Marine Low Molecular Weight Natural Products as Potential Cancer Preventive Compounds
Abstract
:1. Introduction
2. Marine Lipids
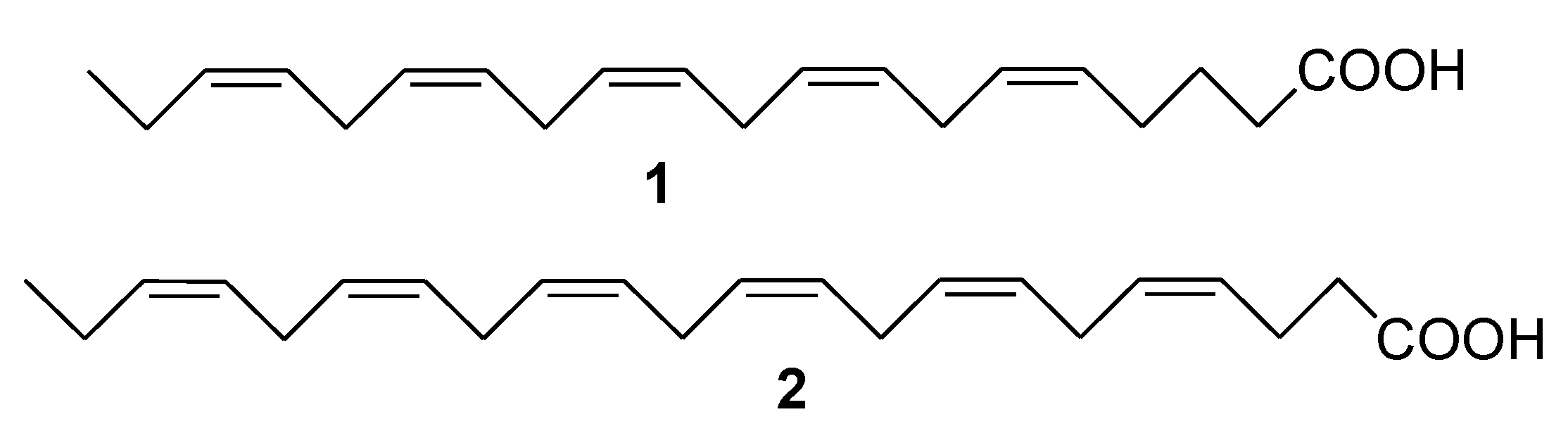
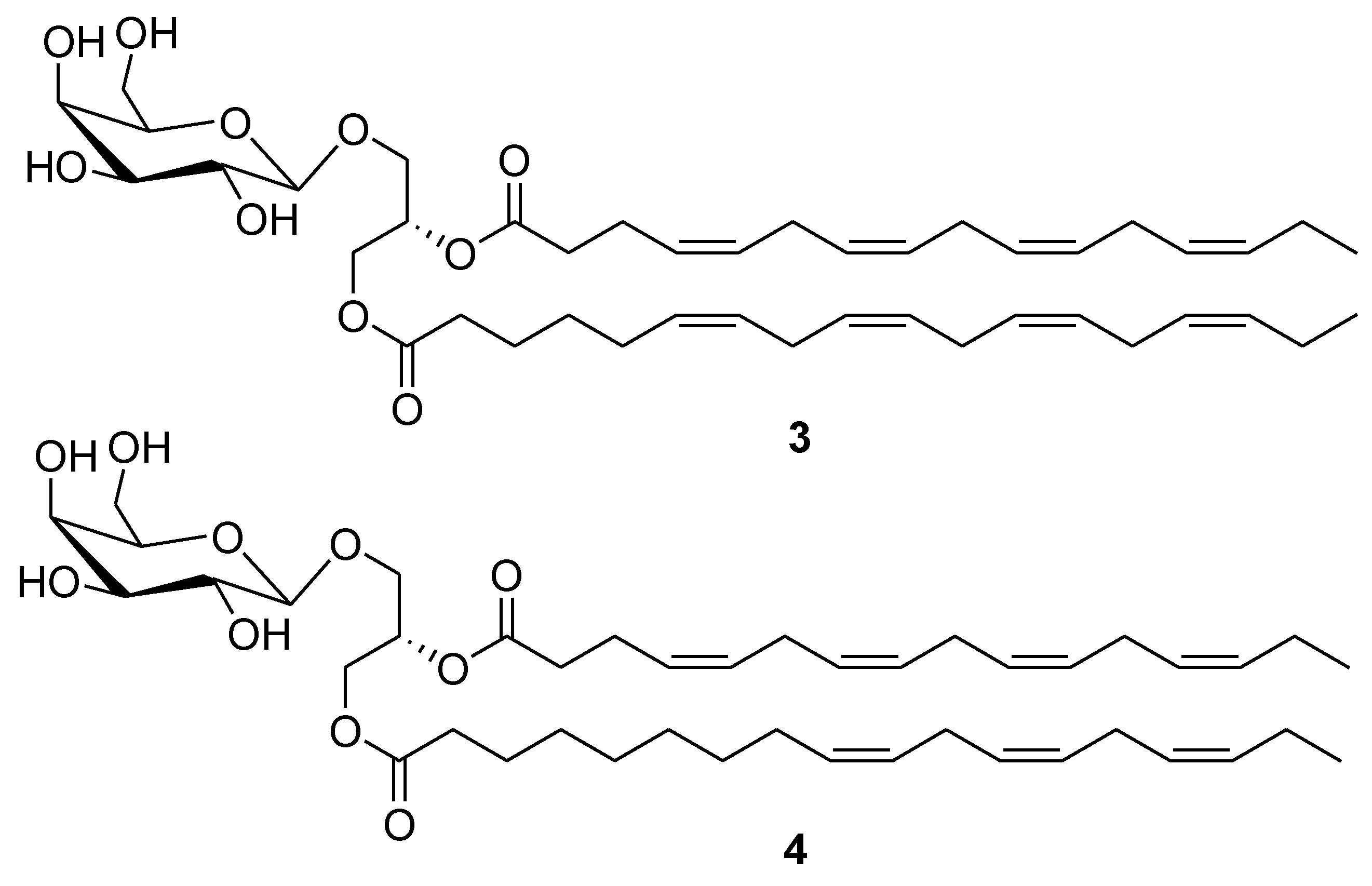
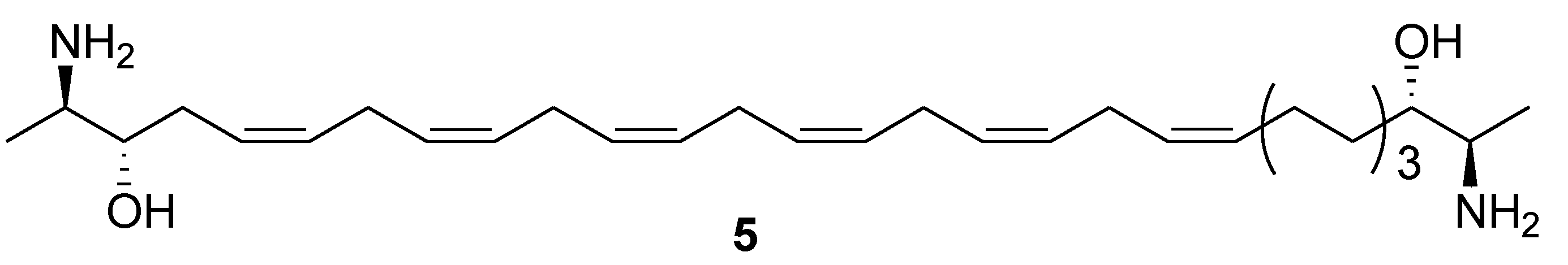
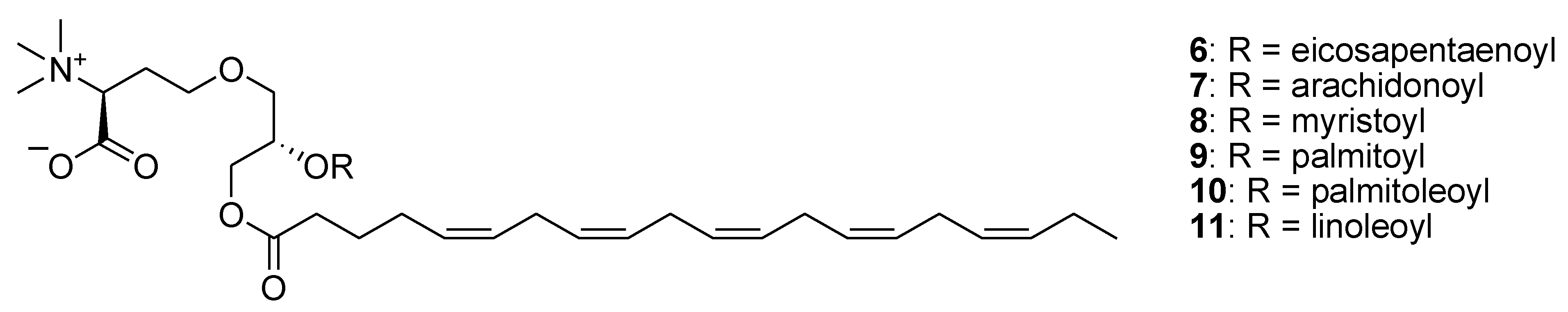
3. Marine Carotenoids
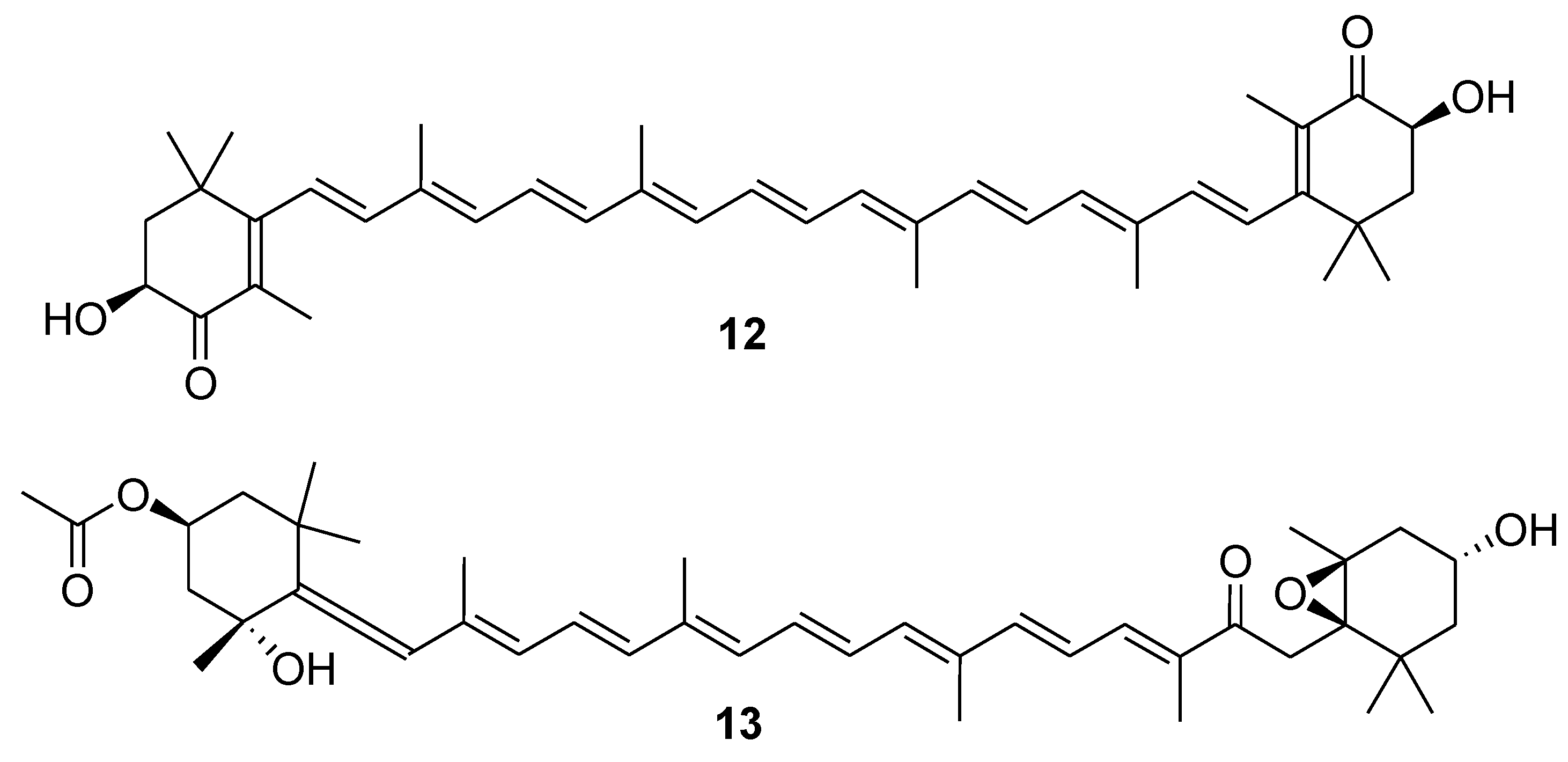
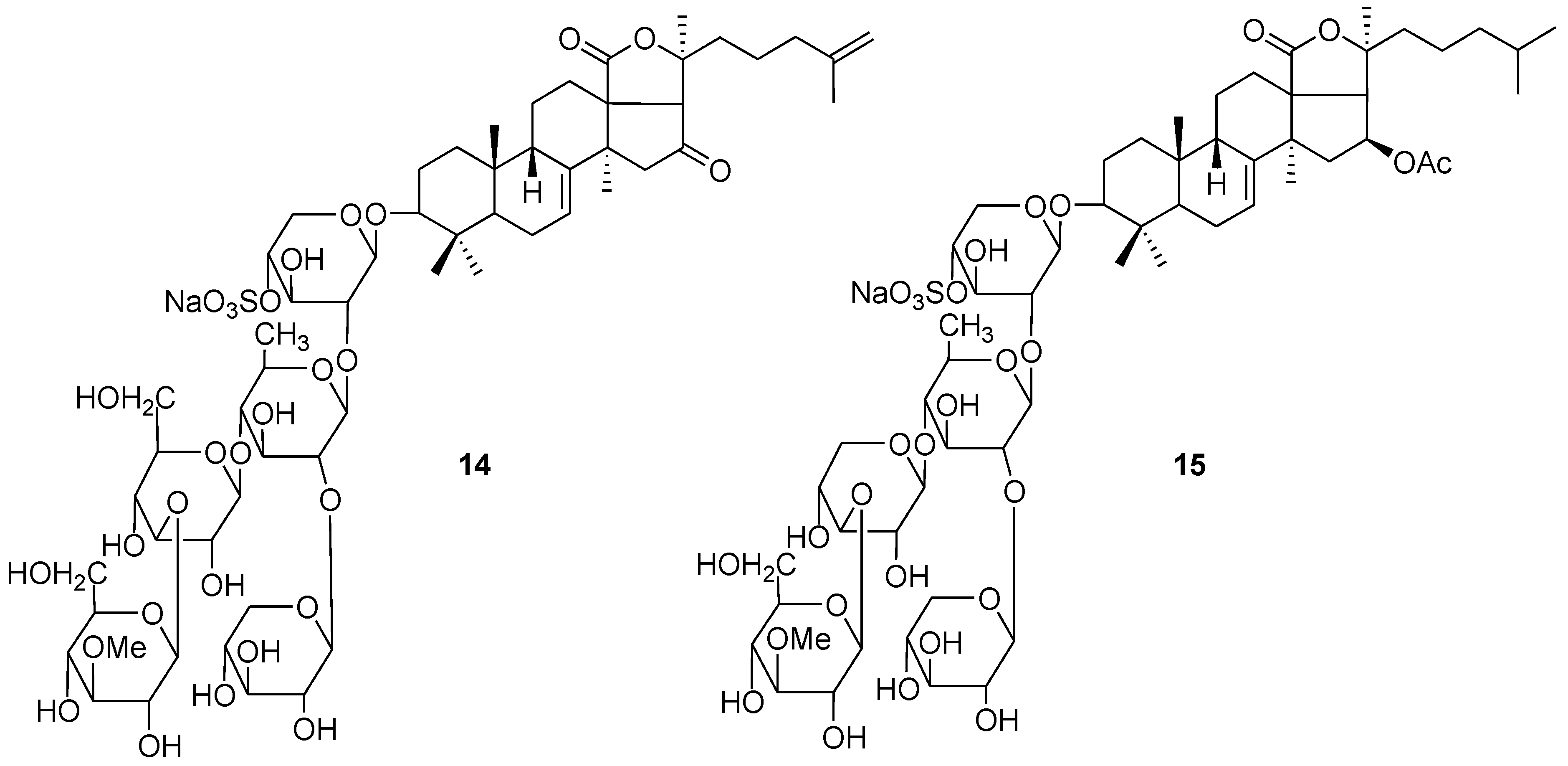
4. Glycosides
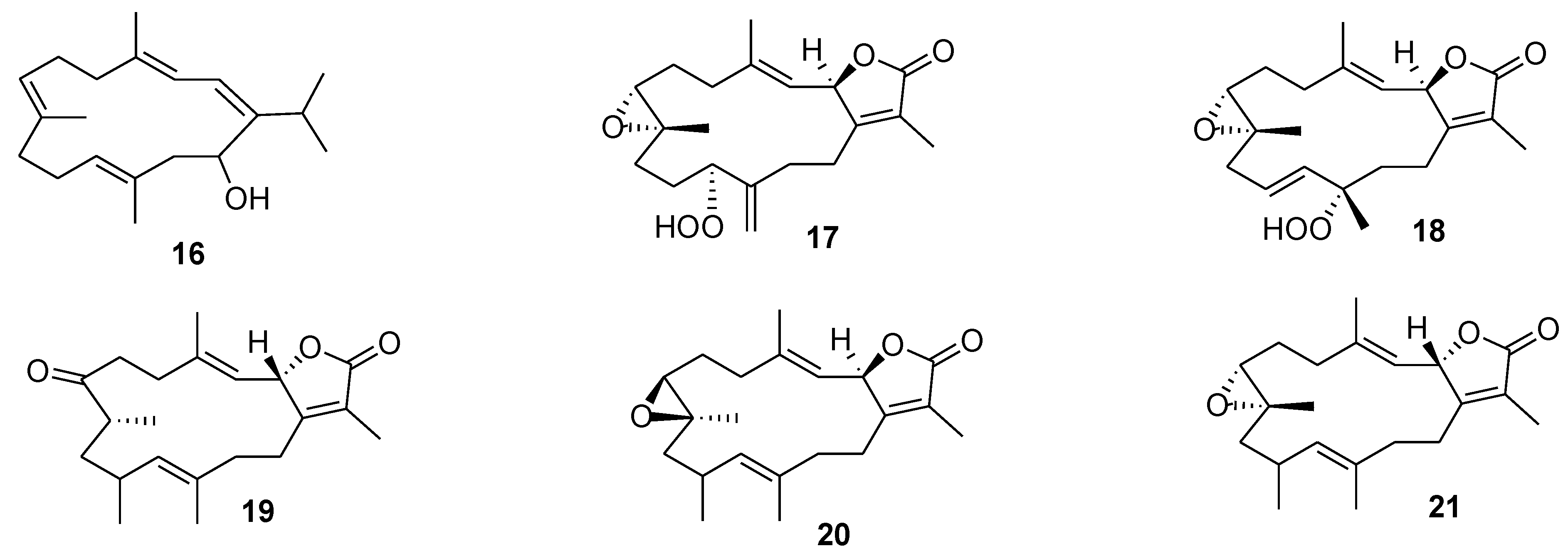
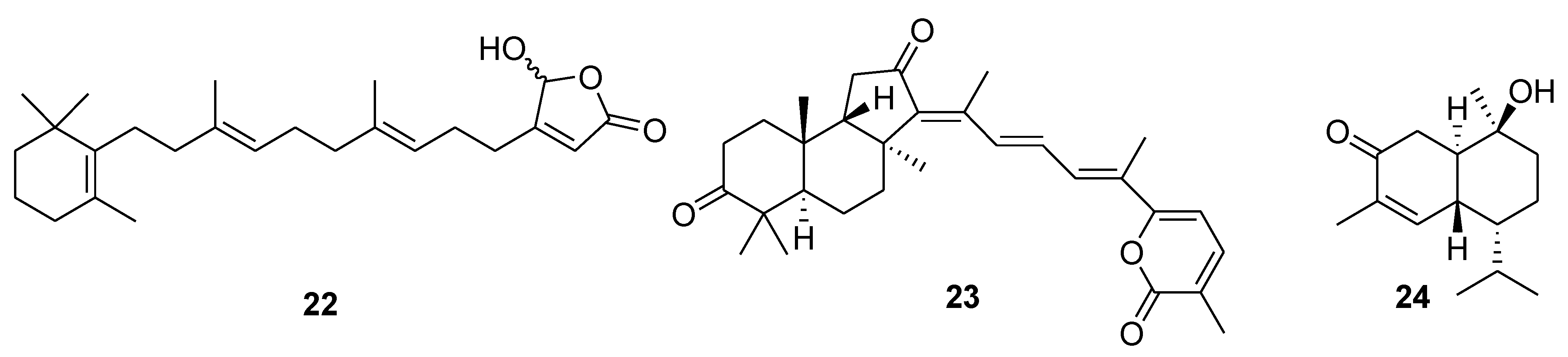
5. Terpenoids
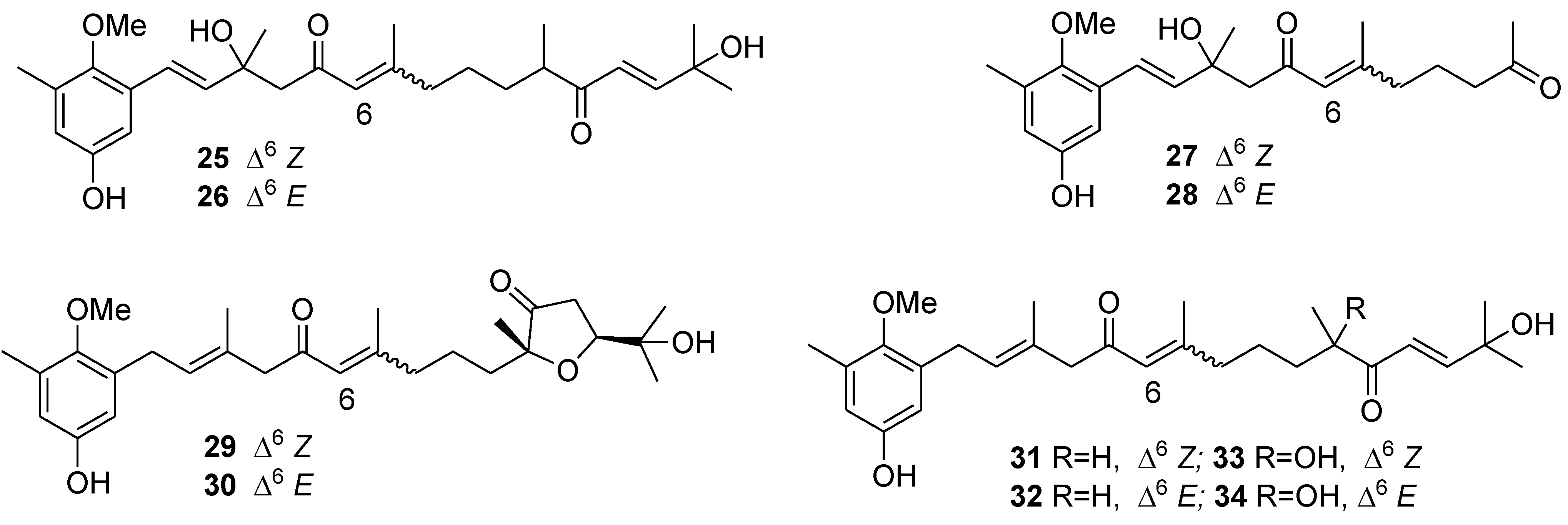

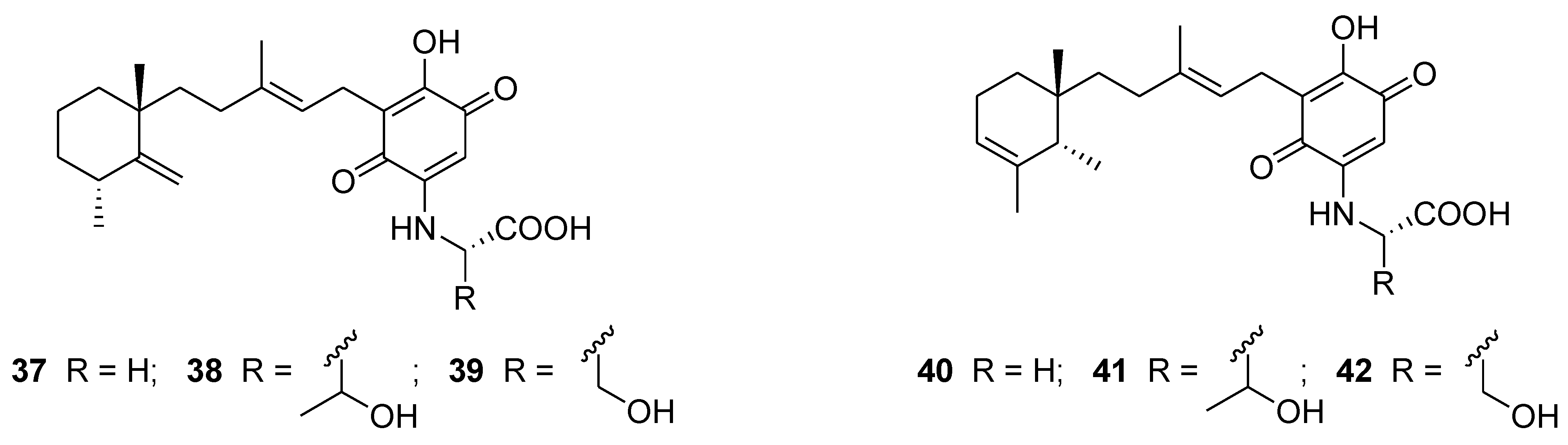
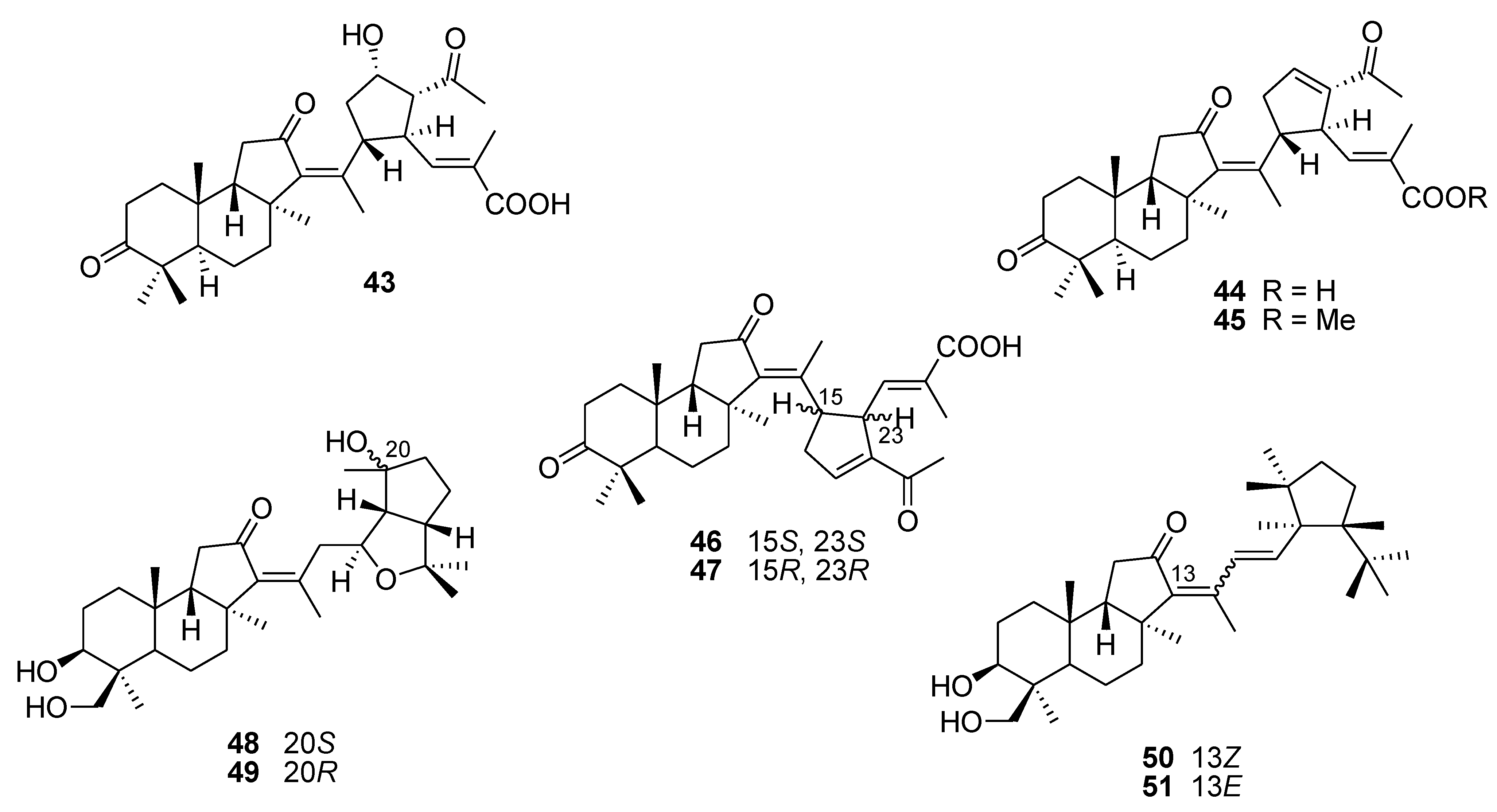
6. Alkaloids

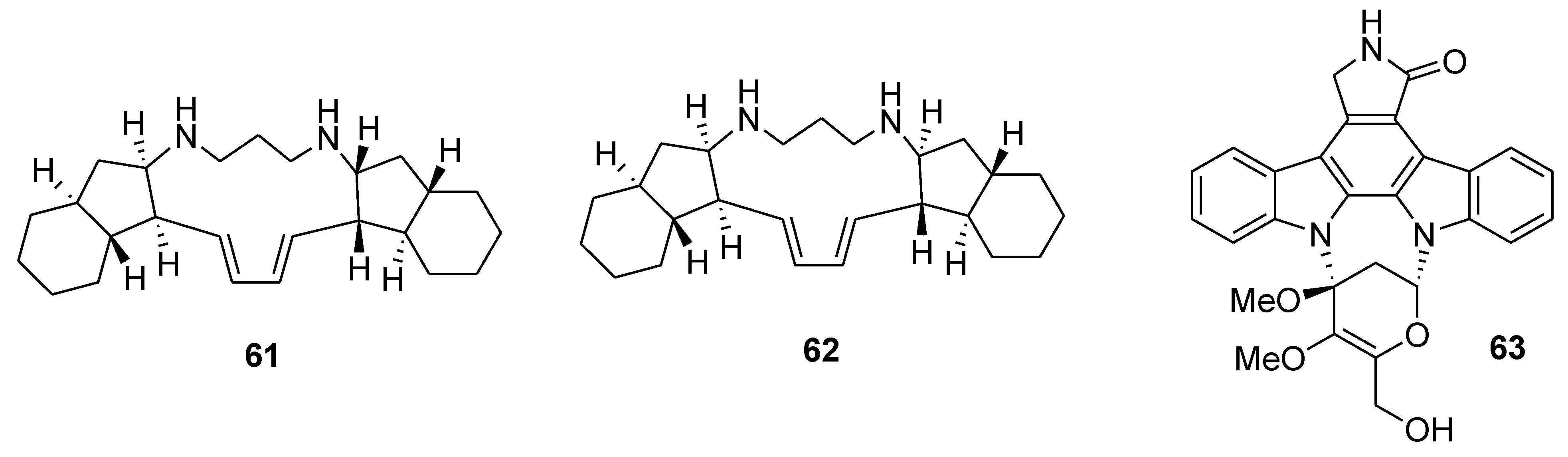
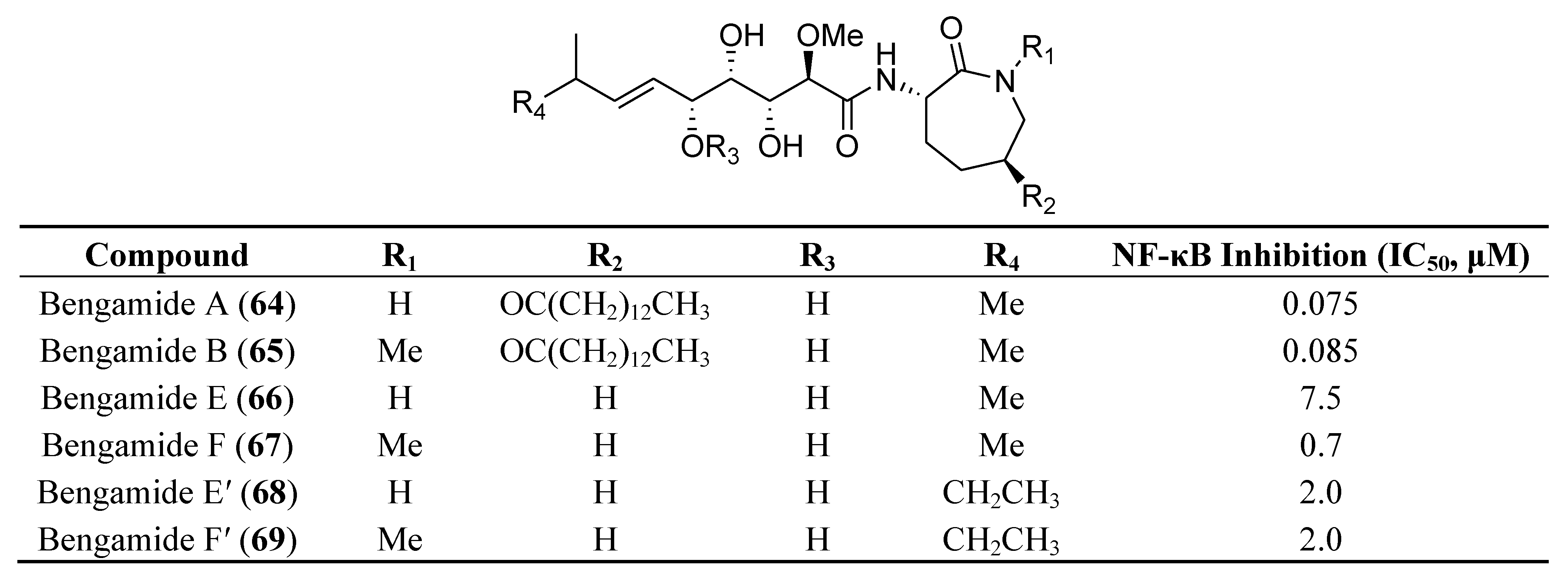

7. Other Low Molecular Weight Marine Natural Compounds
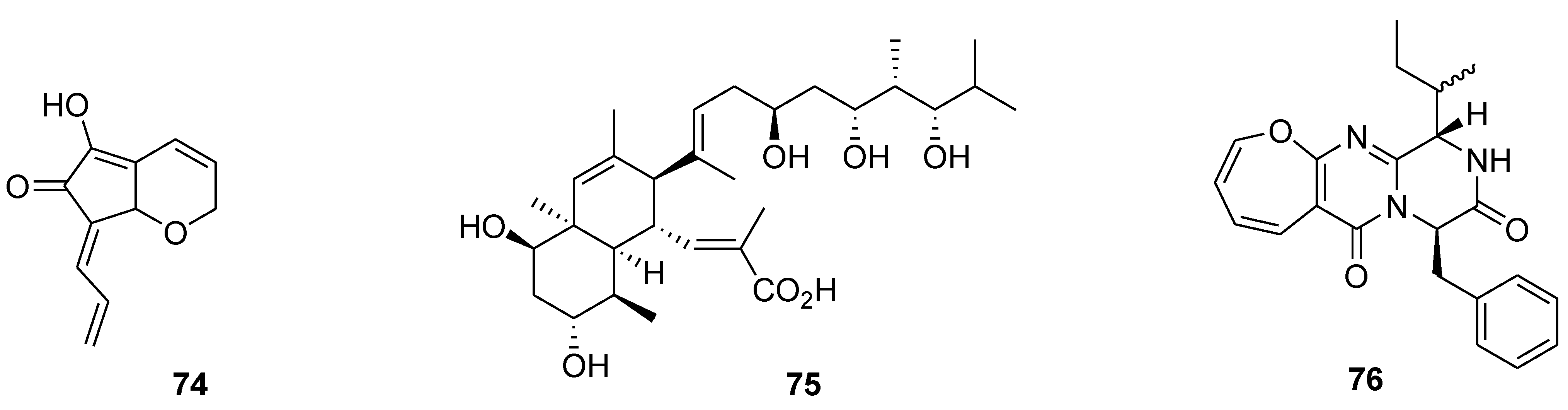
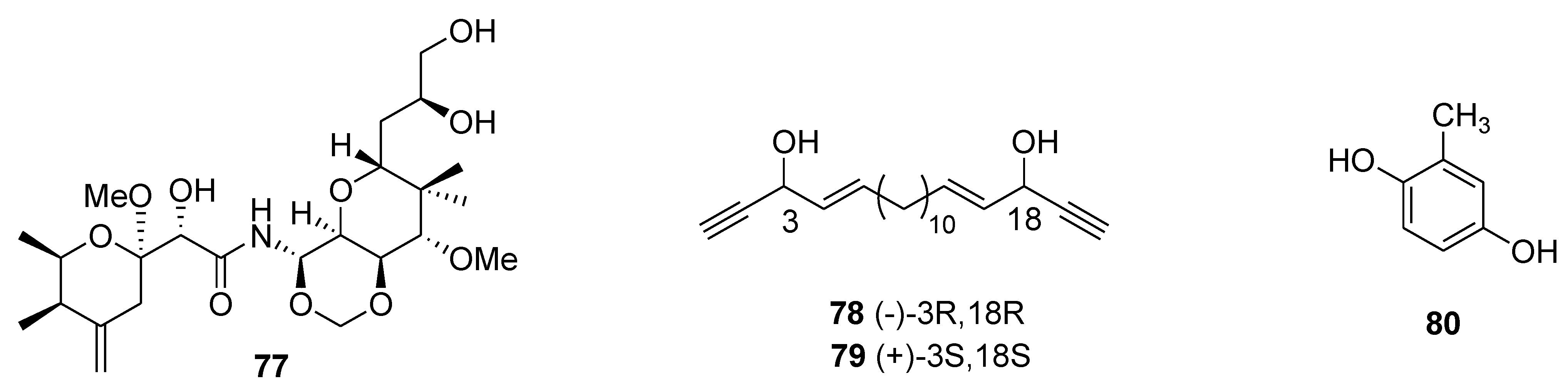
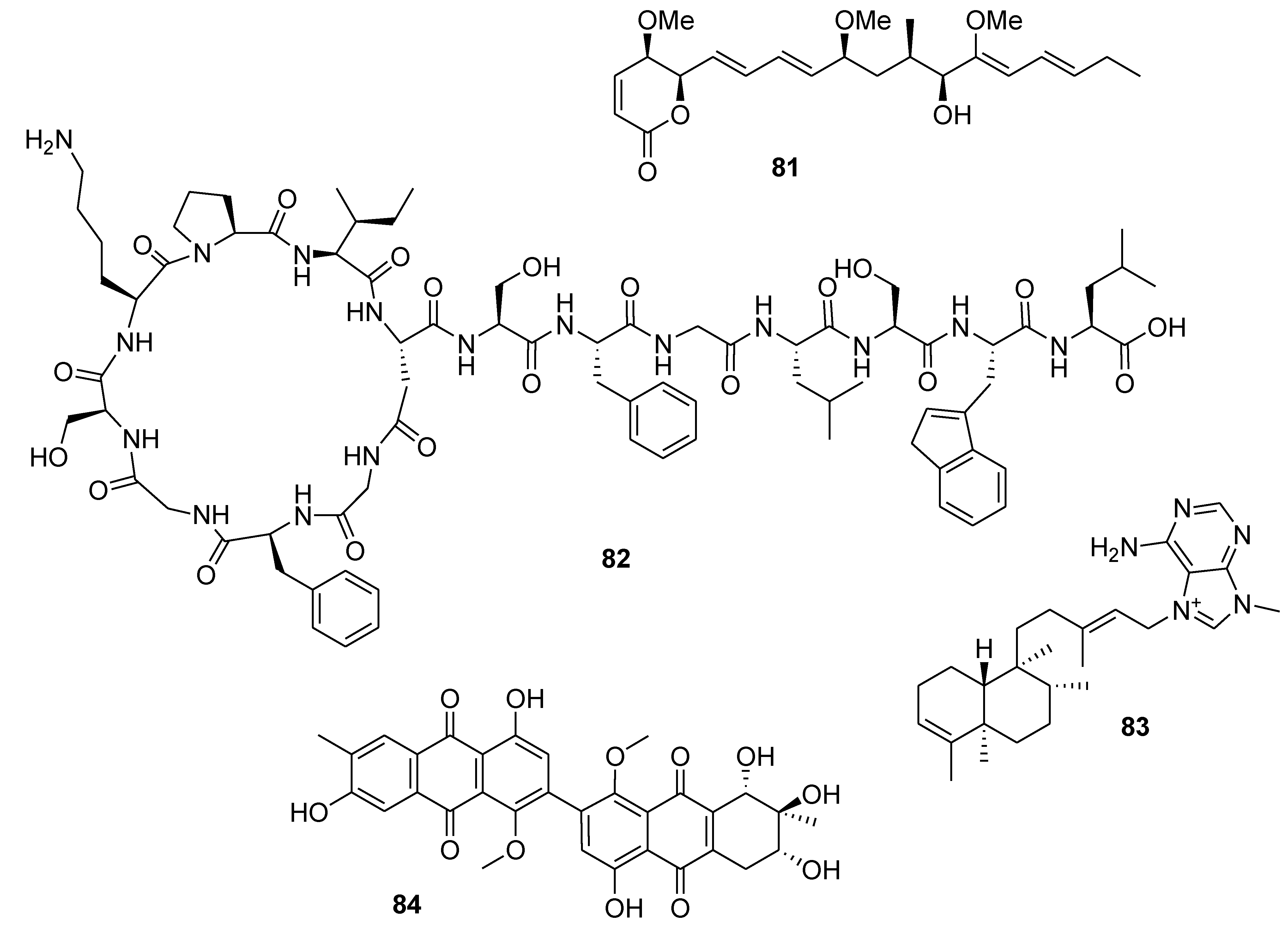

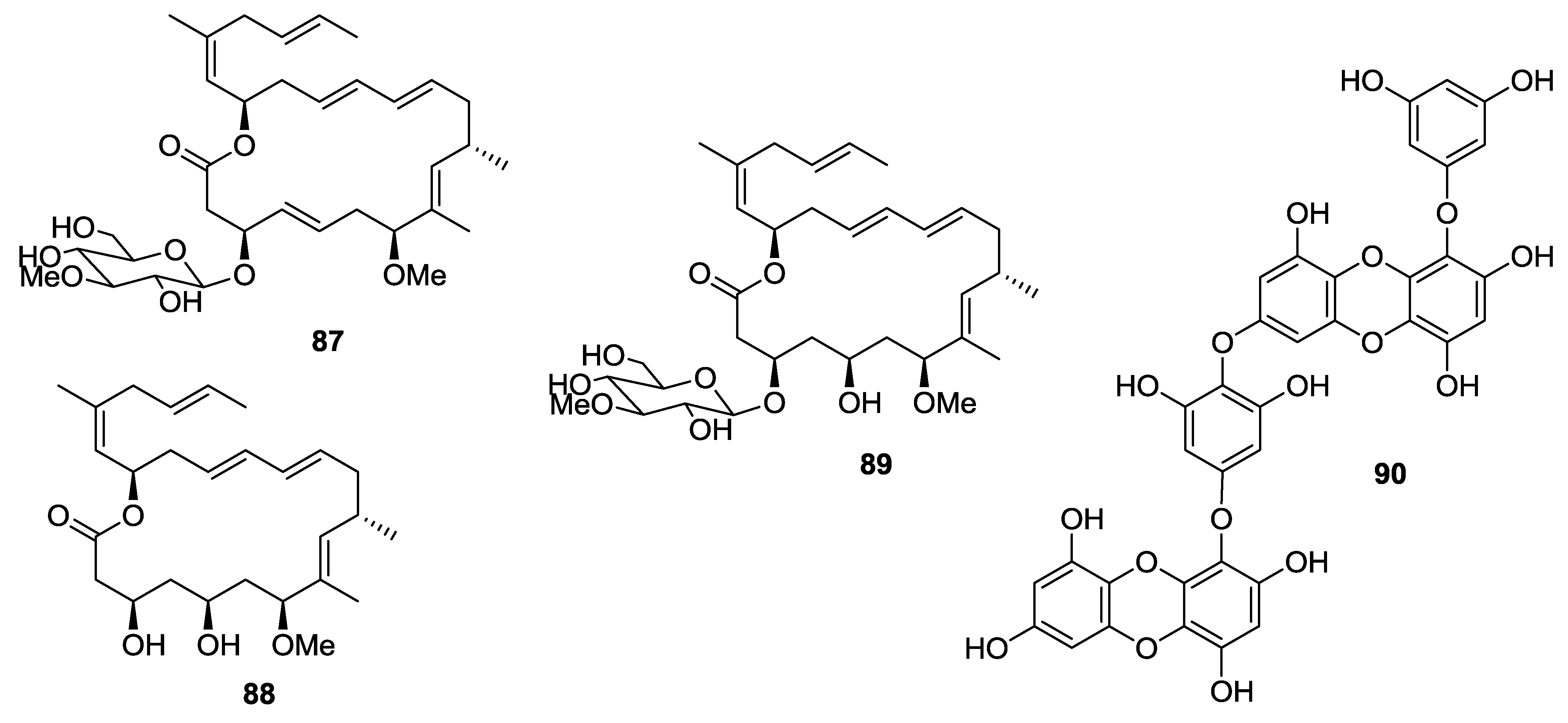
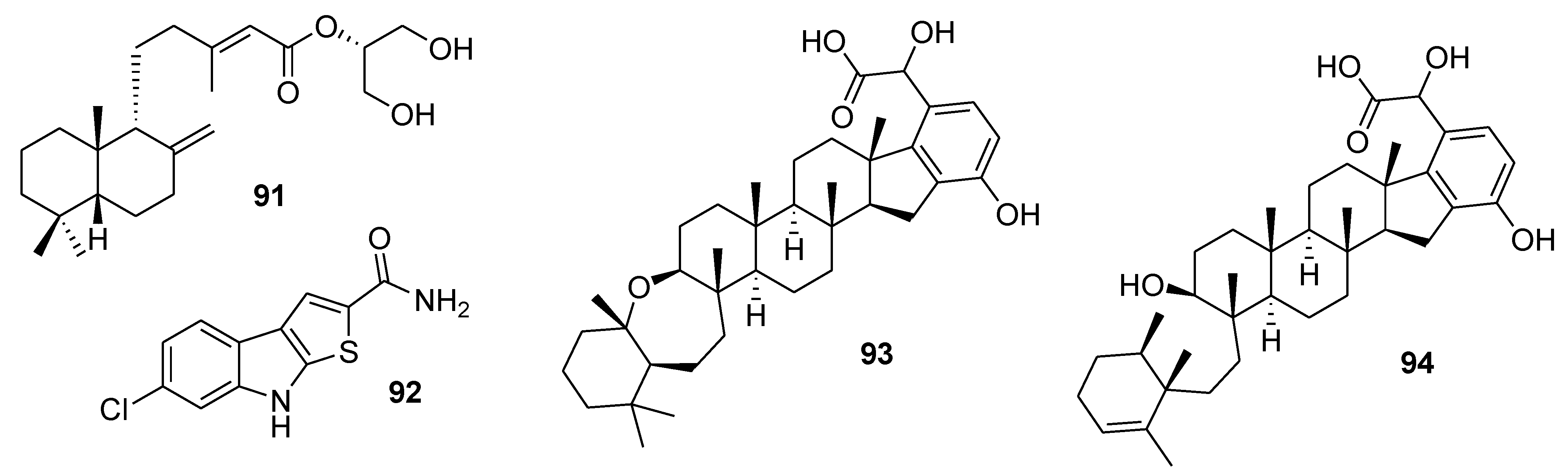
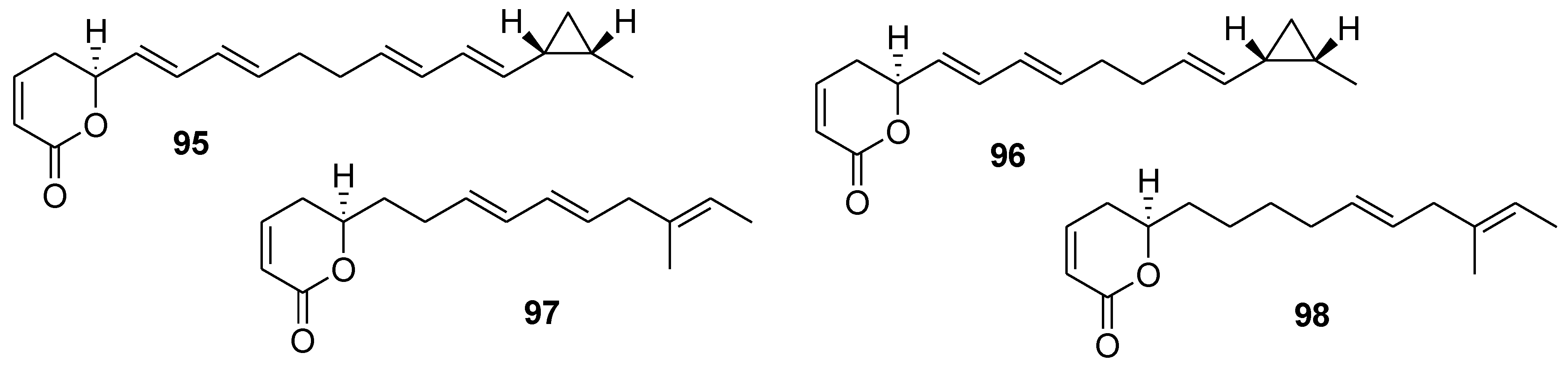
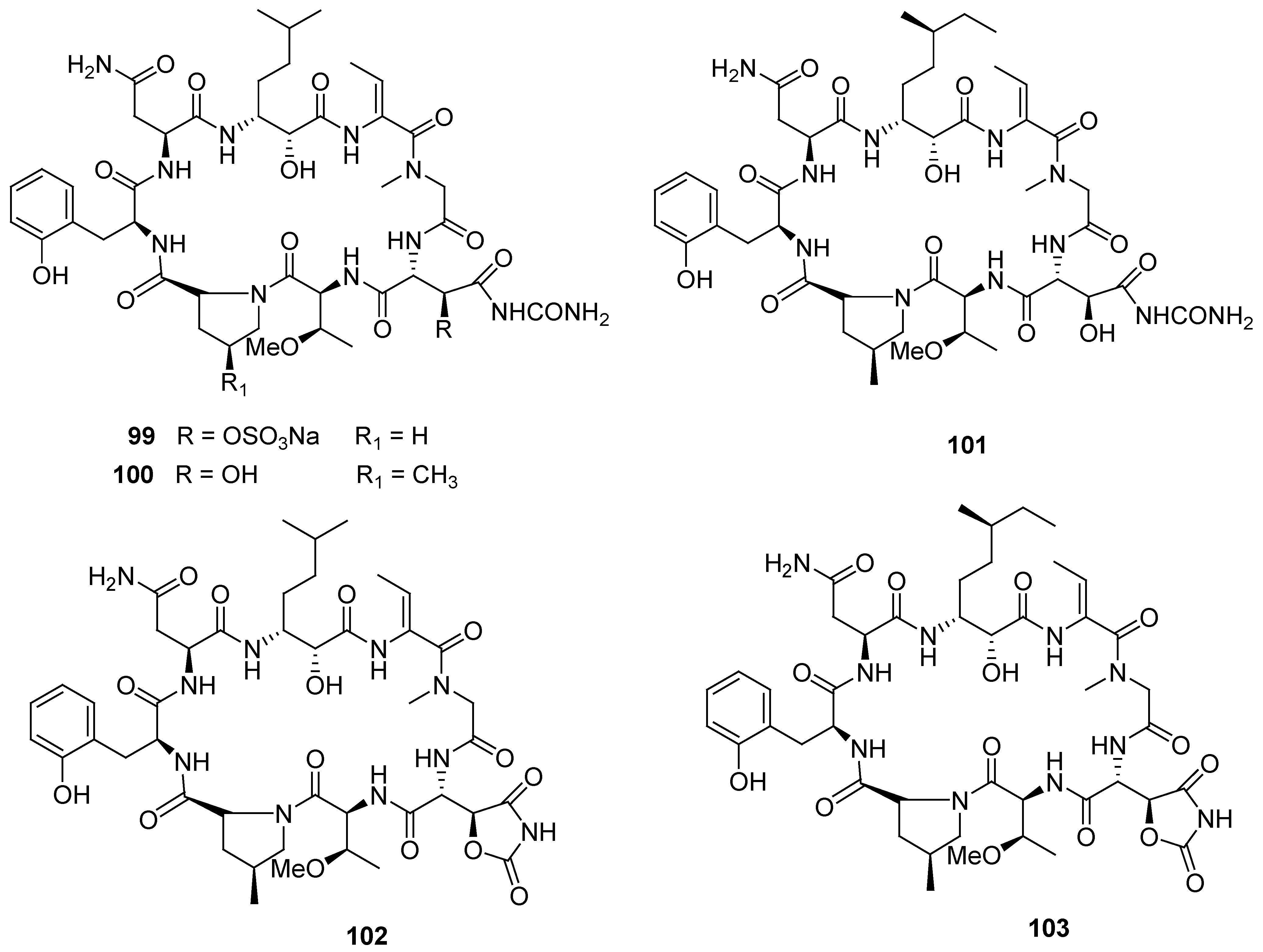
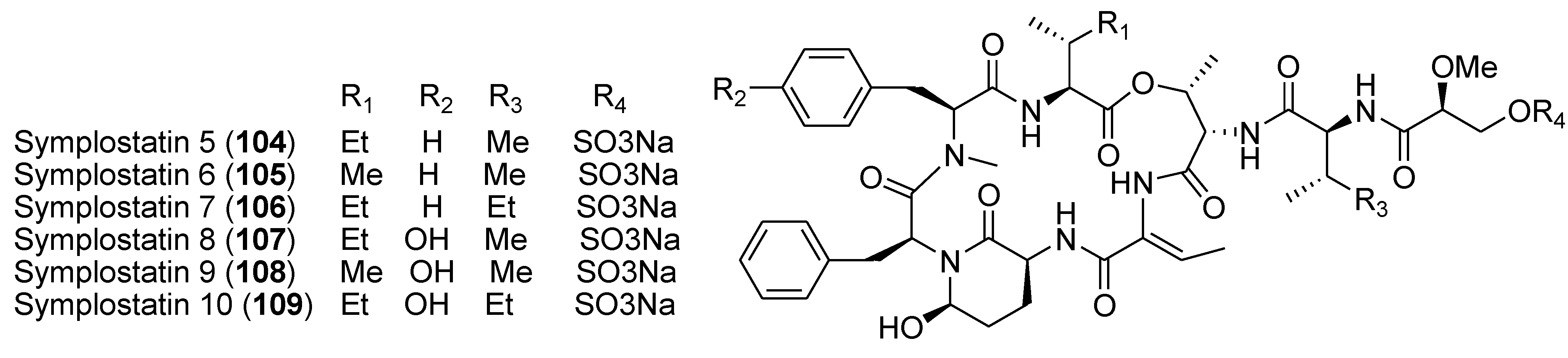
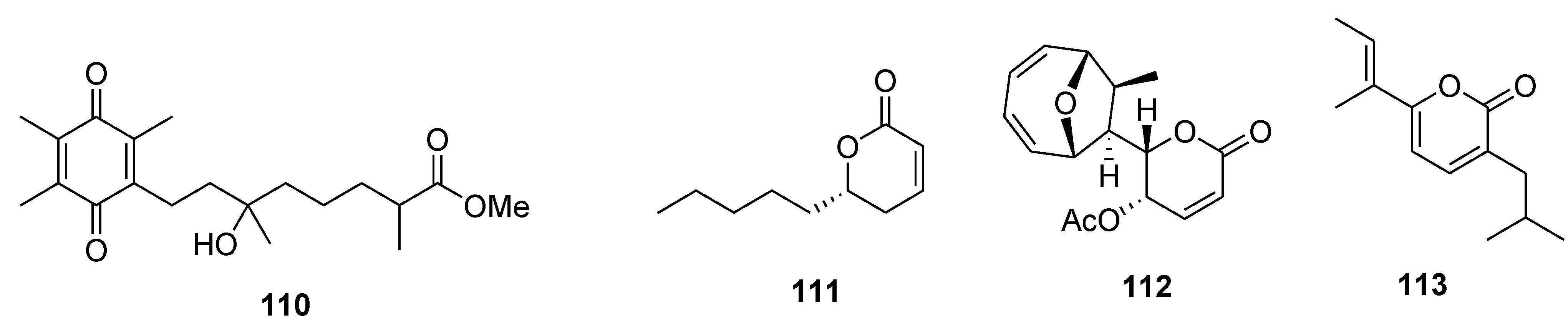
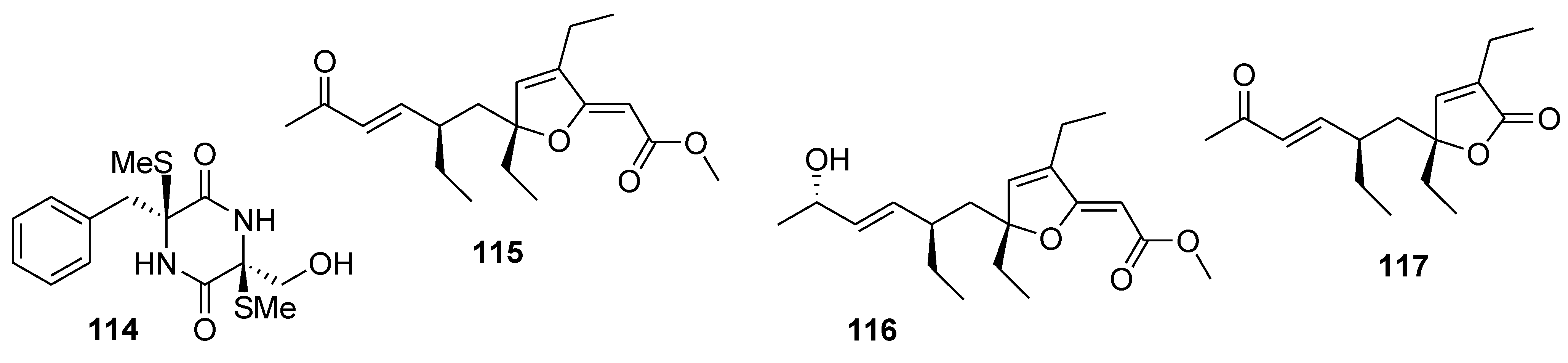
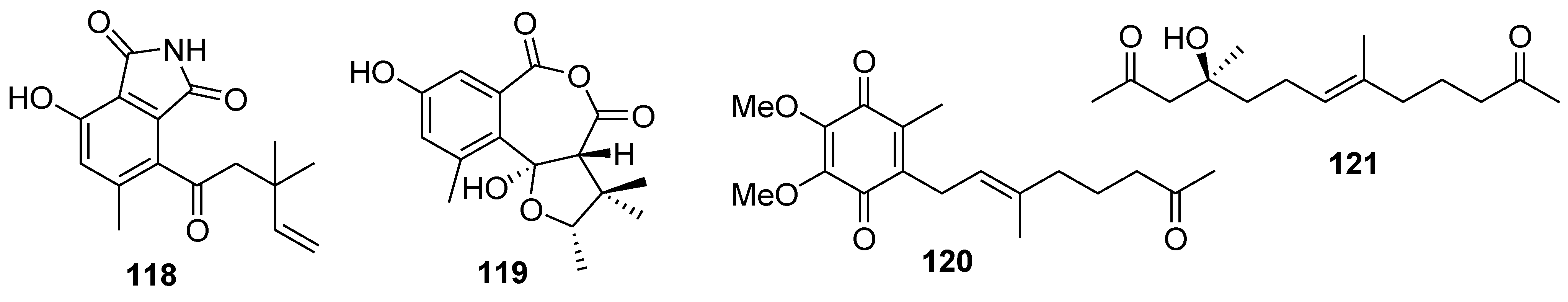
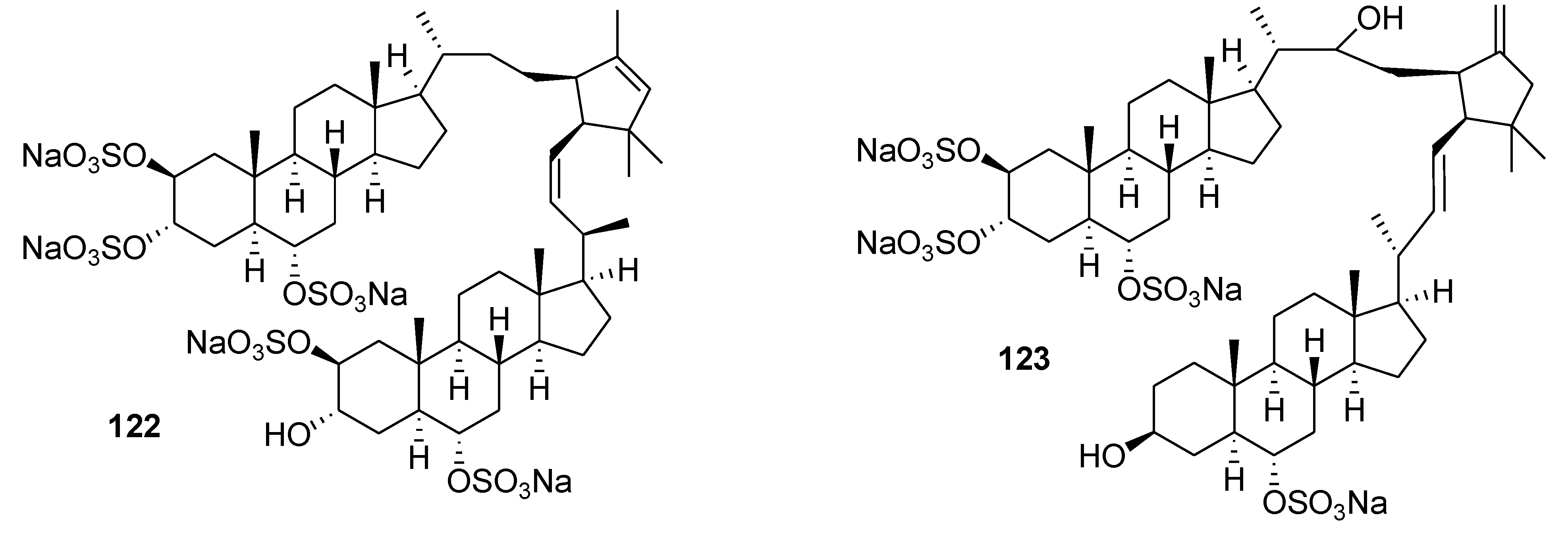
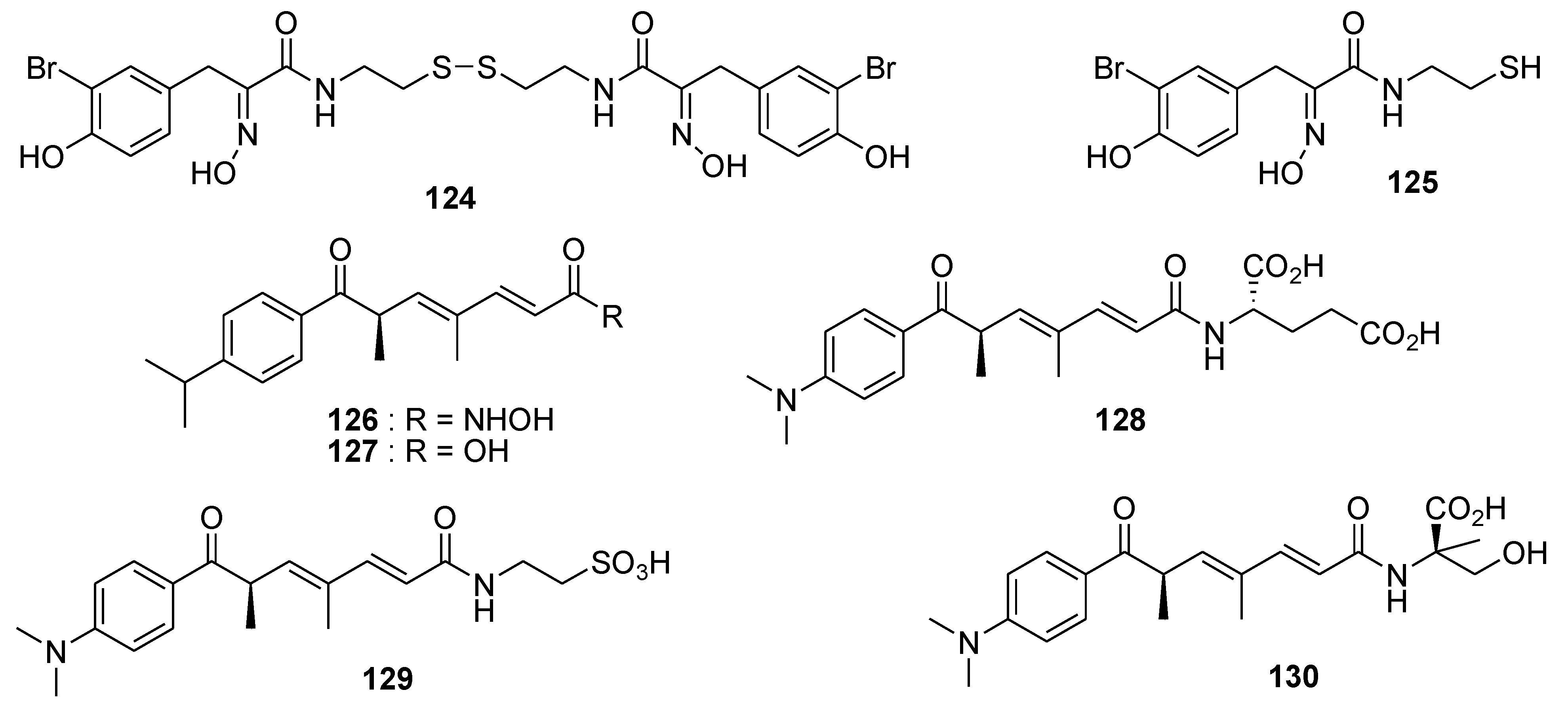
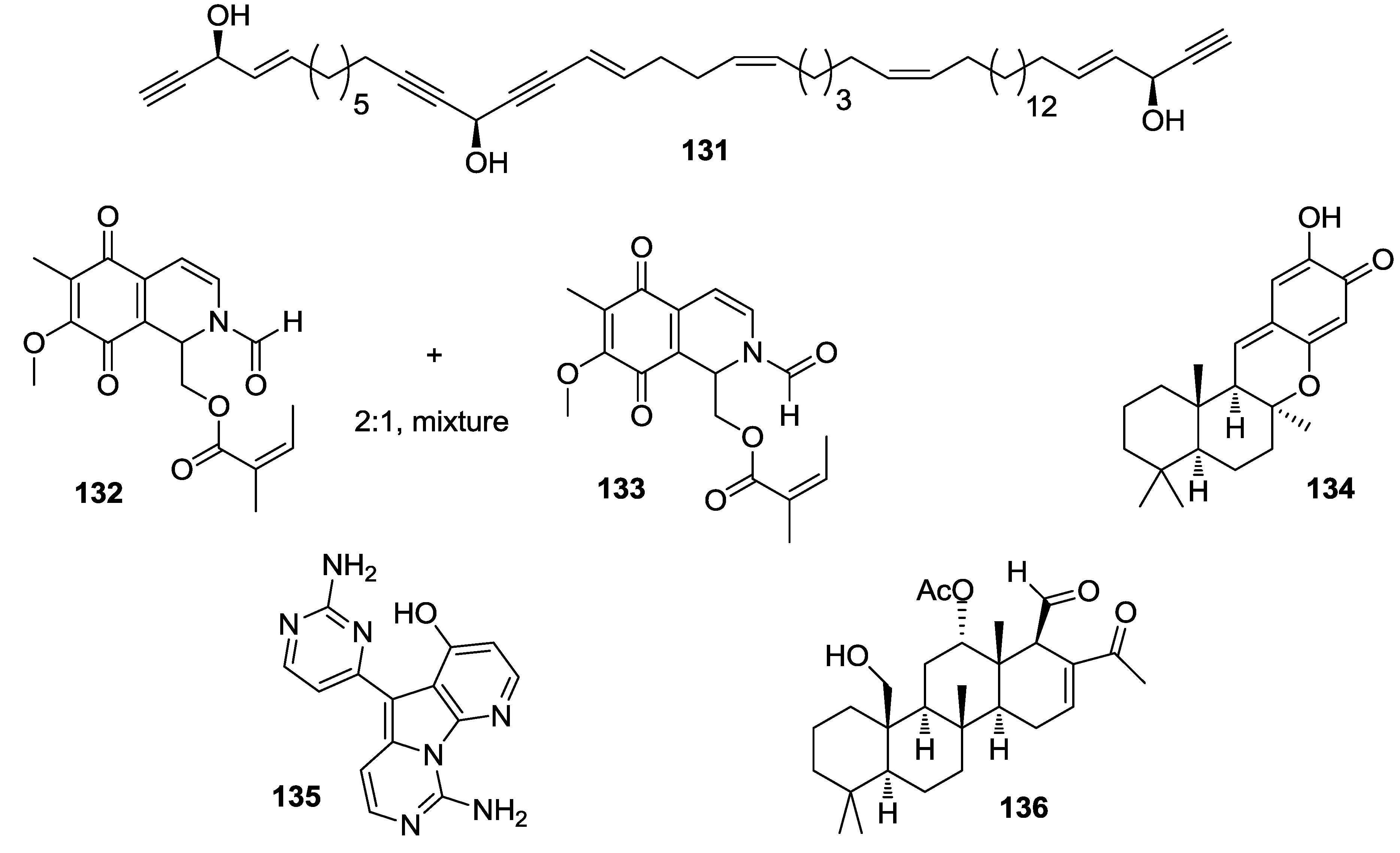
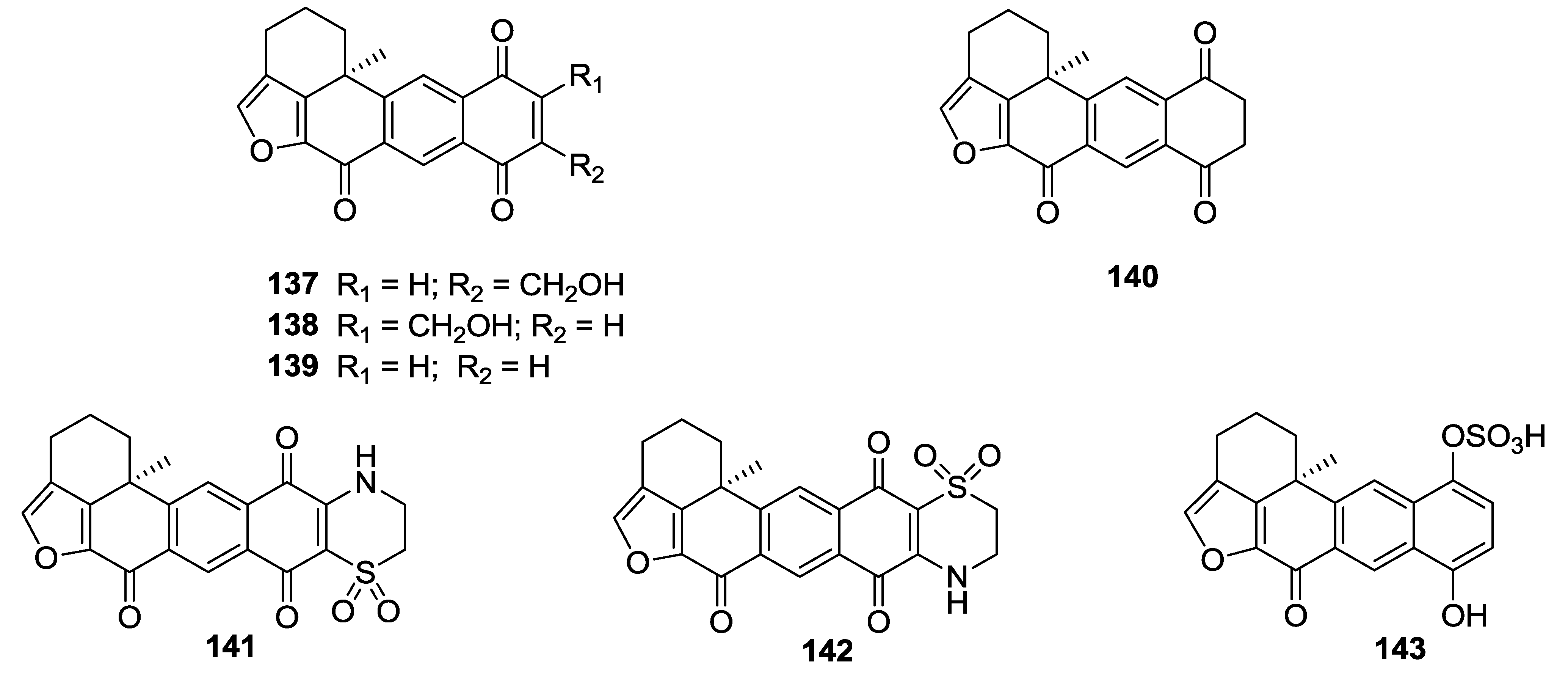
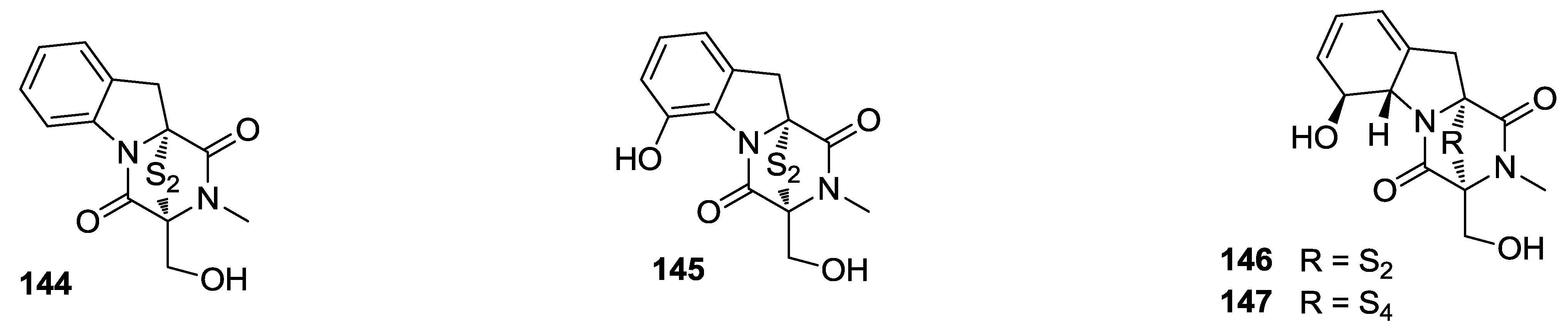
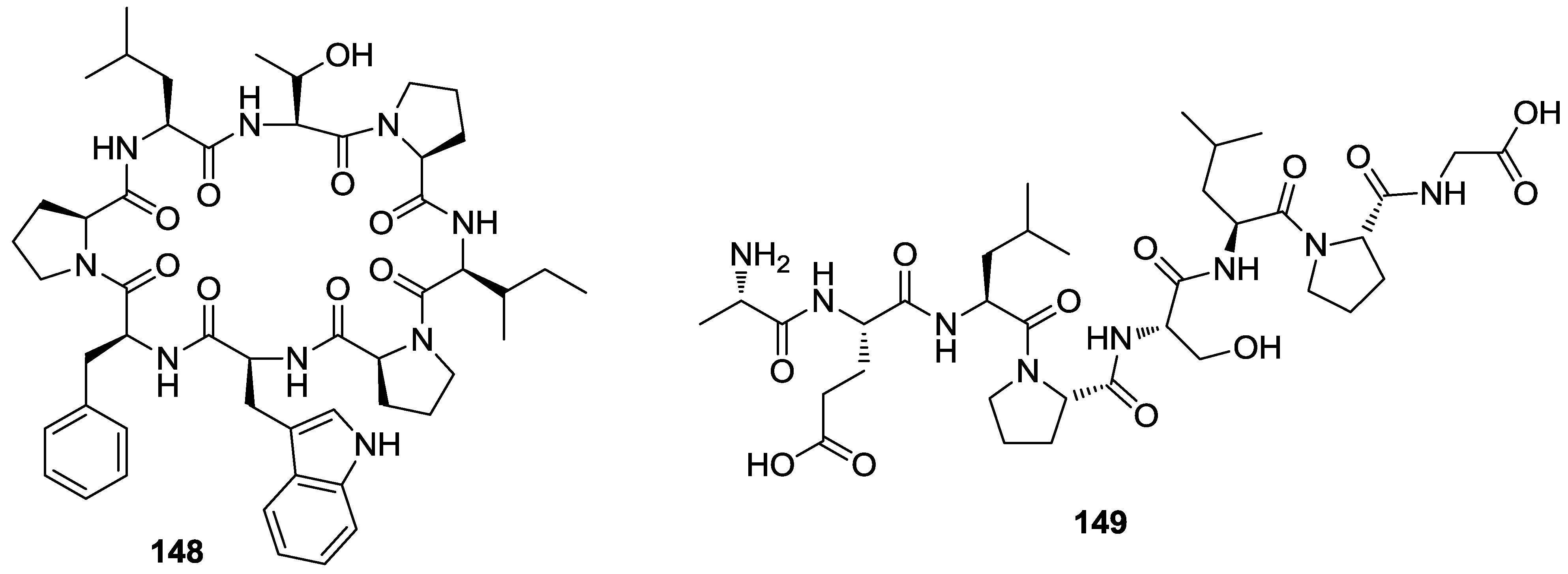
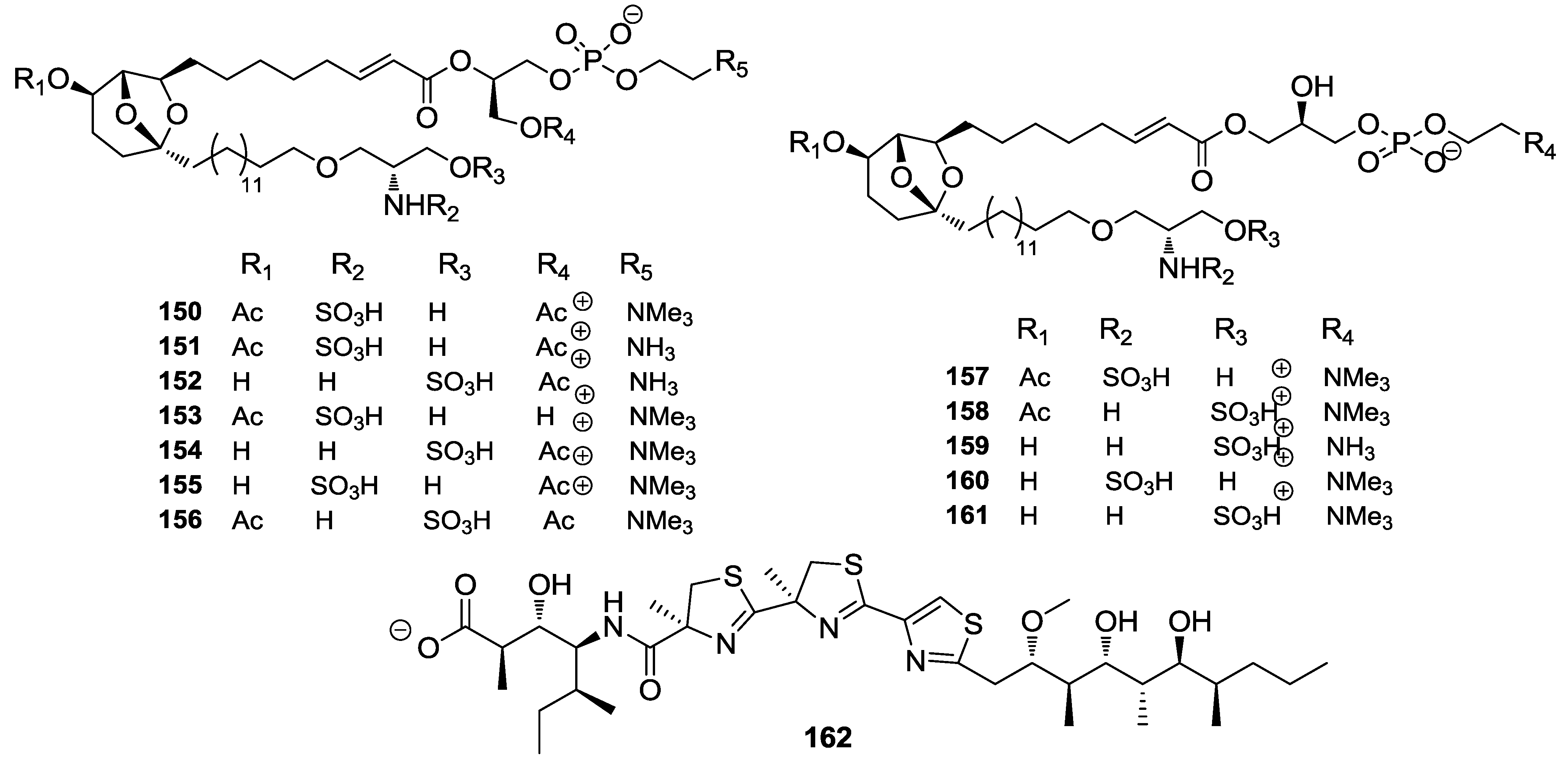
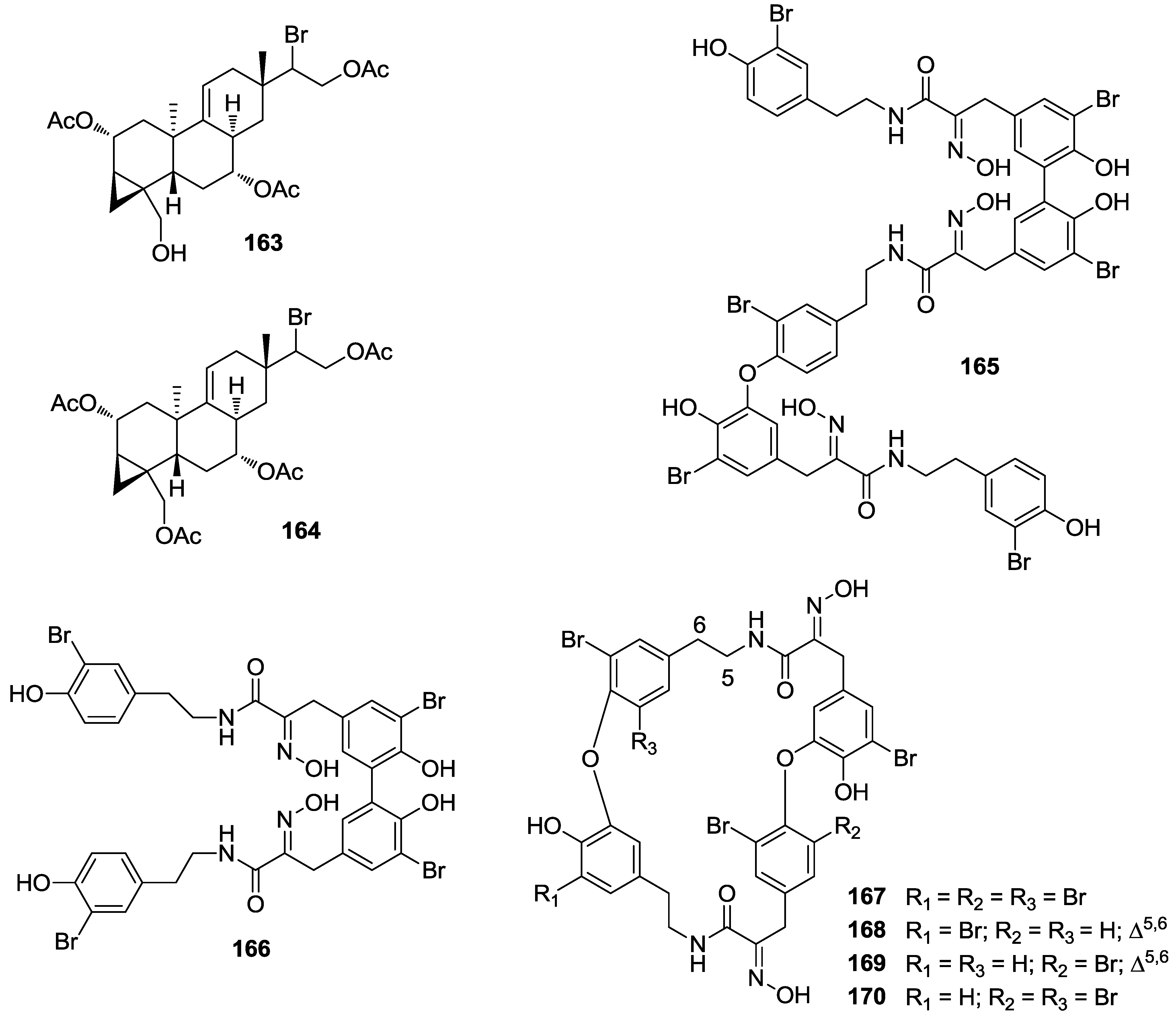
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Fujiki, H.; Suganuma, M.; Yatsunami, J.; Komori, A.; Okabe, S.; Nishiwaki-Matsushima, R.; Ohta, T. Significant marine natural products in cancer research. Gazz. Chim. Ital. 1993, 123, 309–316. [Google Scholar]
- Simmons, T.L.; Andrianasolo, E.; McPhail, K.; Flatt, P.; Gerwick, W.H. Marine natural products as anticancer drugs. Mol. Cancer Ther. 2005, 4, 333–342. [Google Scholar]
- Bhatnagar, I.; Kim, S.-K. Marine antitumor drugs: Status, shortfalls and strategies. Mar. Drugs 2010, 8, 2702–2720. [Google Scholar] [CrossRef]
- Schumacher, M.; Kelkel, M.; Dicato, M.; Diederich, M. Gold from the sea: Marine compounds as inhibitors of the hallmarks of cancer. Biotechnol. Adv. 2011, 29, 531–547. [Google Scholar] [CrossRef]
- Stonik, V.A.; Fedorov, S.N. Cancer preventive marine natural product. In Cellular and Genetic Practices for Translational Medicine; Kwak, J.-Y., Han, J.-Y., Eds.; Research Signpost: Karalla, India, 2011; pp. 1–36. [Google Scholar]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef]
- Vinothkumar, S.; Parameswaran, P.S. Recent advances in marine drug research. Biotechnol. Adv. 2013, 31, 1826–1845. [Google Scholar] [CrossRef]
- Sawadogo, W.R.; Schumacher, M.; Teiten, M.-H.; Cerella, C.; Dicato, M.; Diederich, M. A survey of marine natural compounds and their derivatives with anti-cancer activity reported in 2011. Molecules 2013, 18, 3641–3673. [Google Scholar] [CrossRef]
- Azmi, A.S.; Ahmad, A.; Banerjee, S.; Rangnekar, V.M.; Mohammad, R.M.; Sarkar, F.H. Chemoprevention of pancreatic cancer: characterization of Par-4 and its modulation by 3,3′-diindolylmethane (DIM). Pharm. Res. 2008, 25, 2117–2124. [Google Scholar] [CrossRef]
- Tsuda, H.; Ohshima, Y.; Nomoto, H.; Fujita, K.; Matsuda, E.; Iigo, M.; Takasuka, N.; Moore, M.A. Cancer prevention by natural compounds. Drug Metab. Pharmacokin. 2004, 19, 245–263. [Google Scholar] [CrossRef]
- Berenblum, I.; Armuth, V. Two independent aspects of tumor promotion. Biochim. Biophys. Acta 1981, 651, 51–63. [Google Scholar]
- Heidelberger, C.; Freeman, A.E.; Pienta, R.J.; Sivak, A.; Bertram, J.S.; Casto, B.C.; Dunkel, V.C.; Francis, M.W.; Kakunaga, T.; Little, J.B.; et al. Cell transformation by chemical agents—a review and analysis of the literature. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat. Res. 1983, 114, 283–385. [Google Scholar] [CrossRef]
- Sporn, M.B. Approaches to prevention of epithelial cancer during the preneoplastic period. Cancer Res. 1976, 36, 2699–2702. [Google Scholar]
- Umar, A.; Viner, J.L.; Hawk, E.T. The future of colon cancer prevention. Ann. N. Y. Acad. Sci. 2001, 952, 88–108. [Google Scholar] [CrossRef]
- Mehta, R.G.; Pezzuto, J.M. Discovery of cancer preventive agents from natural products: From plants to prevention. Curr. Oncol. Rep. 2002, 4, 478–486. [Google Scholar] [CrossRef]
- Hong, W.K.; Sporn, M.B. Recent advances in chemoprevention of cancer. Science 1997, 278, 1073–1077. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharm. Res. 2009, 59, 365–378. [Google Scholar] [CrossRef]
