1. Introduction
Gorgonian corals of the family
Ellisellidae are proven to be rich source of briarane diterpenoids that are well known for displaying a wide spectrum of bioactivities, including cytotoxic, anti-inflammatory, antiviral, antifouling, insecticidal and immunomodulatory effects [
1,
2,
3,
4]. In the course of searching for novel and bioactive secondary metabolites from marine organisms of the South China Sea [
5,
6], the tumor cell growth inhibitory activity of briarane diterpenoids attracted our attention, leading to the isolation of a series of briarane-type diterpenes the gorgonians
Dichotella gemmacea and
Junceella gemmacea [
7,
8,
9,
10,
11].
Chart 1.
Structures for compounds 1–20.
Chart 1.
Structures for compounds 1–20.
In particular, gemmacolides J, V and Y showed potential activity against A549 cells, being more active than the positive control adriamycin [
9,
10]. These results encourage subsequent studies of this class of metabolites, leading to the new collection of gorgonian
D. gemmacea, a promising source of briarane diterpenoids. Chemical investigation on the acetone extract of these animals resulted in the isolation and structure elucidation of seven new briaranes, namely gemmacolides AS–AY (
1–
7), together with ten known analogues, gemmacolide L (
12), gemmacolide X (
13), gemmacolide AH (
11), gemmacolide AQ (
8), gemmacolide AJ (
9), gemmacolide AO (
10), Junceellolide C (
17), junceellolide D (
14), junceellin (
15), and frajunolide K (
16) [
8,
9,
10,
12,
13,
14] (
Chart 1). The structures of these compounds were elucidated by detailed analysis of spectroscopic data and comparison with the reported analytical data for the known compounds. The isolates were tested
in vitro for their tumor cell growth inhibitory activity. We report here the isolation, structure elucidation, and biological activity of these new compounds.
2. Results and Discussion
Freshly collected specimens of
Dichotella gemmacea were immediately frozen to −20 °C and stored at this temperature before extraction. The usual workup for the extraction and isolation of briarane diterpenoids [
7,
8,
9,
10,
11] yielded seventeen pure compounds (
1–
17).
Gemmacolide AS (
1) was isolated as a white amorphous powder. Its molecular formula was established as C
36H
50O
14 by HRESI-MS. The IR spectrum showed absorption bands of hydroxy (3478 cm
−1), γ-lactone (1778 cm
−1), and ester (1739 cm
−1) functionalities. This observation was in agreement with the signals in the
13C NMR and DEPT spectra (
Table 1) for ten
sp2 carbon atoms (6 × OC=O, CH=CH, CH=C) at lower field and twenty three
sp3 carbon atoms at higher field (1 × C, 4 × CH, 3 × CH
2, 9 × CH
3, 2 × OC, 5 × OCH, 2 × OCH
2), accounting for eight double bond equivalents. The remaining double bond equivalents were due to the presence of four rings in the molecule.
Table 1.
13C NMR data of gemmacolides AS–AY (1–7) a.
Table 1.
13C NMR data of gemmacolides AS–AY (1–7) a.
