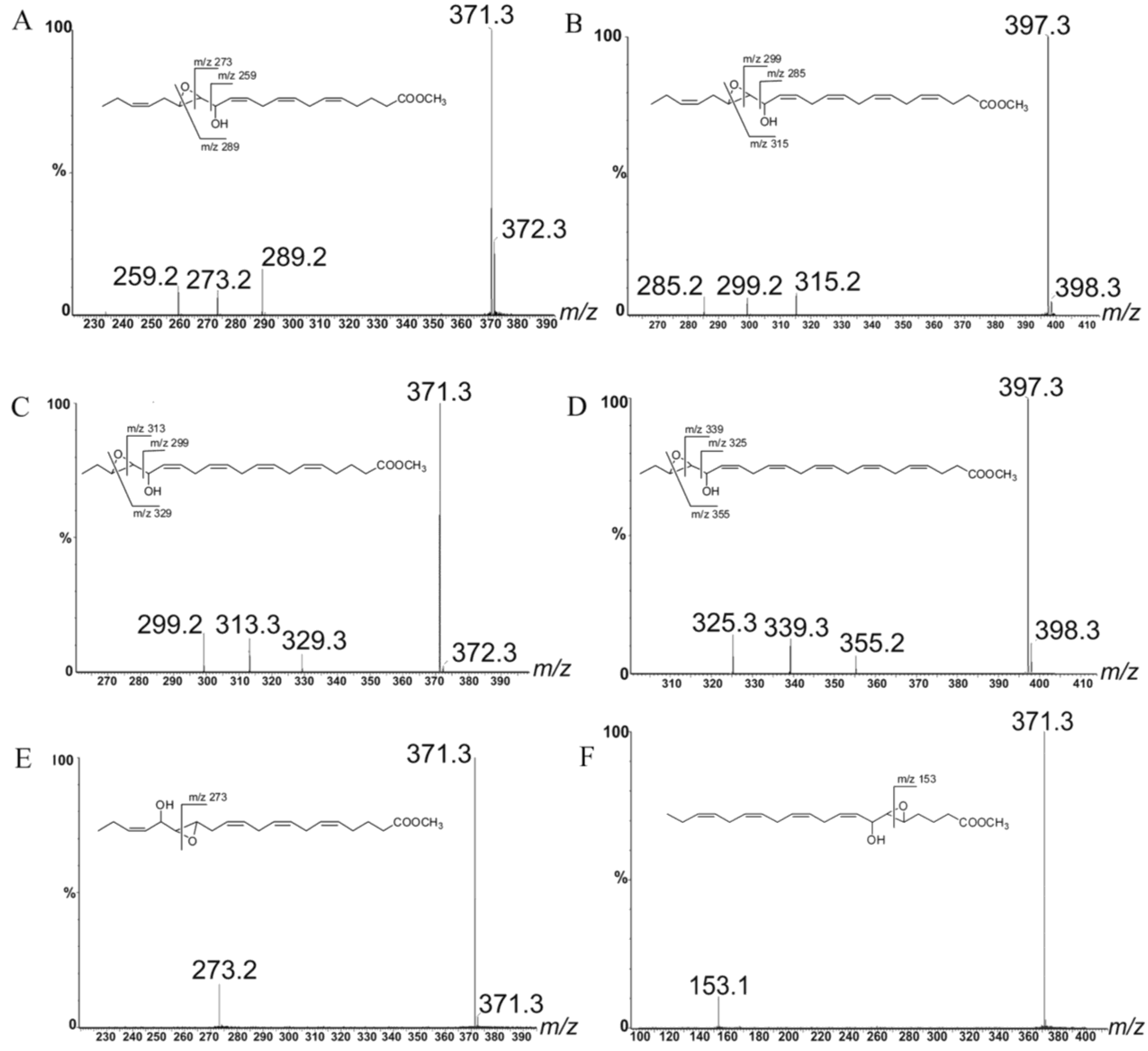
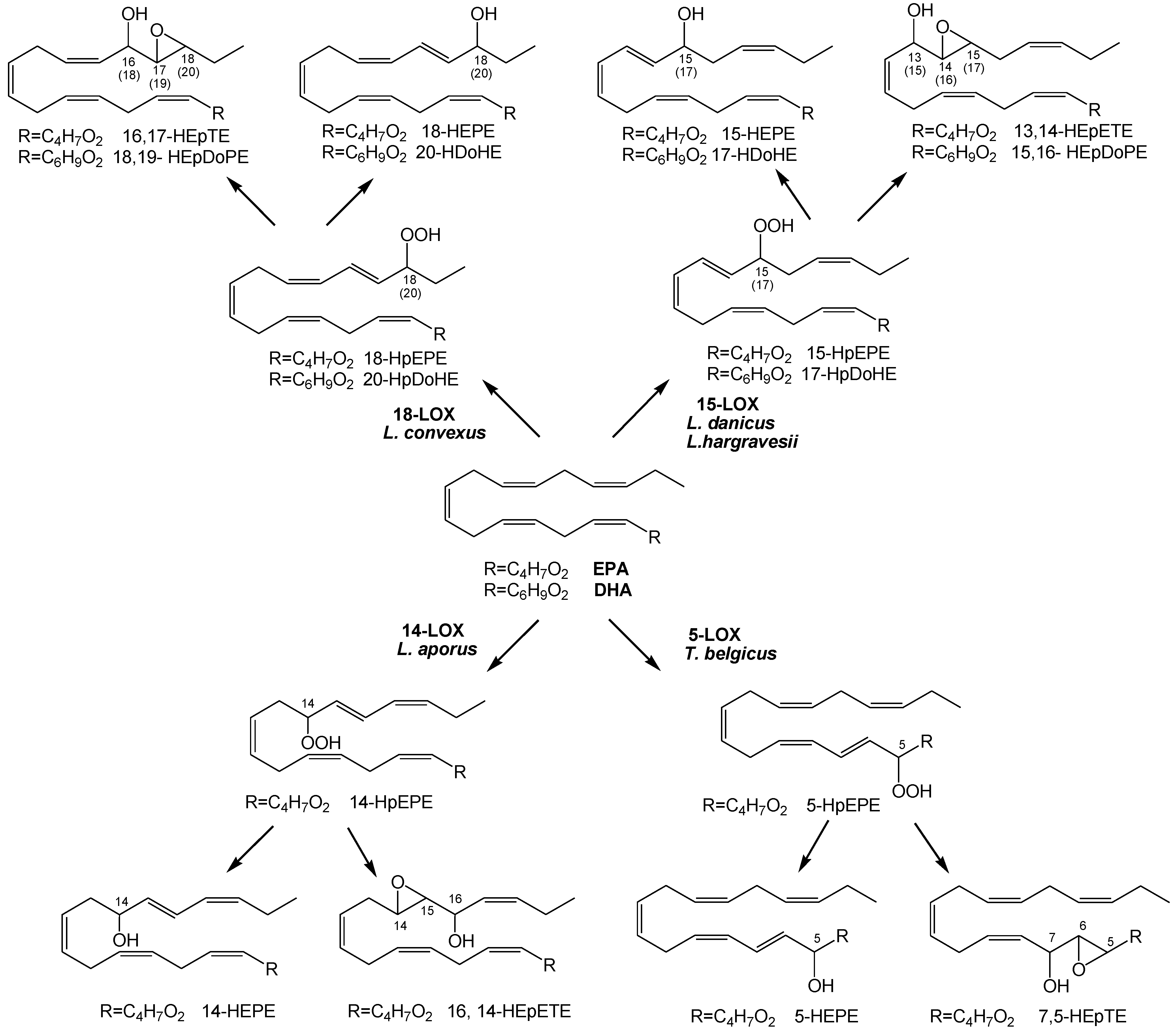
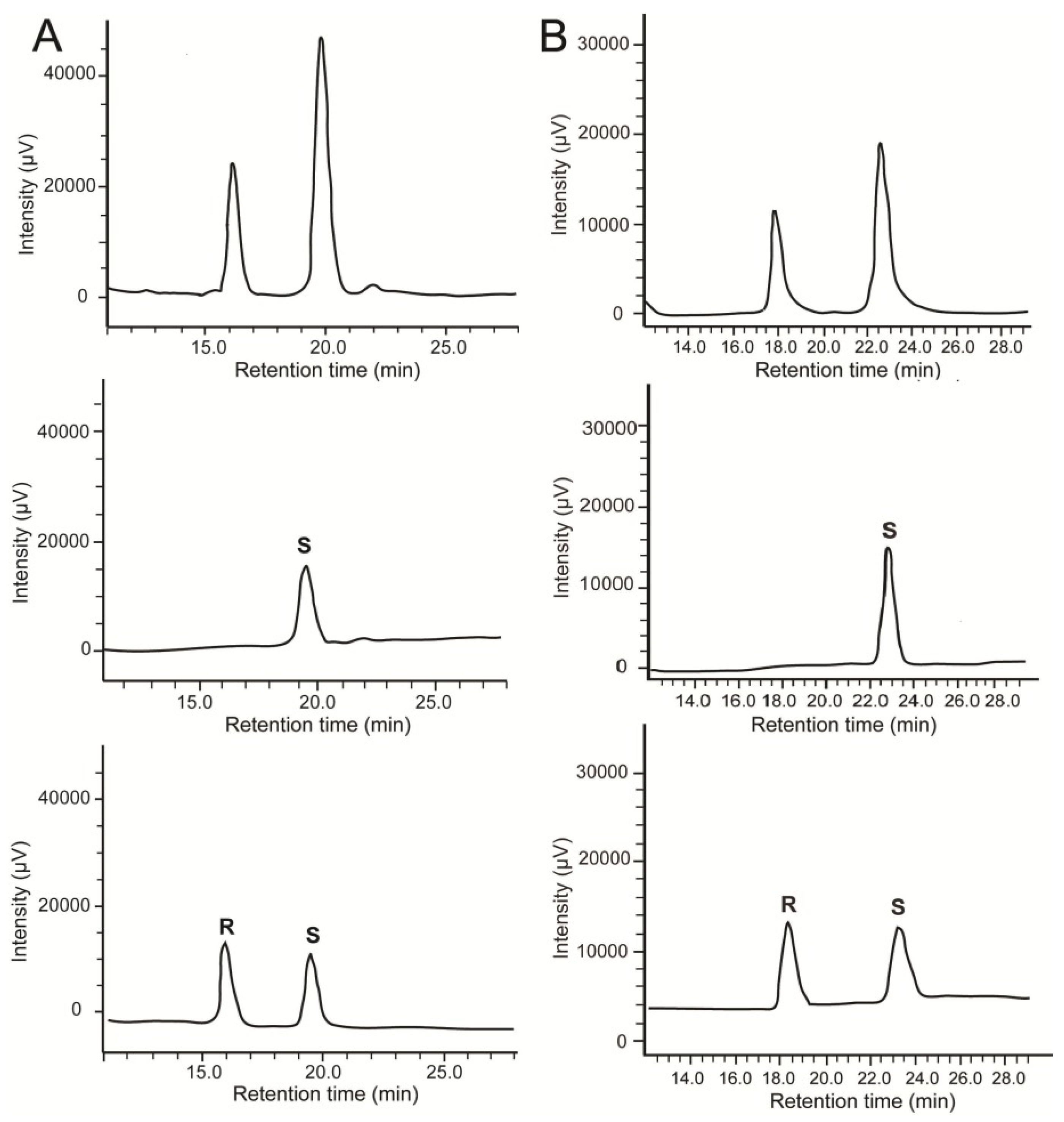
2.2. Stereochemistry of Hydroacids
2.3. Quantitative Analysis of Oxylipins
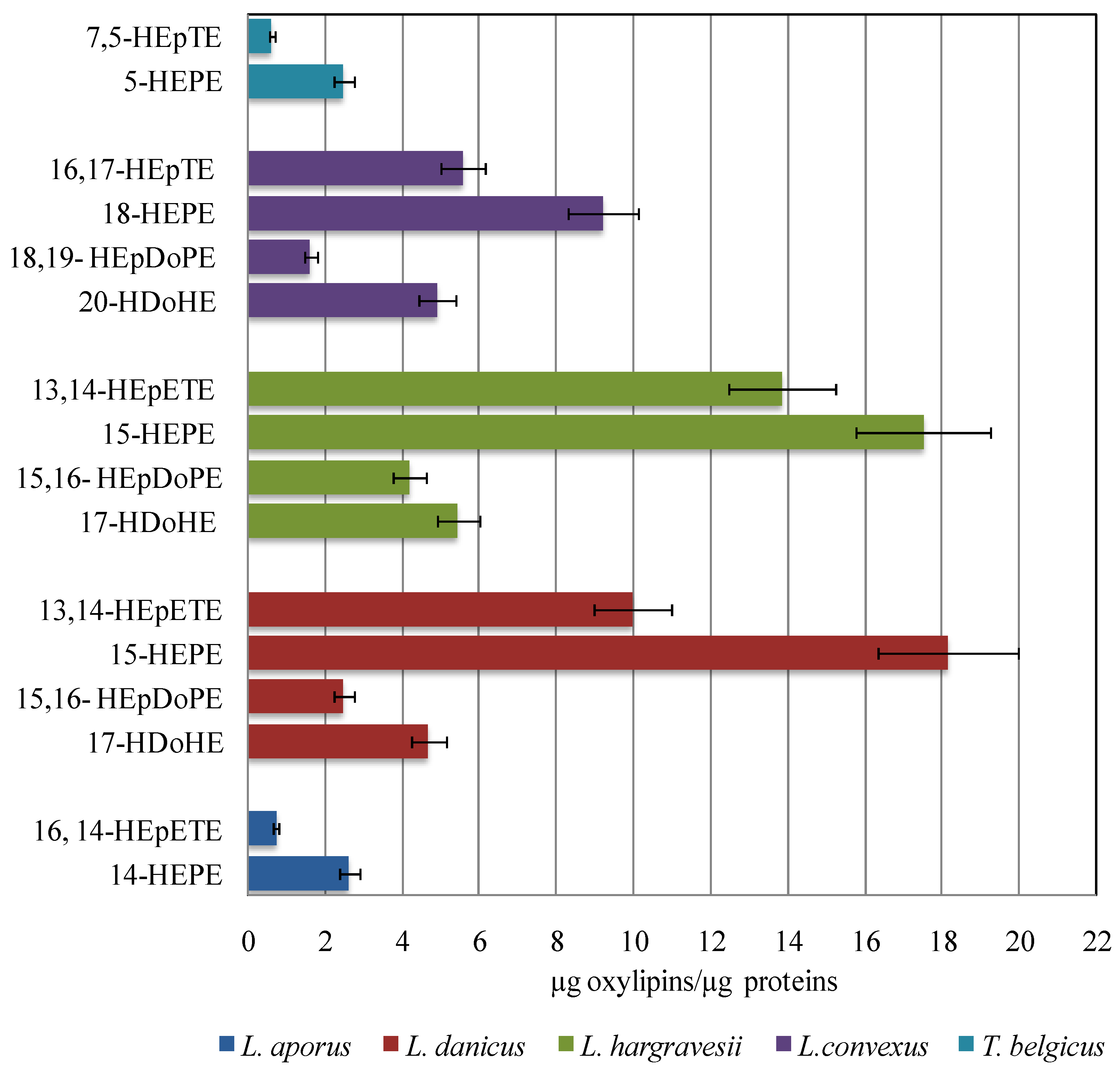
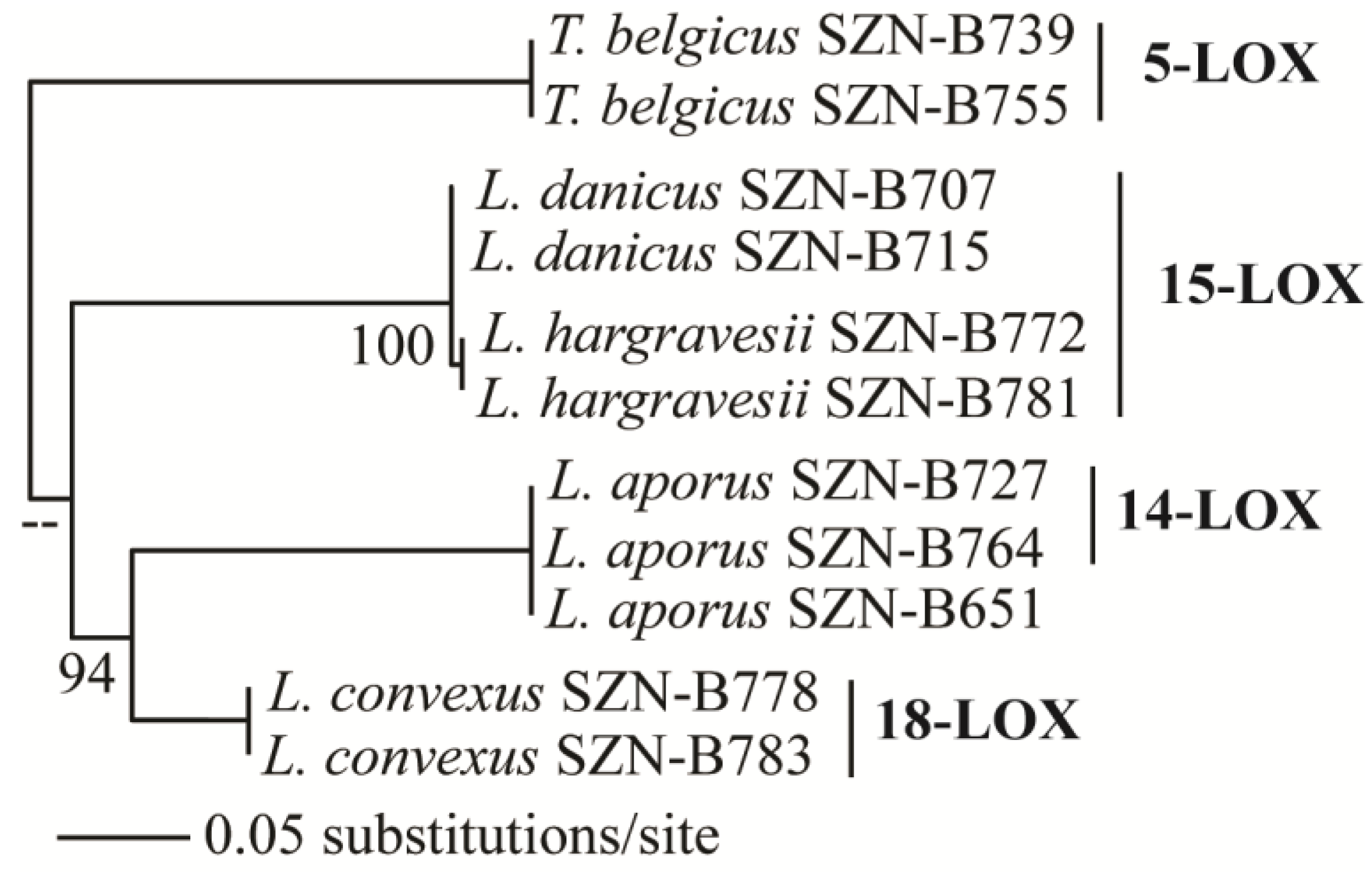
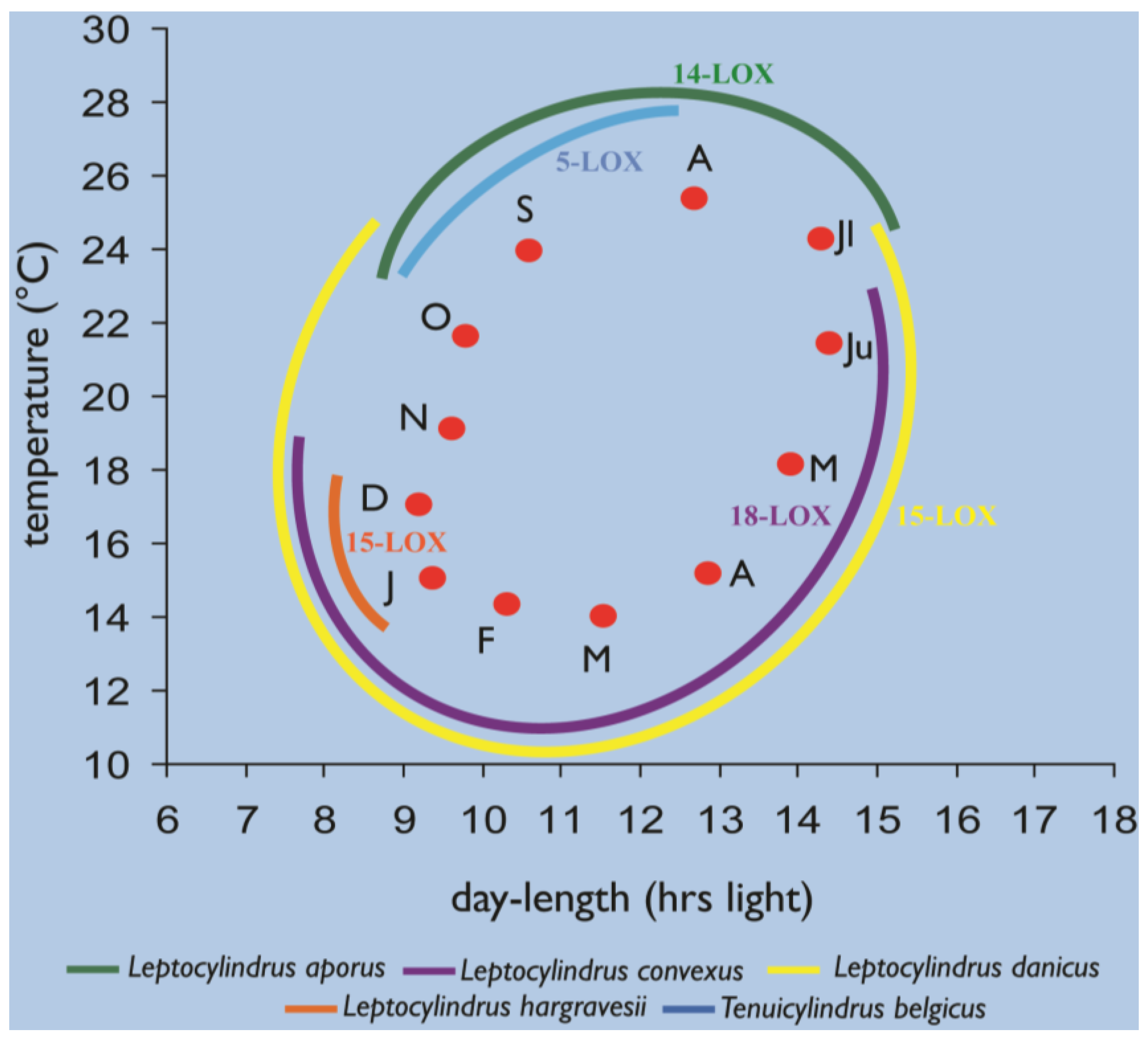
2.4. Oxylipins Data Support the DNA-Based Species Categorization and Functionality
3. Experimental Section
3.1. Cultures
3.2. Oxylipins Analysis
3.3. Protein Content
3.4. Chiral Analysis of HEPEs and HDoHEs
3.5. Phylogenetic Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Nelson, D.M.; Tréguer, P.; Brzezinski, A.; Leynaert, A.; Quéguiner, B. Production and dissolution of biogenic silica in the ocean: Revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Glob. Biogeochem. Cycles 1995, 9, 359–372. [Google Scholar] [CrossRef]
- Alverson, A.J. Molecular systematics and the diatom species. Protist 2008, 159, 339–353. [Google Scholar] [CrossRef]
- Guiry, M.D. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef]
- Mann, D.G.; Vanormelingen, P. An inordinate fondness? The number, distributions, and origins of diatom species. J. Eukaryot. Microbiol. 2013, 60, 414–420. [Google Scholar] [CrossRef]
- Lundholm, N.; Hasle, G.R.; Fryxell, G.A.; Hargraves, P.E. Morphology, phylogeny and taxonomy of species within the Pseudo-nitzschia americana complex (Bacillariophyceae) with descriptions of the two new species P. brasiliana and P. linea. Phycologia 2002, 41, 480–497. [Google Scholar] [CrossRef]