- Surh, Y.-J. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic compounds. Mutat. Res. 1999, 428, 305–327. [Google Scholar] [CrossRef]
- Pan, M.-H.; Ho, C.-T. Chemopreventive effects of natural dietary compounds on cancer development. Chem. Soc. Rev. 2008, 37, 2558–2574. [Google Scholar] [CrossRef]
- Cerella, C.; Sobolewski, C.; Dicato, M.; Diederich, M. Targeting COX-2 expression by natural compounds: A promising alternative strategy to synthetic COX-2 inhibitors for cancer chemoprevention and therapy. Biochem. Pharmacol. 2010, 80, 1801–1815. [Google Scholar] [CrossRef]
- von Schwarzenberg, K.; Vollmar, A.M. Targeting apoptosis pathways by natural compounds in cancer: Marine compounds as lead structures and chemical tools for cancer therapy. Cancer Lett. 2013, 332, 295–303. [Google Scholar] [CrossRef]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta 1799, 775–787. [Google Scholar]
- Bharate, S.B.; Sawant, S.D.; Pal Singh, P.; Vishwakarma, R.A. Kinase inhibitors of marine origin. Chem. Rev. 2013, 113, 6761–6815. [Google Scholar] [CrossRef]
- Muller, A.J.; DuHadaway, J.B.; Chang, M.Y.; Ramalingam, A.; Sutanto-Ward, E.; Boulden, J.; Soler, A.P.; Mandik-Nayak, L.; Gilmour, S.K.; Prendergast, G.C. Nonhematopoietic expression of IDO is integrally required for inflammatory tumor promotion. Cancer Immunol. Immunother. 2010, 59, 1655–1663. [Google Scholar] [CrossRef]
- Marks, P.A.; Richon, V.M.; Rifkind, R.A. Histone deacetylase inhibitors: Inducers of differentiation or apoptosis of transformed cells. J. Natl. Cancer Inst. 2000, 92, 1210–1216. [Google Scholar] [CrossRef]
- Simeone, A.-M.; Tari, A.M. How retinoids regulate breast cancer cell proliferation and apoptosis. Cell. Mol. Life Sci. 2004, 61, 1475–1484. [Google Scholar]
- Xia, Y.; Choi, H.-K.; Lee, K. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur. J. Med. Chem. 2012, 49, 24–40. [Google Scholar] [CrossRef]
- Nencioni, A.; Grunebach, F.; Patrone, F.; Ballestrero, A.; Brossart, P. Proteasome inhibitors: Antitumor effects and beyond. Leukemia 2007, 21, 30–36. [Google Scholar] [CrossRef]
- Zhang, C.; Kim, S.K. Matrix metalloproteinase inhibitors (MMPs) from marine natural products: The current situation and future prospects. Mar. Drugs 2009, 7, 71–84. [Google Scholar] [CrossRef]
- Yasui, Y.; Kim, M.; Tanaka, T. PPAR ligands for cancer chemoprevention. PPAR Res. 2008, 2008. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Krasokhin, V.B.; Shubina, L.K.; Dyshlovoy, S.A.; Nam, N.H.; Minh, C.V. The extracts of some marine invertebrates and algae collected off the coast of Vietnam induce the inhibitory effects on the Activator Protein-1 transcriptional activity in JB6 Cl41 cells. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Candela, C.G.; Lopez, L.; Kohen, V. Importance of a balanced omega 6/omega 3 ratio for the maintenance of health. Nutritional recommendations. Nutr. Hosp. 2011, 26, 323–329. [Google Scholar]
- Wendel, M.; Heller, A.R. Anticancer actions of omega-3 fatty acids-Current state and future perspectives. Anticancer Agents Med. Chem. 2009, 9, 457–470. [Google Scholar] [CrossRef]
- Hall, M.N.; Chavarro, J.E.; Lee, I.-M.; Willett, W.C.; Ma, J. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 1136–1143. [Google Scholar] [CrossRef]
- Cockbain, A.J.; Toogood, G.J.; Hull, M.A. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut 2012, 61, 135–149. [Google Scholar] [CrossRef]
- Murff, H.J.; Shrubsole, M.J.; Cai, Q.; Smalley, W.E.; Dai, Q.; Milne, G.L.; Ness, R.M.; Zheng, W. Dietary intake of PUFAs and colorectal polyp risk. Am. J. Clin. Nutr. 2012, 95, 703–712. [Google Scholar] [CrossRef]
- Spencer, M.; Finlin, B.S.; Unal, R.; Zhu, B.; Morris, A.J.; Shipp, L.R.; Lee, J.; Walton, R.G.; Adu, A.; Erfani, R.; et al. Omega-3 fatty acids reduce adipose tissue macrophages in human subjects with insulin resistance. Diabetes 2013, 62, 1709–1717. [Google Scholar] [CrossRef]
- Rosa, D.D.; Lourenco, F.C.; da Fonseca, A.C.M.; de Sales, R.L.; Ribeiro, S.M.R.; Neves, C.A.; Peluzio, M.C.G. Fish oil improves the lipid profile and reduces inflammatory cytokines in Wistar rats with precancerous colon lesions. Nutr. Cancer 2012, 64, 569–579. [Google Scholar] [CrossRef]
- Monk, J.M.; Jia, Q.; Callaway, E.; Weeks, B.; Alaniz, R.C.; McMurray, D.N.; Chapkin, R.S. Th17 cell accumulation is decreased during chronic experimental colitis by (n-3) PUFA in Fat-1 mice. J. Nutr. 2012, 142, 117–124. [Google Scholar] [CrossRef]
- Chiu, C.-Y.; Gomolka, B.; Dierkes, C.; Huang, N.R.; Schroeder, M.; Purschke, M.; Manstein, D.; Dangi, B.; Weylandt, K.H. Omega-6 docosapentaenoic acid-derived resolvins and 17-hydroxydocosahexaenoic acid modulate macrophage function and alleviate experimental colitis. Inflamm. Res. 2012, 61, 967–976. [Google Scholar] [CrossRef]