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|
| 1 | 47.2, C | 46.5, C | 46.4, C | 46.6, C | 47.0, C | 47.1, C | 48.8, C |
| 2 | 74.5, CH | 75.6, CH | 74.3, CH | 75.3, CH | 71.9, CH | 72.8, CH | 70.4, CH |
| 3 | 132.5, CH | 131.2, CH | 128.6, CH | 131.2, CH | 129.2, CH | 65.3, CH | 42.8, CH2 |
| 4 | 127.6, CH | 129.6, CH | 127.8, CH | 129.1, CH | 129.1, CH | 78.9, CH | 70.5, CH |
| 5 | 140.0, C | 144.5, C | 139.3, C | 139.8, C | 136.8, C | 135.0, C | 140.0, C |
| 6 | 122.4, CH | 123.8, CH | 122.8, CH | 126.3, CH | 64.3, CH | 54.6, CH | 52.0., CH |
| 7 | 78.9, CH | 78.7, CH | 78.8, CH | 78.4, CH | 78.7, CH | 80.2, CH | 81.3, CH |
| 8 | 81.2, C | 81.1, C | 81.2, C | 81.0, C | 80.0, C | 83.8, C | 81.3, C |
| 9 | 64.1, CH | 63.9, CH | 64.1, CH | 63.7, CH | 75.7, CH | 71.7, CH | 70.8, CH |
| 10 | 32.8, CH | 32.7, CH | 31.3, CH | 32.7, CH | 32.7, CH | 41.2, CH | 35.7, CH |
| 11 | 59.3, C | 58.4, C | 60.1, C | 58.2, C | 57.0, C | 56.9, C | 57.2, C |
| 12 | 73.0, CH | 73.2, CH | 76.0, CH | 72.6, CH | 73.3, CH | - | 72.8, CH |
| 13 | 29.0, CH2 | 66.3, CH | 66.3, CH | 66.3, CH2 | 66.6, CH | 24.8, CH | 29.5, CH |
| 14 | 73.1, CH | 73.9, CH | 77.5, CH | 73.7, CH | 72.5, CH | 74.0, CH | 72.6, CH |
| 15 | 14.2, CH3 | 14.4, CH3 | 14.6, CH3 | 14.3, CH3 | 14.7, CH3 | 15.8, CH3 | 14.0, CH3 |
| 16 | 62.8, CH2 | 63.8, CH2 | 63.1, CH2 | 44.4, CH2 | 117.1, CH2 | 118.8, CH2 | 118.4, CH2 |
| 17 | 44.2, CH | 44.1, CH | 44.2, CH | 44.0, CH | 48.5, CH | 49.6, CH | 51.3, CH |
| 18 | 175.4, C | 175.2, C | 175.3, C | 174.9, C | 175.6, C | 175.0, C | 175.8, C |
| 19 | 6.4, CH3 | 6.3, CH3 | 6.4, CH3 | 6.3, CH3 | 8.6, CH3 | 7.2, CH3 | 5.9, CH3 |
| 20 | 49.1, CH2 | 49.0, CH2 | 49.1, CH2 | 49.1, CH2 | 49.7, CH2 | 51.9, CH2 | 50.7, CH2 |
| 9-OAc | 170.2, C | 170.2, C | 170.2, C | 170.1, C | 169.7, C | No. | 169.3, C |
| 21.6, CH3 | 21.6, CH3 | 21.6, CH3 | 21.5, CH3 | 21.0, CH3 | 21.3, CH3 |
| R1 | 169.1, C | 170.9, C | 169.3, C | 171.8, C | see 1′–6′ | 170.3, C | 172.9, C |
| 21.3, CH3 | 21.5, CH3 | 21.3, CH3 | 61.1, CH2 | 20.6, CH3 | 21.6, CH3 |
| R2 | 170.1, C | 169.7, C | No. | see 1′–5′ | 169.5, C | 169.7, C | No. |
| 21.2, CH3 | 20.9, CH3 | 20.8, CH3 | 20.4, CH3 |
| R3 | No. | see 1′–5′ | No. | 169.7, C | 170.5, C | No. | 169.5, C |
| 20.5, CH3 | 20.6, CH3 | 21.3, CH3 |
| R4 | see 1′–5′ | 170.1, C | see 1′–5′ | 170.6, C | see 1ʺ–5ʺ | No. | see 1′–5′ |
| 20.8, CH3 | 21.0, CH3 |
| R5 | see 1ʺ–5ʺ | | 170.2, C | | | 170.1, C | |
| | 20.9, CH3 | | | 21.0, CH3 | |
| 1′ | 172.0, C | 171.8, C | 173.7, C | 171.4, C | 166.1, C | | 172.9, C |
| 2′ | 43.3, CH2 | 42.6, CH2 | 43.1, CH2 | 43.5, CH2 | 60.9, CH2 | | 43.2, CH2 |
| 3′ | 25.7, CH | 25.0, CH | 25.2, CH | 25.7, CH | 172.4, C | | 24.7, CH |
| 4′ | 22.4, CH3 | 22.4, CH3 | 22.6, CH3 | 22.4, CH3 | 42.8, CH2 | | 22.7, CH3 |
| 5′ | 22.3, CH3 | 22.3, CH3 | 22.3, CH3 | 22.3, CH3 | 25.7, CH | | 22.5, CH3 |