- Lundholm, N.; Moestrup, Ø.; Hasle, G.R.; Hoef-Emden, K. A study of the Pseudo-nitzschia pseudodelicatissima/cuspidata complex (Bacillariophyceae): What is P. pseudodelicatissima? J. Phycol. 2003, 39, 797–813. [Google Scholar] [CrossRef]
- Lundholm, N.; Moestrup, Ø.; Kotaki, Y.; Hoef-Emden, K.; Scholin, C.; Miller, P. Inter- and intraspecific variation of the Pseudo-nitzschia delicatissima complex (Bacillariophyceae) illustrated by rRNA probes, morphological data and phylogenetic analyses. J. Phycol. 2006, 42, 464–481. [Google Scholar] [CrossRef]
- Amato, A.; Kooistra, W.H.C.F.; Levialdi Ghiron, J.H.; Mann, D.G.; Pröschold, T.; Montresor, M. Reproductive isolation among sympatric cryptic species in marine diatoms. Protist 2007, 158, 193–207. [Google Scholar] [CrossRef]
- Mann, D.G. The diatom genus Sellaphora: Separation from Navicula. Br. Phycol. J. 1989, 24, 1–20. [Google Scholar] [CrossRef]
- Behnke, A.; Friedl, T.; Chepurnov, V.A.; Mann, D.G. Reproductive compatibility and rDNA sequence analyses in the Sellaphora pupula species complex (Bacillariophyta). J. Phycol. 2004, 40, 193–208. [Google Scholar] [CrossRef]
- Mann, D.G.; McDonald, S.M.; Bayer, M.M.; Droop, S.J.M.; Chepurnov, V.A.; Loke, R.E.; Ciobanu, A.; Du Buf, J.M.H. The Sellaphora pupula species complex (Bacillariophyceae): morphometric analysis, ultrastructure and mating data provide evidence for five new species. Phycologia 2004, 43, 459–482. [Google Scholar] [CrossRef]
- Evans, K.M.; Wortley, A.H.; Simpson, G.E.; Chepurnov, V.A.; Mann, D.G. A molecular systematic approach to explore diversity within the Sellaphora pupula species complex (Balcillariophyta). J. Phycol. 2008, 44, 215–231. [Google Scholar] [CrossRef]