- Fasano, E.; Serini, S.; Piccioni, E.; Toesca, A.; Monego, G.; Cittadini, A.R.; Ranelletti, F.O.; Calviello, G. DHA induces apoptosis by altering the expression and cellular location of GRP78 in colon cancer cell lines. Biochim. Biophys. Acta 2012, 1822, 1762–1772. [Google Scholar] [CrossRef]
- Banskota, A.H.; Gallant, P.; Stefanova, R.; Melanson, R.; O’Leary, S.J.B. Monogalactosyldiacylglycerols, potent nitric oxide inhibitors from the marine microalga Tetraselmis chui. Nat. Prod. Res. 2013, 27, 1084–1090. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Takeuchi, T.; Rotinsulu, H.; Mangindaan, R.E.P.; van Soest, R.W.M.; Ukai, K.; Kobayashi, H.; Namikoshi, M.; Ohta, T.; Yokosawa, H. Leucettamol A: A new inhibitor of Ubc13-Uev1A interaction isolated from a marine sponge, Leucetta aff. Microrhaphis. Bioorg.Med. Chem. Lett. 2008, 18, 6319–6320. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; McGinn, P.J. New diacylglyceryltrimethyl-homoserines from the marine microalga Nannochloropsis granulata and their nitric oxide inhibitory activity. J. Appl. Phycol. 2013, 25, 1513–1521. [Google Scholar] [CrossRef]
- Tang, F.-Y. The silver bullet for cancer prevention: Chemopreventive effects of carotenoids. Bio. Med. 2012, 2, 117–121. [Google Scholar]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef]
- Nishino, H.; Tokuda, H.; Satomi, Y.; Masuda, M.; Bu, P.; Onozuka, M.; Yamaguchi, S.; Okuda, Y.; Takayasu, J.; Tsuruta, J.; et al. Cancer prevention by carotenoids. Pure Appl. Chem. 1999, 71, 2273–2278. [Google Scholar] [CrossRef]
- Nishino, H.; Murakoshi, M.; Ii, T.; Takemura, M.; Kuchide, M.; Kanazawa, M.; Mou, X.Y.; Wada, S.; Masuda, M.; Ohsaka, Y.; et al. Carotenoids in cancer chemoprevention. Cancer Metastasis Rev. 2002, 21, 257–264. [Google Scholar] [CrossRef]
- Amaro, H.M.; Barros, R.; Guedes, A.C.; Sousa-Pinto, I.; Malcata, F.X. Microalgal compounds modulate carcinogenesis in the gastrointestinal tract. Trends Biotechnol. 2013, 31, 92–98. [Google Scholar] [CrossRef]
- Mikami, K.; Hosokawa, M. Biosynthetic pathway and health benefits of fucoxanthin, an algae-specific xanthophyll in brown seaweeds. Int. J. Mol. Sci. 2013, 14, 13763–13781. [Google Scholar] [CrossRef]
- Abd El Baky, H.H.; El-Baroty, G.S. Healthy Benefit of Microalgal Bioactive Substances. J. Aquat. Sci. 2013, 1, 11–23. [Google Scholar]
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; de Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A treasure from the sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [CrossRef]
- Cantrell, A.; McGarvey, D.J.; Truscott, T.G., Rancan; Böhm, F. Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch. Biochem. Biophys. 2003, 412, 47–54. [Google Scholar] [CrossRef]
- Edge, R.; Land, E.J.; McGarvey, D.; Mulroy, L.; Truscott, T.G. Relative one-electron reduction potentials of carotenoid radical cations and the interactions of carotenoids with the vitamin E radical cation. J. Am. Chem. Soc. 1998, 120, 4087–4090. [Google Scholar]
- Halliwell, B. Free radicals, antioxidants, and human disease: Curiosity, cause or consequence? Lancet 1994, 344, 721–724. [Google Scholar] [CrossRef]
- Maoka, T.; Tokuda, H.; Suzuki, N.; Kato, H.; Etoh, H. Anti-oxidative, anti-tumor-promoting, and anti-carcinogensis activities of nitroastaxanthin and nitrolutein, the reaction products of astaxanthin and lutein with peroxynitrite. Mar. Drugs 2012, 10, 1391–1399. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef]
- Heo, S.-J.; Jeon, Y.-J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B 2009, 95, 101–107. [Google Scholar]
- Miyashita, K. Function of marine carotenoids. Forum Nutr. 2009, 61, 136–146. [Google Scholar] [CrossRef]
- Yasui, Y.; Hosokawa, M.; Mikami, N.; Miyashita, K.; Tanaka, T. Dietary astaxanthin inhibits colitis and colitis-associated colon carcinogenesis in mice via modulation of the inflammatory cytokines. Chem. Biol. Interact. 2011, 193, 79–87. [Google Scholar]
- Palozza, P.; Torelli, C.; Boninsegna, A.; Simone, R.; Catalano, A.; Mele, M.C.; Picci, N. Growth-inhibitory effects of the astaxanthin-rich alga Haematococcus pluvialis in human colon cancer cells. Cancer Lett. 2009, 283, 108–117. [Google Scholar] [CrossRef]
- Kim, K.N.; Heo, S.J.; Kang, S.M.; Ahn, G.; Jeon, Y.J. Fucoxanthin induces apoptosis in human leukemia HL-60 cells through a ROS-mediated Bcl-xL pathway. Toxicol. in Vitro 2010, 24, 1648–1654. [Google Scholar] [CrossRef]
- Yu, R.X.; Hu, X.M.; Xu, S.Q.; Jiang, Z.J.; Yang, W. Effects of fucoxanthin on proliferation and apoptosis in human gastric adenocarcinoma MGC-803 cells via JAK/STAT signal pathway. Eur. J. Pharmacol. 2011, 657, 10–19. [Google Scholar] [CrossRef]