| 6′ | | | | | 22.3, 2 × CH3 | | |
| 1ʺ | 172.3, C | | | | 172.6, C | | |
| 2ʺ | 42.9, CH | | | | 43.3, CH2 | | |
| 3ʺ | 24.8, CH3 | | | | 25.1, CH | | |
| 4ʺ and 5ʺ | 22.5, 2 × CH3 | | | | 22.4, 2 × CH3 | | |
1H and
13C NMR spectra of
1 showed great similarity to those of gemmacolide N (
18) [
11], the difference was the acetyl group at C-14 and the methoxy group at C-16 in gemmacolide N (
18) being replaced by two isovaleric acetyl in
1 due to the obvious HMBC correlations from the secondary alcohol protons to the respective ester carbonyl groups. The established planar structure of
1 was further supported by the COSY and HMBC spectra as shown in
Figure 1. The relative configuration of
1 at the stereogenetic centers was proved the same as that of
18 by a NOESY experiment (
Figure 2), showing a β configuration of H-7, H-12, H-14, Me-15, H-17, and CH
2-20, and an α configuration of H-2, H-9, H-10, and Me-18. The geometry of the Δ
3 double bond was assigned as
Z based on the proton coupling constant between H-3 and H-4 (
J = 10.1 Hz) while that of Δ
5 was determined as
E due to the NOESY correlation between H-6 and H
2-16. The relative configuration of
1 was thus determined as (1
R*,2
S*,7
S*,8
S*,9
S*,10
S*,11
R*,12
R*,14
S*,17
R*).
As gemmacolide AS (
1) contained the same lactone and diene chromophores as gemmacolide N (
18) and they differed only in the nature of substitutions for R
4 and R
5, the ECD spectrum of gemmacolide N could therefore be used as an ECD reference for the configurational assignment of gemmacolide AS (
1) and its analogues. Because the absolute configuration of gemmacolide N had been unambiguously determined by a TDDFT calculation of its solution ECD spectrum [
11], the absolute configuration of
1 was therefore determined as (1
R,2
S,7
S,8
S,9
S,10
S,11
R,12
R,14
S,17
R) due to the congruent ECD curves for
1 and that of gemmacolide N (
18).
Figure 1.
Key HMBC (arrow H→C) and COSY (bond) spin coupling systems for compound 1.
Figure 1.
Key HMBC (arrow H→C) and COSY (bond) spin coupling systems for compound 1.
Figure 2.
Key NOESY correlations for compound 1.
Figure 2.
Key NOESY correlations for compound 1.
Gemmacolide AT (
2), a white amorphous powder, had a molecular formula of C
33H
44O
15 as established by HRESI-MS. The
1H and
13C NMR spectra data (
Table 1 and
Table 2) of
2 were almost identical to those of gemmacolide AQ (
8) [
8], showing the same functional groups for both compounds. The isovaleryl group, however, was found to be attached to C-13 of
2 instead of C-14 in gemmacolide AQ based on the analysis of HMBC spectra (see
Supplementary Information). The structure of
2 had the same relative and absolute stereochemistry as that of
8 assessed by the NOESY and ECD measurements.
Table 2.
1H NMR data for gemmacolides AS–AV (1–4) a.
Table 2.