- Zingone, A.; Wyatt, T. Harmful algal blooms: keys to the understanding of the phytoplankton ecology. In The Sea: Ideas and Observations on Progress in the Study of the Seas; Robinson, A.R., Brink, K.H., Eds.; Harvard University Press: Harvard, Cambridge, MA, USA, 2005; Volume 13, pp. 867–926. [Google Scholar]
- Sarno, D.; Kooistra, W.C.H.F.; Medlin, L.K.; Percopo, I.; Zingone, A. Diversity in the genus Skeletonema (Bacillariophyceae). II. An assessment of the taxonomy of S. costatum-like species, with the description of four new species. J. Phycol. 2005, 41, 151–176. [Google Scholar] [CrossRef]
- Sarno, D.; Kooistra, W.C.H.F.; Balzano, S.; Hargraves, P.E.; Zingone, A. Diversity in the genus Skeletonema (Bacillariophyceae): III. Phylogenetic position and morphological variability of Skeletonema costatum and Skeletonema grevillei, with the description of Skeletonema ardens sp. nov. J. Phycol. 2007, 43, 156–170. [Google Scholar] [CrossRef]
- Nanjappa, D.; Kooistra, W.H.C.F.; Zingone, A. A reappraisal of the genus Leptocylindrus (Bacillariophyta), with the addition of three species and the erection of Tenuicylindrus gen. nov. J. Phycol. 2013, 49, 917–936. [Google Scholar]
- Créach, V.; Ernst, A.; Sabbe, K.; Vanelslander, B.; Vyverman, W.; Stal, L.J. Using quantitative PCR to determine the distribution of a semicryptic benthic diatom, Navicula phyllepta Bacillariophyceae. J. Phycol. 2006, 42, 1142–1154. [Google Scholar] [CrossRef]