- Satomi, Y. Fucoxanthin induces GADD45A expression and G1 arrest with SAPK/JNK activation in LNCap human prostate cancer cells. Anticancer Res. 2012, 32, 807–813. [Google Scholar]
- Yamamoto, K.; Ishikawa, C.; Katano, H.; Yasumoto, T.; Mori, N. Fucoxanthin and its deacetylated product, fucoxanthinol, induce apoptosis of primary effusion lymphomas. Cancer Lett. 2011, 300, 225–234. [Google Scholar] [CrossRef]
- Ganesan, P.; Noda, K.; Manabe, Y.; Ohkubo, T.; Tanaka, Y.; Maoka, T.; Sugawara, T.; Hirata, T. Siphonaxanthin, a marine carotenoid from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. Biochim. Biophys. Acta 2011, 1810, 497–503. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Stonik, V.A. Steroid glycosides from marine organisms. Steroids 2011, 76, 425–454. [Google Scholar] [CrossRef]
- Stonik, V.A.; Kalinin, V.I.; Avilov, S.A. Toxins from sea cucumbers (holothuroids): Chemical structures, properties, taxonomic distribution, biosynthesis, and evolution. J. Nat. Toxins 1999, 8, 235–248. [Google Scholar]
- Kalinin, V.I.; Aminin, D.L.; Avilov, S.A.; Silchenko, A.S.; Stonik, V.A. Triterpene glycosides from sea cucumbers (Holothurioidea, Echinodermata), biological activities and functions. In Studies in Natural Product Chemistry; ur Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 135–196. [Google Scholar]
- Aminin, D.L.; Pislyagin, E.A.; Menchinskaya, E.S.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Immunomodulatory and Anticancer Activity of Sea Cucumber Triterpene Glycosides. In Studies in Natural Products Chemistry; ur Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 73–91. [Google Scholar]
- Aminin, D.L.; Agafonova, I.G.; Berdyshev, E.V.; Isachenko, E.G.; Avilov, S.A.; Stonik, V.A. Immunomodulatory properties of cucumariosides from the edible Far-Eastern holothurian Cucumaria japonica. J. Med. Food 2001, 4, 127–135. [Google Scholar] [CrossRef]
- Aminin, D.L.; Pinegin, B.V.; Pichugina, L.V.; Zaporozhets, T.S.; Agafonova, I.G.; Boguslavski, V.M.; Silchenko, A.S.; Avilov, S.A.; Stonik, V.A. Immunomodulatory properties of cumaside. Int. Immunopharm. 2006, 6, 1070–1082. [Google Scholar] [CrossRef]
- Aminin, D.L.; Chaikina, E.L.; Agafonova, I.G.; Avilov, S.A.; Kalinin, V.I.; Stonik, V.A. Antitumor activity of the immunomodulatory lead cumaside. Int. Immunopharm. 2010, 10, 648–654. [Google Scholar] [CrossRef]
- Menchinskaya, E.S.; Pislyagin, E.A.; Kovalchyk, S.N.; Davydova, V.N.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I.; Aminin, D.L. Antitumor Activity of Cucumarioside A2-2. Chemotherapy 2013, 59, 181–191. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Menchinskaya, E.S.; Aminin, D.L.; Kalinin, V.I. Structure of cucumarioside I2 from the sea cucumber Eupentacta fraudatrix (Djakonov et Baranova) and cytotoxic and immunostimulatory activities of this saponin and relative compounds. Nat. Prod. Res. 2013, 27, 1776–1783. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and biological action of cucumariosides A1, A3, A4, A5, A6, A12 and A15, seven new minor non-sulfated tetraosides and unprecedented 25-keto, 27-norholostane aglycone. Nat. Prod. Commun. 2012, 7, 517–525. [Google Scholar]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and cytotoxic action of cucumariosides A2, A7, A9, A10, A11, A13 and A14, seven new minor non-sulfated tetraosides and an aglycone with an uncommon 18-hydroxy group. Nat. Prod. Commun. 2012, 7, 845–852. [Google Scholar]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and biological activity of cucumariosides B1 and B2, two new minor non-sulfated unprecedented triosides. Nat. Prod. Commun. 2012, 7, 1157–1162. [Google Scholar]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I.; Jayasandhya, P.; Rajan, G.C.; Padmakumar, K.P. Structures and biological activities of typicosides A1, A2, B1, C1 and C2, triterpene glycosides from the sea cucumber Actinocucumis typical. Nat. Prod. Commun. 2013, 8, 301–310. [Google Scholar]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and biological action of cucumariosides I1, I3, I4, three new minor disulfated pentaosides. Nat. Prod. Commun. 2013, 8, 1053–1058. [Google Scholar]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Dolmatov, I.Yu.; Kalinin, V.I.; Stonik, V.A. Structure and biological action of cladolosides B1, B2, C, C1, C2 and D, six new triterpene glycosides from the sea cucumber Cladolabes schmeltzii. Nat. Prod. Commun. 2013, 8, 1527–1534. [Google Scholar]
- Menchinskaya, E.S.; Aminin, D.L.; Avilov, S.A.; Silchenko, A.S.; Andryjashchenko, P.V.; Kalinin, V.I.; Stonik, V.A. Inhibition of tumor cells multidrug resistance by cucumarioside A2-2, frondoside A and their complexes with cholesterol. Nat. Prod. Commun. 2013, 8, 1377–1380. [Google Scholar]