1H NMR data for gemmacolides AS–AV (1–4) a.
| No. | 1 | 2 | 3 | 4 |
|---|
| 2 | 5.67, d (9.7) | 5.62, ov | 5.55, d (9.7) | 5.64, ov |
| 3 | 5.60, t (10.1, 9.7) | 5.61, ov | 5.60, t (9.7, 10.0) | 5.65, ov |
| 4 | 6.28, d (10.1) | 6.35, d (9.1) | 6.28, d (10.0) | 6.41, d (7.7) |
| 6 | 5.75, d (8.6) | 5.83, d (8.5) | 5.75, d (8.5) | 6.07, d (8.7) |
| 7 | 4.98, d (8.6) | 4.96, d (8.5) | 4.98, d (8.5) | 4.94, d (8.7) |
| 9 | 4.80, br d (4.7) | 4.74, br d (4.8) | 4.80, br d (4.5) | 4.75, br d (3.8) |
| 10 | 3.68, br d (4.7) | 3.61, br d (4.8) | 3.56, br d (4.5) | 3.61, ov |
| 12 | 4.53, br s | 4.90, br d (2.8) | 3.47, m | 4.92, br d (2.9) |
| 13β | 1.96, ov | 5.10, t (2.7, 2.8) | 3.95, q (3.6) | 5.07, t (2.8, 2.9) |
| 13α | 2.29, ov | | | |
| 14 | 4.96, br d (3.0) | 5.22, br d (2.7) | 5.30, m | 5.18, br d (2.8) |
| 15 | 1.05, s | 1.14, s | 1.08, s | 1.14, s |
| 16a | 5.37, d (15.7) | 4.47, br s | 5.28, d (15.9) | 4.67, d (13.5) |
| 16b | 4.68, d (15.7) | 4.47, br s | 4.69, d (15.9) | 4.54, d (13.5) |
| 17 | 2.30, ov | 2.29, q (7.1) | 2.31, ov | 2.31, ov |
| 19 | 1.16, d (7.1) | 1.15, d (7.1) | 1.17, d (7.1) | 1.14, d (6.9) |
| 20a | 3.53, br d (2.4) | 3.63, br d (2.5) | 3.51, br d (2.8) | 3.60, ov |
| 20b | 2.79, br d (2.4) | 2.94, br d (2.5) | 2.73, br d (2.8) | 2.94, br d (2.0) |
| 9-OAc | 2.19, s | 2.20, s | 2.18, s | 2.19, s |
| R1 | 1.95, s | 1.99, s | 1.97, s | 4.16, d (16.8) |
| 4.02, d (16.8) |
| R2 | 2.11, s | 2.16 s | No. | see 2′–5′ |
| R3 | No. | see 2′–5′ | No. | 1.93, s |
| R4 | see 2′–5′ | 2.09 s | see 2′–5′ | 2.09, s |
| R5 | see 2ʺ–5ʺ | No. | 2.14, s | No. |
| 2′a | 2.28, ov | 2.09, ov (×2) | 2.30, ov (×2) | 2.34, ov |
| 2′b | 2.01, ov | | | 2.20, ov |
| 3′ | 2.17, m | 1.99, m | 2.08, m | 2.14, m |
| 4′ | 0.99, d (6.6) | 0.92, d (6.6) (×2 Me) | 1.00, d (6.7) | 0.99, d (6.8) |
| 5′ | 0.99, d (6.6) | | 0.99, d (6.7) | 1.01 (d, 6.8) |
| 2ʺa | 2.15, ov | | | |
| 2ʺb | 2.06, ov | | | |
| 3ʺ | 2.04, ov | | | |
| 4ʺ | 0.96, d (6.6) | | | |
| 5ʺ | 0.94, d (6.6) | | | |
Gemmacolide AU (
3) was isolated as a white amorphous powder and found to have a molecular formula of C
31H
42O
14 established by HRESI-MS.
1H and
13C NMR spectra of
3 were similar to those of compound gemmacolide AR (
19) [
8]. The acetyl group at C-12 and C-13 in
19 was replaced by two hydroxy groups in
3. This conclusion was supported by extensive 2D NMR (see
Supplementary Information) and HRESIMS analysis. The structure of
3 was thus determined as (−)-(1
S,2
S,3
Z,5
E,7
S,8
S,9
S,10
S,11
R,12
R,13
S,14
R,17
R) based on the ECD experiment.
Gemmacolide AV (
4) was obtained as a white amorphous powder. Its HRESI-MS demonstrated the same molecular formula as C
33H
43O
15Cl by HRESI-MS.