- Vanelslander, B.; Créach, V.; Vanormelingen, P.; Ernst, A.; Chepurnov, V.A.; Sahan, E.; Muyzer, G.; Stal, L.J.; Vyverman, W.; Sabbe, K. Ecological differentiation between sympatric pseudocryptic species in the estuarine benthic diatom Navicula phyllepta (Bacillariophyceae). J. Phycol. 2009, 45, 1278–1289. [Google Scholar] [CrossRef]
- Balzano, S.; Sarno, D.; Kooistra, W.H.C.F. Effects of salinity on the growth rate and morphology of ten Skeletonema strains. J. Plankton Res. 2011, 33, 937–945. [Google Scholar] [CrossRef]
- Degerlund, M.; Huseby, S.; Zingone, A.; Sarno, D.; Landfald, B. Functional diversity in cryptic species of Chaetoceros socialis Lauder (Bacillariophyceae). J. Plankton Res. 2012, 34, 416–431. [Google Scholar]
- Huseby, S.; Degerlund, M.; Zingone, A.; Hansen, E. Metabolic fingerprinting reveals differences between northern and southern strains of the cryptic diatom Chaetoceros socialis. Eur. J. Phycol. 2012, 47, 480–489. [Google Scholar] [CrossRef]
- Fontana, A.; d’Ippolito, G.; Cutignano, A.; Miralto, A.; Ianora, A.; Romano, G.; Cimino, G. Chemistry of oxylipin pathways in marine diatoms. Pure Appl. Chem. 2007, 71, 481–490. [Google Scholar]
- Andreou, A.; Brodhun, F.; Feussner, I. Biosynthesis of oxylipins in non-mammals. Prog. Lipid Res. 2009, 48, 148–170. [Google Scholar] [CrossRef]
- Wichard, T.; Poulet, S.A.; Halsband-Lenk, C.; Albaina, A.; Harris, R.; Liu, D.; Pohnert, G. Survey of the chemical defence potential of diatoms: Screening of fifty species for α,β,γ,δ-unsaturated aldehydes. J. Chem. Ecol. 2005, 31, 949–958. [Google Scholar] [CrossRef]