- Yun, S.-H.; Park, E.-S.; Shin, S.-W.; Na, Y.-W.; Han, J.-Y.; Jeong, J.-S.; Shastina, V.V.; Stonik, V.A.; Park, J.-I.; Kwak, J.-Y. Stichoposide C induces apoptosis through the generation of ceramide in leukemia and colorectal cancer cells and shows in vivo antitumor activity. Clin. Cancer Res. 2012, 18, 5934–5948. [Google Scholar] [CrossRef]
- Fujiki, H.; Cope, F.O.; Issinger, O.G. Sarcophytol-A, a potent tumor promoter inhibitor, prevents phosphorylation of a proteolytic fragment of C23 induced by the non-TPA type tumor promoter, okadaic acid. Proc. Am. Assoc. Cancer Res. 1988, 29, 155–155. [Google Scholar]
- Fujiki, H.; Suganuma, M.; Suguri, H.; Yoshizawa, S.; Takagi, K.; Kobayashi, M. Sarcophytol-A and sarcophytol-B inhibit tumor promotion by teleocidin in 2-stage carcinogenesis in mouse skin. J. Cancer Res. Clin. Oncol. 1989, 115, 25–28. [Google Scholar] [CrossRef]
- Wei, H.; Frenkel, K. Suppression of tumor promoter-induced oxidative events and DNA damage in vivo by sarcophytol A: A possible mechanism of antipromotion. Cancer Res. 1992, 52, 2298–2303. [Google Scholar]
- Fujiki, H.; Suganuma, M.; Komori, A.; Yatsunami, J.; Okabe, S.; Ohta, T.; Sueoka, E. A new tumor promotion pathway and its inhibitors. Cancer Det. Prev. 1994, 18, 1–7. [Google Scholar]
- Bhimani, R.S.; Troll, W.; Grunberger, D.; Frenkel, K. Inhibition of oxidative stress in HeLa cells by chermopreventive agents. Cancer Res. 1993, 53, 4528–4533. [Google Scholar]
- Yokomatsu, H.; Satake, K.; Hiura, A.; Tsutsumi, M.; Suganuma, M. Sarcophytol-A—a new chemotherapeutic and chemopreventive agent for pancreatic-cancer. Pancreas 1994, 9, 526–530. [Google Scholar] [CrossRef]
- Narisawa, T.; Takahashi, M.; Niwa, M.; Fukaura, Y.; Fujiki, H. Inhibition of methylnitrosourea-induced large bowel-cancer development in rats by sarcophytol A, a product from a marine soft coral Sarcophyton glaucum. Cancer Res. 1989, 49, 3287–3289. [Google Scholar]
- Yamauchi, O.; Omori, M.; Ninomiya, M.; Okuno, M.; Moriwaki, H.; Suganuma, M.; Fujiki, H.; Muto, Y. Inhibitory effect of sarcophytol-A on development of spontaneous hepatomas in mice. Jpn. J. Cancer Res. 1992, 82, 1234–1238. [Google Scholar]
- Hegazy, M.-E.F.; Gamal Eldeen, A.M.; Shahat, A.A.; Abdel-Latif, F.F.; Mohamed, T.A.; Whittlesey, B.R.; Paré, P.W. Bioactive hydroperoxyl cembranoids from the Red Sea soft coral Sarcophyton glaucum. Mar. Drugs 2012, 10, 209–222. [Google Scholar] [CrossRef]
- Albizat, K.F.; Holman, T.; Faulkner, D.J.; Glaser, K.B.; Jacobs, R.S. Luffariellolide, an anti-inflammatory sesterterpene from the marine sponge Luffariella sp. Experientia 1987, 45, 388–390. [Google Scholar]
- Wang, S.; Wang, Z.; Lin, S.; Zheng, W.; Wang, R.; Jin, S.; Chen, J.; Jin, L.; Li, Y. Revealing a natural marine product as a novel agonist for retinoic acid receptors with a unique binding mode and inhibitory effects on cancer cells. Biochem. J. 2012, 446, 79–87. [Google Scholar] [CrossRef]
- Liu, W.K.; Ling, Y.H.; Cheung, F.W.K.; Che, C.-T. Stellettin A induces endoplasmic reticulum stress in murine B16 melanoma cells. J. Nat. Prod. 2012, 75, 586–590. [Google Scholar] [CrossRef]
- Zhu, H.; Hua, X.; Gong, T.; Pang, J.; Hou, Q.; Zhu, P. Hypocreaterpenes A and B, cadinane-type sesquiterpenes from a marine-derived fungus, Hypocreales sp. Phytochem. Lett. 2013, 6, 392–396. [Google Scholar] [CrossRef]
- de los Reyes, C.; Zbakh, H.; Motilva, V.; Zubía, E. Antioxidant and anti-inflammatory meroterpenoids from the brown alga Cystoseira usneoides. J. Nat. Prod. 2013, 76, 621–629. [Google Scholar] [CrossRef]
- Wang, W.; Lee, Y.; Lee, T.G.; Mun, B.; Giri, A.G.; Lee, J.; Kim, H.; Hahn, D.; Yang, I.; Chin, J.; et al. Phorone A and isophorbasone A, sesterterpenoids isolated from the marine sponge Phorbas sp. Org. Lett. 2012, 14, 4486–4489. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kubota, T.; Yamamoto, S.; Kobayashi, J. Inhibitory effects of metachromins L–Q and its related analogs against receptor tyrosine kinases EGFR and HER2. Bioorg. Med. Chem. Lett. 2013, 23, 117–118. [Google Scholar] [CrossRef]
- Li, J.; Zhu, H.; Ren, J.; Deng, Z.; de Voogd, N.J.; Proksch, P.; Lin, W. Globostelletins J–S, isomalabaricanes with unusual cyclopentane sidechains from the marine sponge Rhabdastrella globostellata. Tetrahedron 2012, 68, 559–565. [Google Scholar] [CrossRef]
- Shubina, L.K.; Kalinovsky, A.I.; Fedorov, S.N.; Radchenko, O.S.; Denisenko, V.A.; Dmitrenok, P.S.; Dyshlovoy, S.A.; Krasokhin, V.B.; Stonik, V.A. Aaptamine alkaloids from the Vietnamese sponge Aaptos sp. Nat. Prod. Commun. 2009, 4, 1085–1088. [Google Scholar]