1H and
13C NMR spectroscopic data of
1 showed great similarity to those of gemmacolide AJ (
9) [
8], the only difference of the isovaleric acetyl at C-2 in
9 being replaced by a glycolyl group in
4. The location of the glycolyl group at C-2 was indicated by the distinct HMBC correlations (see
Supplementary Information). The established structure of
4 was further supported by detailed analysis of its 2D NMR data. Its absolute configuration was proven the same as that of
9 based on their similar ECD spectrum.
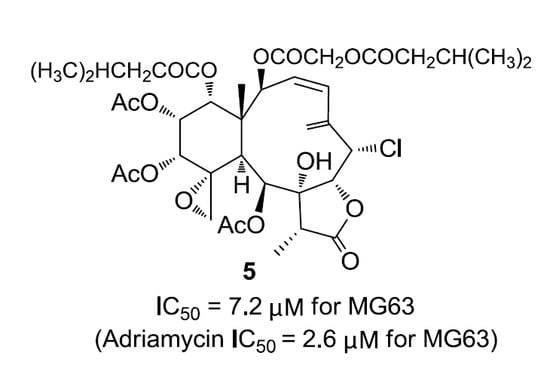
Gemmacolide AW (
5) was found to be a white amorphous powder, having the molecular formula C
38H
51ClO
16 based on the HRESIMS data. The
1H and
13C NMR data of
5 greatly resembled those of gemmacolide L (
12) [
10]. However, the substitutions of isovaleric acetyl group at C-12 and acetyl group at C-14 in
12 had to be interchanged in
5 based on the HMBC experiment (see
Supplementary Information). The relative configuration for all chiral centers remained intact due to the NOEY experiment. Its absolute configuration was proved the same as that of
12 due to their similarity in ECD spectrum.
Gemmacolide AX (
6) was isolated as a white amorphous powder. The molecular formula C
26H
33ClO
11 was established by HRESI-MS. Comparison of overall
1H and
13C NMR data (
Table 1 and
Table 3) of
6 with those of gemmacolide X (
13) [
9] revealed great similarity except for the absence of two acetyl group. The acetyl group at C-9 and C-12 in
13 was replaced by a hydroxy group and a hydrogen atom in
6. This conclusion was supported by extensive 2D NMR analysis. The relative configuration of all the asymmetric centers was determined as (1
R*,2
R*,3
R*,4
R*,6
S*,7
R*,8
R*,9
S*,10
S*,11
R*,14
S*,17
R*) based on a NOESY experiment (see
Supplementary Information). The absolute configuration of
6 was tentatively suggested as (1
R,2
R,3
R,4
R,6
S,7
R,8
R,9
S,10
S,11
R,14
S,17
R) (
Chart 1) due to its biogenetic correlation with compounds
1–
5.
Table 3.
1H NMR data for gemmacolides AW–AY (5–7) a.
Table 3.
1H NMR data for gemmacolides AW–AY (5–7) a.
| Proton | 5 | 6 | 7 |
|---|
| 2 | 6.24, d (10.1) | 5.38, d (6.8) | 5.90, ov |
| 3β | 5.74, ov | 6.13, dd (6.8, 10.6) | 2.07, ov |
| 3α | | | 2.11, ov |
| 4 | 6.00, d (11.5) | 4.43, d (10.6) | 4.43, ov |
| 6 | 5.10, br s | 5.48, br d (3.1) | 4.43, ov |
| 7 | 4.72, br d (3.6) | 4.57, br d (3.1) | 4.43, ov |
| 9 | 4.95, br s | 4. 37, br d (6.8) | 5.79, br s |
| 10 | 3.96, br s | 2.44, br d (6.8) | 3.62, br s |
| 12 | 4.88, br d (3.4) | 2.19, ov (×2) | 4.61, br s |
| 13β | 5.23, t (3.4) | 1.92, ov (×2) | 2.19, ov |
| 13α | | | 2.09, ov |
| 14 | 5.28, br s | 4.97, br s | 4.96, br s |
| 15 | 1.31, s | 1.33, s | 1.21, s |
| 16a | 5.72, br s | 5.54, br d (2.0) | 6.04, br s |
| 16b | 5.70, br s | 5.31, br d (2.0) | 5.81, br s |
| 17 | 2.93, ov | 2.59, q (7.1) | 2.99, ov |
| 19 | 1.22, d (7.6) | 1.30, d (7.1) | 1.26, d (6.4) |
| 20a | 2.92, br d (2.9) | 2.72, br d (3.0) | 2.86, br d (2.1) |
| 20b | 2.64, br d (2.9) | 2.82, br d (3.0) | 2.35, br d (2.1) |
| 9-OAc | 2.17, s | | 2.23, s |
| R1 | 4.59, d (15.6) | 2.04, s | 2.03, s |
| 4.44, d (15.6) |
| 2.28, ov |
| 2.15, ov |
| 0.98, d (6.6) (×2 Me) |
| R2 | 2.11, s | 1.98, s | 3.08, ov |
| R3 | 1.95, s | No. | 2.00, s |
| R4 | 2.25, m | | 2.18, ov |
| 2.15, m | 2.06, ov |
| 2.15, m | 2.04, ov |
| 0.98, d (6.6) (×2 Me) | 0.98, d (6.3) |
| | 0.94, d (6.3) |