- Fontana, A.; d’Ippolito, G.; Cutignano, A.; Romano, G.; Lamari, N.; Gallucci, A.M.; Cimino, G.; Miralto, A.; Ianora, A. LOX-induced lipid peroxidation mechanism responsible for the detrimental effect of marine diatoms on zooplankton grazers. Chembiochem 2007, 8, 1810–1818. [Google Scholar] [CrossRef]
- Lamari, N.; Ruggiero, M.V.; d’Ippolito, G.; Kooistra, W.H.C.F.; Fontana, A.; Montresor, M. Specificity of lipoxygenase pathways supports species delineation in the marine diatom genus Pseudo-nitzschia. PLoS One 2013, 8, e73281. [Google Scholar]
- Cutignano, A.; Lamari, N.; d’ippolito, G.; Manzo, E.; Cimino, G.; Fontana, A. Lipoxygenase products in marine diatoms: A concise analytical method to explore the functional potential of oxylipins. J. Phycol. 2011, 47, 233–243. [Google Scholar] [CrossRef]
- Gillmor, S.A.; Villasenor, A.; Fletterick, R.; Sigal, E.; Browner, M.F. The structure of mammalian 15-lipoxygenase reveals similarity to the lipases and the determinants of substrate specificity. Nat. Struct. Mol. Biol. 1997, 4, 1003–1009. [Google Scholar] [CrossRef]
- Sloane, D.L.; Leung, R.; Barnett, J.; Craik, C.S.; Sigal, E. Conversion of human 15-lipoxygenase to an efficient 12-lipoxygenase: the side-chain geometry of amino acids 417 and 418 determine positional specificity. Protein Eng. 1995, 8, 275–282. [Google Scholar] [CrossRef]
- Chang, M.S.; Boeglin, W.E.; Guengerich, F.P.; Brash, A.R. Cytochrome P450-Dependent transformations of 15R- and 15S-hydroperoxyeicosatetraenoic acids: Stereoselective formation of epoxy alcohol products. Biochemistry 1996, 35, 464–471. [Google Scholar] [CrossRef]