- Shubina, L.K.; Makarieva, T.N.; Dyshlovoy, S.A.; Fedorov, S.N.; Dmitenok, P.S.; Stonik, V.A. Three new aaptamines from the marine sponge Aaptos sp. and their proapoptotic properties. Nat. Prod. Commun. 2010, 5, 1881–1884. [Google Scholar]
- Utkina, N.K. Antioxidant activity of aromatic alkaloids from the marine sponges Aaptos aaptos and Hyrtios sp. Chem. Nat. Comp. 2009, 45, 849–853. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Naeth, I.; Venz, S.; Preukschas, M.; Sievert, H.; Jacobsen, C.; Shubina, L.K.; Salazar, M.G.; Scharf, C.; Walther, R.; et al. Proteomic profiling of germ cell cancer cells treated with aaptamine, a marine alkaloid with antiproliferative activity. J. Proteome Res. 2012, 11, 2316–2330. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Venz, S.; Shubina, L.K.; Fedorov, S.N.; Walther, R.; Jacobsen, C.; Stonik, V.A.; Bokemeyer, C.; Balabanov, S.; Honecker, F. Activity of aaptamine and two derivatives, demethyloxyaaptamine and isoaaptamine, in cisplatin-resistant germ cell cancer. J. Proteomics 2014, 96, 223–239. [Google Scholar] [CrossRef]
- Yamazaki, H.; Wewengkang, D.S.; Kanno, S.; Ishikawa, M.; Rotinsulu, H.; Mangindaan, R.E.P.; Namikoshi, M. Papuamine and haliclonadiamine, obtained from an Indonesian sponge Haliclona sp., inhibited cell proliferation of human cancer cell lines. Nat. Prod. Res. 2013, 27, 1012–1015. [Google Scholar] [CrossRef]
- Fu, P.; Yang, C.; Wang, Y.; Liu, P.; Ma, Y.; Xu, L.; Su, M.; Hong, K.; Zhu, W. Streptocarbazoles A and B, two novel indolocarbazoles from the marine-derived actinomycete strain Streptomyces sp. FMA. Org. Lett. 2012, 14, 2422–2425. [Google Scholar] [CrossRef]
- Johnson, T.A.; Sohn, J.; Vaske, Y.M.; White, K.N.; Cohen, T.L.; Vervoort, H.C.; Tenney, K.; Valeriote, F.A.; Bjeldanes, L.F.; Crews, P. Myxobacteria versus sponge-derived alkaloids: The bengamide family identified as potent immune modulating agents by scrutiny of LC–MS/ELSD libraries. Bioorg. Med. Chem. 2012, 20, 4348–4355. [Google Scholar] [CrossRef]
- Hwang, B.S.; Oh, J.S.; Jeong, E.J.; Sim, C.J.; Rho, J.-R. Densanins A and B, new macrocyclic pyrrole alkaloids isolated from the marine sponge Haliclona densaspicula. Org. Lett. 2012, 14, 6154–6157. [Google Scholar] [CrossRef]
- Sakai, E.; Kato, H.; Rotinsulu, H.; Losung, F.; Mangindaan, R.E.P.; de Voogd, N.J.; Yokosawa, H.; Tsukamoto, S. Variabines A and B: New β-carboline alkaloids from the marine sponge Luffariella variabilis. J. Nat. Med. 2014, 68, 215–219. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Miyazaki, M.; Kodrasov, M.P.; Rotinsulu, H.; Losung, F.; Mangindaan, R.E.P.; de Voogd, N.J.; Yokosawa, H.; Nicholson, B.; Tsukamoto, S. Spongiacidin C, a pyrrole alkaloid from the marine sponge Stylissa massa, functions as a USP7 inhibitor. Bioorg. Med. Chem. Lett. 2013, 23, 3884–3886. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Dyshlovoy, S.А.; Shubina, L.K.; Guzii, A.G.; Kuzmich, A.S.; Makarieva, T.N. C11 cyclopentenone from the ascidian Diplosoma sp. prevents epidermal growth factor-induced transformation of JB6 cells. Drugs Ther. Stud. 2012, 2, e4. [Google Scholar]
- Williams, D.E.; Dalisay, D.S.; Li, F.; Amphlett, J.; Maneerat, W.; Chavez, M.A.G.; Wang, Y.A.; Matainaho, T.; Yu, W.; Brown, P.J.; et al. Nahuoic acid A produced by a Streptomyces sp. isolated from a marine sediment is a selective SAM-competitive inhibitor of the histone metyltransferase SETD8. Org. Lett. 2013, 15, 414–417. [Google Scholar] [CrossRef]
- Takawa, M.; Cho, H.S.; Hayami, S.; Toyokawa, G.; Kogure, M.; Yamane, Y.; Iwai, Y.; Maejima, K.; Ueda, K.; Masuda, A.; et al. Histone lysine methyltransferase SETD8 promotes carcinogenesis by deregulating PCNA expression. Cancer Res. 2012, 72, 3217–3227. [Google Scholar] [CrossRef]
- Asami, Y.; Jang, J.-H.; Soung, N.-K.; He, L.; Moon, D.O.; Kim, J.W.; Oh, H.; Muroi, M.; Osada, H.; Kim, B.Y.; et al. Protuboxepin A, a marine fungal metabolite, inducing metaphase arrest and chromosomal misalignment in tumor cells. Bioorg. Med. Chem. 2012, 20, 3799–3806. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Fedorov, S.N.; Kalinovsky, A.I.; Shubina, L.K.; Bokemeyer, C.; Stonik, V.A.; Honecker, F. Mycalamide A shows cytotoxic properties and prevents EGF-induced neoplastic transformation through inhibition of nuclear factors. Mar. Drugs 2012, 10, 1212–1224. [Google Scholar] [CrossRef]
- Shirouzu, T.; Watari, K.; Ono, M.; Koizumi, K.; Saiki, I.; Tanaka, C.; van Soest, R.W.M.; Miyamoto, T. Structure, synthesis, and biological activity of a C-20 bisacetylenic alcohol from a marine sponge Callyspongia sp. J. Nat. Prod. 2013, 76, 1337–1342. [Google Scholar] [CrossRef]
- Garcia-Caballero, M.; Mari-Beffa, M.; Canedo, L.; Medina, M.A.; Quesada, A.R. Toluquinol, a marine fungus metabolite, is a new angiosuppresor that interferes the Akt pathway. Biochem. Pharmacol. 2013, 85, 1727–1740. [Google Scholar] [CrossRef]