| R5 | | 2.07, s | |
Gemmacolide AY (
7) was obtained as a white amorphous powder. The molecular formula C
31H
43ClO
13 was established by HRE-SIMS. Comparison of overall
1H and
13C NMR data (
Table 1 and
Table 3) of
7 with those of gemmacolide U (
20) [
9] revealed great similarity with the only difference of the acetyl group at C-4 in
20 being replaced by a hydroxy group. This conclusion was supported by extensive 2D NMR and HRESIMS analysis (see
Supplementary Information). The absolute configuration of
20 was tentatively assigned as that drawn in
Chart 1 due to its biogenetic correlation with the co-isolates.
Compounds
1–
7 were evaluated for their tumor cell growth inhibitory activity against A549 and MG63 cells. Compounds
4 and
7 showed moderate growth inhibitory effect against A549 while compound
5 displayed a promising growth inhibitory activity toward MG63 (
Table 4).
Table 4.
Tumor cell growth inhibitory activity of compounds 1–7 (IC50 in μM).
Table 4.
Tumor cell growth inhibitory activity of compounds 1–7 (IC50 in μM).
| | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Adriamycin a |
|---|
| A549 | >30.0 | >30.0 | >30.0 | 25.3 | >30.0 | >30.0 | 24.6 | 3.6 |
| MG63 | >30.0 | >30.0 | >30.0 | >30.0 | 7.2 | >30.0 | >30.0 | 2.6 |
3. Experimental Section
3.1. General Experimental Procedures
Commercial silica gel (Yantai, China, 200–300; 400–500 mesh) and RP silica gel (Merck, Darmstadt, Germany, 43–60 μm) were used for column chromatography (CC). Precoated silica gel plates (Yantai, China, HSGF-254) and RP silica gel (Macherey-Nagel, Düren, Germany, RP-18 F254) were used for analytical thin-layer chromatography (TLC). Spots were detected on TLC under UV or by heating after spraying with anisaldehyde-sulphuric acid reagent. The NMR spectra were recorded at 300 K on a Bruker DRX 400 spectrometer (Bruker Biospin Inc., Ettlingen, Germany). Chemical shifts are reported in parts per million (δ), with use of the residual CHCl3 signal (δH 7.26 ppm) as an internal standard for 1H NMR and CDCl3 (δC 77.0 ppm) for 13C NMR; Coupling constants (J) are reported in Hz. 1H NMR and 13C NMR assignments were complemented 1H-1H COSY, HSQC, HMBC and NOESY experiments. The following abbreviations are used to describe spin multiplicity: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br s = broad singlet, dd = doublet of doublets, ov = overlapped signals. Optical rotations were measured in CHCl3 with an Autopol IV polarimeter (Rudolph, Flanders, NJ, USA) at the sodium D line (590 nm). Infrared spectra were recorded in thin polymer films on a Nexus 470 FT-IR spectrophotometer (Nicolet, Madison, WI, USA); peaks are reported in cm−1. UV absorption spectra were recorded on a Varian Cary 100 UV-Vis spectrophotometer (Varian, Palo Alto, CA, USA); peaks wavelengths are reported in nm. Circular dichroism spectra were recorded with a JASCO J-715 circular dichroism spectropolarimeter (JASCO, Mary’s Court Easton, MD, USA). The MS and HRMS were performed on a Q-TOF Micro mass spectrometer (Bruker, Bremen, Germany), resolution 5000. An isopropyl alcohol solution of sodium iodide (2 mg/mL) was used as a reference compound. Semi-preparative RP-HPLC was performed on an Agilent 1100 system (Agilent Technologies, Santa Clara, CA, USA) equipped with a refractive index detector using an YMC Pack ODS-A column (particle size 5 μm, 250 × 10 mm, YMC Co., Ltd., Kyoto, Japan).