- Jin, J.; Boeglin, W.E.; Cha, J.K.; Brash, A.R. 8R-Lipoxygenase-catalyzed synthesis of a prominent cis-epoxyalcohol from dihomo-γ-linolenic acid: a distinctive transformation compared with S-lipoxygenases. J. Lipid Res. 2012, 53, 292–299. [Google Scholar] [CrossRef]
- d’Ippolito, G.; Cutignano, A.; Briante, R.; Febbraio, F.; Cimino, G.; Fontana, A. New C16 fatty-acid-based oxylipin pathway in the marine diatom Thalassiosira rotula. Organ. Biomol. Chem. 2005, 3, 4065–4070. [Google Scholar] [CrossRef]
- d’Ippolito, G.; Lamari, N.; Montresor, M.; Romano, G.; Cutignano, A.; Gerecht, A.; Cimino, G.; Fontana, A. 15S-Lipoxygenase metabolism in the marine diatom Pseudo-nitzschia delicatissima. New Phytol. 2009, 183, 1064–1071. [Google Scholar] [CrossRef]
- Gerwick, W.; Roberts, M.; Vulpanovici, A.; Ballantine, D. Biogenesis and Biological Function of Marine Algal Oxylipins. In Lipoxygenases and Their Metabolites; Nigam, S., Pace-Asciak, C., Eds.; Springer US: Heidelberg, Germany, 1999; Volume 447, pp. 211–218. [Google Scholar]
- Gerwick, W.H. Structure and biosynthesis of marine algal oxylipins. Biochim. Biophys. Acta 1994, 1211, 243–255. [Google Scholar]
- Serhan, C.; Arita, M.; Hong, S.; Gotlinger, K. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids 2004, 39, 1125–1132. [Google Scholar] [CrossRef]
- Serhan, C.N.; Yang, R.; Martinod, K.; Kasuga, K.; Pillai, P.S.; Porter, T.F.; Oh, S.F.; Spite, M. Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2009, 206, 15–23. [Google Scholar] [CrossRef]