- Towle, K.M.; Chaytor, J.L.; Liu, H.; Austin, P.; Roberge, M.; Roskelley, C.D.; Vederas, J.C. Synthesis and biological studies of neopetrosiamides as inhibitors of cancer cell invasion. Org. Biomol. Chem. 2013, 11, 1476–1481. [Google Scholar] [CrossRef]
- Igarashi, Y.; Asano, D.; Furihata, K.; Oku, N.; Miyanaga, S.; Sakurai, H.; Saiki, I. Absolute configuration of pterocidin, a potent inhibitor of tumor cell invasion from a marine-derived Streptomyces. Tetrahedron Lett. 2012, 53, 654–656. [Google Scholar] [CrossRef]
- Um, S.; Kim, Y.-J.; Kwon, H.; Wen, H.; Kim, S.-H.; Kwon, H.C.; Park, S.; Shin, J.; Oh, D.-C. Sungsanpin, a Lasso Peptide from a Deep-Sea Streptomycete. J. Nat. Prod. 2013, 76, 873–879. [Google Scholar] [CrossRef]
- Pimentel, A.A.; Felibertt, P.; Sojo, F.; Colman, L.; Mayora, A.; Silva, M.L.; Rojas, H.; Dipolo, R.; Suarez, A.I.; Compagnone, R.S.; et al. The marine sponge toxin agelasine B increases the intracellular Ca2+ concentration and induces apoptosis in human breast cancer cells (MCF-7). Cancer Chemother. Pharmacol. 2012, 69, 71–83. [Google Scholar] [CrossRef]
- Huang, C.; Jin, H.; Song, B.; Zhu, X.; Zhao, H.; Cai, J.; Lu, Y.; Chen, B.; Lin, Y. The cytotoxicity and anticancer mechanisms of alterporriol L, a marine bianthraquinone, against MCF-7 human breast cancer cells. Appl. Microbiol. Biotechnol. 2012, 93, 777–785. [Google Scholar] [CrossRef]
- Ohno, O.; Morita, M.; Kitamura, K.; Teruya, T.; Yoneda, K.; Kita, M.; Kigoshi, H.; Suenaga, K. Apoptosis-inducing activity of the actin-depolymerizing agent aplyronine A and its side-chain derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 1467–1471. [Google Scholar] [CrossRef]
- Morita, M.; Ohno, O.; Teruya, T.; Yamori, T.; Inuzuka, T.; Suenaga, K. Isolation and structures of biselyngbyasides B, C, and D from the marine cyanobacterium Lyngbya sp., and the biological activities of biselyngbyasides. Tetrahedron 2012, 68, 5984–5990. [Google Scholar]
- Park, S.J.; Jeon, Y.J. Dieckol from Ecklonia cava suppresses the migration and invasion of HT1080 cells by inhibiting the focal adhesion kinase pathway downstream of Rac1-ROS signaling. Mol. Cells 2012, 33, 141–149. [Google Scholar] [CrossRef]
- Maschek, J.A.; Mevers, E.; Diyabalanage, T.; Chen, L.; Ren, Y.; McClintock, J.B.; Amsler, C.D.; Wu, J.; Baker, B.J. Palmadorin chemodiversity from the antarctic nudibranch Austrodoris kerguelenensis and inhibition of Jak2/STAT5-dependent HEL leukemia cells. Tetrahedron 2012, 68, 9095–9104. [Google Scholar] [CrossRef]
- Park, E.-J.; Pezzuto, J.M.; Jang, K.H.; Nam, S.-J.; Bucarey, S.A.; Fenical, W. Suppression of nitric oxide synthase by thienodolin in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. Nat. Prod. Commun. 2012, 7, 789–794. [Google Scholar]
- Williams, D.E.; Steino, A.; de Voogd, N.J.; Mauk, A.G.; Andersen, R.J. Halicloic acids A and B isolated from the marine sponge Haliclona sp. collected in the Philippines inhibit indoleamine 2,3-dioxygenase. J. Nat. Prod. 2012, 75, 1451–1458. [Google Scholar] [CrossRef]
- Balunas, M.J.; Grosso, M.F.; Villa, F.A.; Engene, N.; McPhail, K.L.; Tidgewell, K.; Pineda, L.M.; Gerwick, L.; Spadafora, C.; Kyle, D.E.; et al. Coibacins A–D, antileishmanial marine cyanobacterial polyketides with intriguing biosynthetic origins. Org. Lett. 2012, 14, 3878–3881. [Google Scholar] [CrossRef]
- Festa, C.; De Marino, S.; D’Auria, M.V.; Monti, M.C.; Bucci, M.; Vellecco, V.; Debitus, C.; Zampella, A. Anti-inflammatory cyclopeptides from the marine sponge Theonella swinhoei. Tetrahedron 2012, 68, 2851–2857. [Google Scholar] [CrossRef]
- Salvador, L.A.; Taori, K.; Biggs, J.S.; Jakoncic, J.; Ostrov, D.A.; Paul, V.J.; Luesch, H. Potent elastase inhibitors from cyanobacteria: Structural basis and mechanisms mediating cytoprotective and anti-inflammatory effects in bronchial epithelial cells. J. Med. Chem. 2013, 56, 1276–1290. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Kuo, C.-Y.; Wen, Z.-H.; Lin, Y.-Y.; Wang, W.-H.; Su, J.-H.; Sheu, J.-H.; Sung, P.-J. Flexibilisquinone, a new anti-inflammatory quinone from the cultured soft coral Sinularia flexibilis. Molecules 2013, 18, 8160–8167. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, B.; Yi, Y.; Zhang, W.; Wu, X.; Zhang, L.; Shen, Y. Mycoepoxydiene, a fungal polyketide inhibits MCF-7 cells through simultaneously targeting p53 and NF-κB pathways. Biochem. Pharmacol. 2012, 84, 891–899. [Google Scholar] [CrossRef]
- Kim, M.C.; Kwon, O.-W.; Park, J.-S.; Kim, S.Y.; Kwon, H.C. Nocapyrones H-J, 3,6-disubstituted α-pyrones from the marine actinomycete Nocardiopsis sp. KMF-001. Chem. Pharm. Bull. 2013, 61, 511–515. [Google Scholar] [CrossRef]