3.2. Animal Material
The gorgonian coral Dichotella gemmacea. (2.0 kg, wet weight) was collected from the South China Sea (20°54′ N, 109°05′ E), in August 2007 and identified by Dr. Xiu-Bao Li, the South China Sea Institute of Oceanology, Academia Sinica. A voucher specimen was deposited in the Second Military Medical University, Shanghai, China.
3.3. Extraction and Isolation
The frozen specimen was extracted with acetone and methanol (3 × 2.0 L) by ultrasonication. The solvent was combined and removed in vacuo. The resultant residue was partitioned between H2O and EtOAc. The layers were separated, and then EtOAc was removed to afford 14.0 g of residue. The crude extract was further partitioned between MeOH and hexane, affording 10.0 g from the MeOH fraction. This residue was subjected to silicagel CC and eluted with hexane/acetone (from 100:0 to 0:100) as eluent. Fraction 11 was further fractionated by RP-silical gel CC (MeOH/H2O, 20:80 to 75:25, in 5% increments) to give three subfractions (A–C). Subfraction B was purified by HPLC (MeOH/H2O, 55:45, 1.0 mL·min−1) to yield 4 (2.9 mg, 40.8 min), 2 (2.0 mg, 36.5 min) and 7 (0.6 mg, 34.2 min). Fraction 10 was purified by HPLC (MeOH/H2O, 58:42, 1.2 mL·min−1), yielding 14 (10.3 mg, 36.4 min). Fraction 9 were repeatedly subjected to silica gel and Sephadex LH-20 CC, and then purified by HPLC (MeOH/H2O, 60:40, 1.5 mL·min−1) to yield 3 (0.7 mg, 25.3 min), 6 (0.5 mg, 28.5 min), 8 (1.1 mg, 32.6 min), and 17 (2.4 mg, 35.4 min). Fraction 8 was further fractionated by RP-silical gel CC (gradient elution from MeOH//H2O, 3:7 to MeOH, in 5% increments) and purified by HPLC (MeOH/H2O, 67:33, 1.2 mL·min−1), yielding 1 (2.3 mg, 36.4 min), 9 (4.9 mg, 40.1 min), 10 (0.5 mg, 28.5 min), 15 (1.1 mg, 45.6 min), and 16 (2.4 mg, 49.4 min). Fraction 7 was purified by HPLC (MeOH/H2O, 60:40, 1.5 mL·min−1), yielding 5 (7.0 mg, 49.4 min), 11 (4.3 mg, 56.2 min), 12 (52.7 mg, 36.4 min).
Gemmacolide AS (1): white amorphous powder;
= −31 (
c 0.13, CHCl
3); UV (MeOH) 204 nm; CD (CH
3CN,
c 3.0 × 10
−4) λ
max (Δε) positive below 195 nm, 197 (−15.41) nm; IR (film) ν
max 3478, 1778, 1739 cm
−1;
1H NMR spectroscopic data, see
Table 2;
13C NMR spectroscopic data, see
Table 1; ESI-MS
m/z 729 [M + Na]
+; HRESI-MS
m/z 729.3094 [M + Na]
+ (calcd for C
36H
50O
14Na, 729.3098).