- Gerecht, A.; Romano, G.; Ianora, A.; d’Ippolito, G.; Cutignano, A.; Fontana, A. Plasticity of oxylipin metabolism among of the marine diatom Skeletonema marinoi (Bacillariophyceae). J. Phycol. 2011, 47, 1050–1056. [Google Scholar] [CrossRef]
- Ribalet, F.; Vidoudez, C.; Cassin, D.; Pohnert, G.; Ianora, A.; Miralto, A.; Casotti, R. High plasticity in the production of diatom-derived polyunsaturated aldehydes under nutrient limitation: Physiological and ecological Iimplications. Protist 2009, 160, 444–451. [Google Scholar] [CrossRef]
- Nanjappa, D. Genetic, physiological and ecological diversity in the diatom genus Leptocylindrus. Ph.D. Thesis, The Open University, London, UK, December 2012. [Google Scholar]
- Sieg, R.D.; Poulson-Ellestad, K.L.; Kubanek, J. Chemical ecology of the marine plankton. Nat. Prod. Rep. 2011, 28, 388–399. [Google Scholar] [CrossRef]
- Ianora, A.; Bentley, M.G.; Caldwell, G.S.; Casotti, R.; Cembella, A.D.; Engström-Öst, J.; Halsband, C.; Sonnenschein, E.; Legrand, C.; Llewellyn, C.A.; et al. The relevance of marine chemical ecology to plankton and ecosystem function: An emerging field. Mar. Drugs 2011, 9, 1625–1648. [Google Scholar] [CrossRef]
- Keller, M.D.; Selvin, R.C.; Claus, W.; Guillard, R.R.L. Media for the culture of oceanic ultraphytoplankton. J. Phycol. 1987, 23, 633–638. [Google Scholar]
- Swofford, D.L. PAUP*. Phylogenetic Analysis Using Parimony (* and Other Methods), 4th ed.; Sinauer Associates: Sunderland, MA, USA, 1998. [Google Scholar]
- Posada, D.; Crandall, K.A. Selecting the best-fit model of nucleotide substitution. Syst. Biol. 2001, 50, 580–601. [Google Scholar] [CrossRef]