Gemmacolide AT (2): white amorphous powder;
= −20 (
c 0.075, CHCl
3); UV (MeOH) 207 nm; CD (CH
3CN,
c 1.3 × 10
−3) λ
max (Δε) positive below 190 nm, 201 (−14.26) nm, 217 (−13.70) nm; IR (film) ν
max 3468, 1774, 1743 cm
−1;
1H NMR spectroscopic data, see
Table 2;
13C NMR spectroscopic data, see
Table 1; ESI-MS
m/z 703 [M + Na]
+; HRESI-MS
m/z 703.2576 [M + Na]
+ (calcd for C
33H
44O
15Na, 703.2578).
Gemmacolide AU (3): white amorphous powder;
= −0 (
c 0.04, CHCl
3); UV (MeOH) 204 nm; CD (CH
3CN,
c 3.6 × 10
−4) λ
max (Δε) positive below 190 nm, 217.5 (−10.22) nm; IR (film) ν
max 3467, 1777, 1740 cm
−1;
1H NMR spectroscopic data, see
Table 2;
13C NMR spectroscopic data, see
Table 1; ESI-MS
m/z 661 [M + Na]
+; HRESI-MS
m/z 661.2470 [M + Na]
+ (calcd for C
33H
44O
16Na, 661.2472).
Gemmacolide AV (4): white amorphous powder;
= −28 (
c 0.15, CHCl
3); UV (MeOH) 205, 273 nm; CD (CH
3CN,
c 1.3 × 10
−3) λ
max (Δε) positive below 190 nm, 199.5 (−28.02) nm; IR (film) ν
max 3406, 1778, 1745 cm
−1;
1H NMR spectroscopic data, see
Table 2;
13C NMR spectroscopic data, see
Table 1; ESI-MS
m/z 737 [M + Na]
+; HRESI-MS
m/z 737.2195 [M + Na]
+ (calcd for C
33H
43O
15ClNa, 737.2188).
Gemmacolide AW (5): white amorphous powder;
= −104 (
c 0.5, CHCl
3); UV (MeOH) 203 nm; CD (CH
3CN,
c 2.6 × 10
−4) λ
max (Δε) positive below 197 nm, 194 (−31.04) nm, 219 (−21.22) nm; IR (film) ν
max 3550, 1782, 1744 cm
−1;
1H NMR spectroscopic data, see
Table 3;
13C NMR spectroscopic data, see
Table 1; ESI-MS
m/z 821 [M + Na]
+; HRESI-MS
m/z 821.2759 [M + Na]
+ (calcd for C
38H
51O
16ClNa, 821.2763).
Gemmacolide AX (6): white amorphous powder;
= 0 (
c 0.02, CHCl
3); UV (MeOH) 202 nm; CD (CH
3CN,
c 1.4 × 10
−4) λ
max (Δε) positive below 191 nm, 208.5 (−9.64) nm; IR (film) ν
max 3467, 1787, 1743 cm
−1;
1H NMR spectroscopic data, see
Table 3;
13C NMR spectroscopic data, see
Table 1; ESI-MS
m/z 579 [M + Na]
+; HRESI-MS
m/z 579.1613 [M + Na]
+ (calcd for C
26H
33O
11ClNa, 579.1609).
Gemmacolide AY (7): white amorphous powder;
= 0 (
c 0.03, CHCl
3); UV (MeOH) 204 nm; CD (CH
3CN,
c 1.4 × 10
−4) λ
max (Δε) negative below 191 nm, 209 (15.06) nm; IR (film) ν
max 3488, 1788, 1742 cm
−1;
1H NMR spectroscopic data, see
Table 3;
13C NMR spectroscopic data, see
Table 1; ESI-MS
m/z 681 [M + Na]
+; HRESI-MS
m/z 681.2293 [M + Na]
+ (calcd for C
31H
43O
13ClNa, 681.2290).
3.4. Cytotoxicity Assay
Compounds
1–
7 were evaluated for cytotoxicity against human lung adenocarcinoma (A549) and human osteosarcoma cell (MG63), using a modification of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric method [
15]. Adriamycin was used as positive control.