- Kim, H.Y.; Karanian, J.W.; Salem, N., Jr. Formation of 15-lipoxygenase product from docosahexaenoic acid (22:6w3) by human platelets. Prostaglandins 1990, 40, 539–549. [Google Scholar]
- Sun, Y.-P.; Oh, S.F.; Uddin, J.; Yang, R.; Gotlinger, K.; Campbell, E.; Colgan, S.P.; Petasis, N.A.; Serhan, C.N. Resolvin D1 and its aspirin-triggered 17R epimer: Steriochemical assignments, anti-inflammatory properties and enzymatic inactivation. J. Biol. Chem. 2007, 282, 9323–9334. [Google Scholar] [CrossRef]
Supplementary Files
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nanjappa, D.; D'Ippolito, G.; Gallo, C.; Zingone, A.; Fontana, A. Oxylipin Diversity in the Diatom Family Leptocylindraceae Reveals DHA Derivatives in Marine Diatoms. Mar. Drugs 2014, 12, 368-384. https://doi.org/10.3390/md12010368
Nanjappa D, D'Ippolito G, Gallo C, Zingone A, Fontana A. Oxylipin Diversity in the Diatom Family Leptocylindraceae Reveals DHA Derivatives in Marine Diatoms. Marine Drugs. 2014; 12(1):368-384. https://doi.org/10.3390/md12010368
Chicago/Turabian StyleNanjappa, Deepak, Giuliana D'Ippolito, Carmela Gallo, Adriana Zingone, and Angelo Fontana. 2014. "Oxylipin Diversity in the Diatom Family Leptocylindraceae Reveals DHA Derivatives in Marine Diatoms" Marine Drugs 12, no. 1: 368-384. https://doi.org/10.3390/md12010368
APA StyleNanjappa, D., D'Ippolito, G., Gallo, C., Zingone, A., & Fontana, A. (2014). Oxylipin Diversity in the Diatom Family Leptocylindraceae Reveals DHA Derivatives in Marine Diatoms. Marine Drugs, 12(1), 368-384. https://doi.org/10.3390/md12010368