- Song, Y.; Dou, H.; Gong, W.; Liu, X.; Yu, Z.; Li, E.; Tan, R.; Hou, Y. Bis-N-norgliovictin, a small-molecule compound from marine fungus, inhibits LPS-induced inflammation in macrophages and improves survival in sepsis. Eur. J. Pharm. 2013, 705, 49–60. [Google Scholar] [CrossRef]
- Festa, C.; Lauro, G.; De Marino, S.; D’Auria, M.V.; Monti, M.C.; Casapullo, A.; D’Amore, C.; Renga, B.; Mencarelli, A.; Petek, S.; et al. Plakilactones from the marine sponge Plakinastrella mamillaris. Discovery of a new class of marine ligands of peroxisome proliferator-activated receptor γ. J. Med. Chem. 2012, 55, 8303–8317. [Google Scholar] [CrossRef]
- Elsebai, M.F.; Nazir, M.; Kehraus, S.; Egereva, E.; Ioset, K.N.; Marcourt, L.; Jeannerat, D.; Gütschow, M.; Wolfender, J.-L.; König, G.M. Polyketide skeletons from the marine alga-derived fungus Coniothyrium cereale. Eur. J. Org. Chem. 2012, 2012, 6197–6203. [Google Scholar]
- Chen, Y.-H.; Lu, M.-C.; Chang, Y.-C.; Hwang, T.-L.; Wang, W.-H.; Weng, C.-F.; Kuo, J.; Sung, P.-J. Pseudoalteromone A: A novel bioactive ubiquinone from a marine bacterium Pseudoalteromonas sp. CGH2XX (Pseudoalteromonadaceae). Tetrahedron Lett. 2012, 53, 1675–1677. [Google Scholar]
- Chen, Y.-H.; Kuo, J.; Su, J.-H.; Hwang, T.-L.; Chen, Y.-H.; Lee, C.-H.; Weng, C.-F.; Sung, P.-J. Pseudoalteromone B: A novel 15C compound from a marine bacterium Pseudoalteromonas sp CGH2XX. Mar. Drugs 2012, 10, 1566–1571. [Google Scholar] [CrossRef]
- Ushiyama, S.; Umaoka, H.; Kato, H.; Suwa, Y.; Morioka, H.; Rotinsulu, H.; Losung, F.; Mangindaan, R.E.P.; de Voogd, N.J.; Yokosawa, H.; et al. Manadosterols A and B, sulfonated sterol dimers inhibiting the Ubc13–Uev1A interaction, isolated from the marine sponge Lissodendryx fibrosa. J. Nat. Prod. 2012, 75, 1495–1499. [Google Scholar] [CrossRef]
- Pina, I.C.; Gautschi, J.T.; Wang, G.-Y.-S.; Sanders, M.L.; Schmitz, F.J.; France, D.; Cornell-Kennon, S.; Sambucetti, L.C.; Remiszewski, S.W.; Perez, L.B.; et al. Psammaplins from the sponge Pseudoceratina purpurea:Inhibition of both histone deacetylase and DNA methyltransferase. J. Org. Chem. 2003, 68, 3866–3873. [Google Scholar] [CrossRef]
- Hentschel, F.; Sasseb, F.; Lindel, T. Fluorescent analogs of the marine natural product psammaplin A: Synthesis and biological activity. Org. Biomol. Chem. 2012, 10, 7120–7133. [Google Scholar] [CrossRef]
- Hosoya, T.; Hirokawa, T.; Takagi, M.; Shin-ya, K. Trichostatin analogues JBIR-109, JBIR-110, and JBIR-111 from the marine sponge-derived Streptomyces sp. RM72. J. Nat. Prod. 2012, 75, 285–289. [Google Scholar] [CrossRef]
- McKee, T.C.; Rabe, D.; Bokesch, H.R.; Grkovic, T.; Whitson, E.L.; Diyabalanage, T.; Van Wyk, A.W.W.; Marcum, S.R.; Gardella, R.S.; Gustafson, K.R.; et al. Inhibition of Hypoxia Inducible Factor-2 transcription: Isolation of active modulators from marine sponges. J. Nat. Prod. 2012, 75, 1632–1636. [Google Scholar] [CrossRef]
- Du, L.; Mahdi, F.; Datta, S.; Jekabsons, M.B.; Zhou, Y.-D.; Nagle, D.G. Structures and mechanisms of antitumor agents: Xestoquinones uncouple cellular respiration and disrupt HIF signaling in human breast tumor cells. J. Nat. Prod. 2012, 75, 1553–1559. [Google Scholar] [CrossRef]
- Sun, Y.; Takada, K.; Takemoto, Y.; Yoshida, M.; Nogi, Y.; Okada, S.; Matsunaga, S. Gliotoxin analogues from a marine-derived fungus, Penicillium sp., and their cytotoxic and histone methyltransferase inhibitory activities. J. Nat. Prod. 2012, 75, 111–114. [Google Scholar] [CrossRef]
- Arai, M.; Yamano, Y.; Fujita, M.; Setiawan, A.; Kobayashi, M. Stylissamide X, a new proline-rich cyclic octapeptide as an inhibitor of cell migration, from an Indonesian marine sponge of Stylissa sp. Bioorg. Med. Chem. Lett. 2012, 22, 1818–1821. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Qian, Z.-J.; Ryu, B.M.; Kim, K.-N.; Kim, D.; Kim, Y.-M.; Jeon, Y.-J.; Park, W.S.; Choi, I.-W.; Kim, G.H.; et al. Matrix metalloproteinases (MMPs) inhibitory effects of an octameric oligopeptide isolated from abalone Haliotis discus hannai. Food Chem. 2013, 141, 503–509. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kato, H.; Nishikawa, T.; Iwasaki, N.; Suwa, Y.; Rotinsulu, H.; Losung, F.; Maarisit, W.; Mangindaan, R.E.P.; Morioka, H.; et al. Siladenoserinols A–L: New sulfonated serinol derivatives from a tunicate as inhibitors of p53-Hdm2 interaction. Org. Lett. 2013, 15, 322–325. [Google Scholar] [CrossRef]
- Malloy, K.L.; Choi, H.; Fiorilla, C.; Valeriote, F.A.; Matainaho, T.; Gerwick, W.H. Hoiamide D, a marine cyanobacteria-derived inhibitor of p53/MDM2 interaction. Bioorg. Med. Chem. Lett. 2012, 22, 683–688. [Google Scholar] [CrossRef]
- Huang, X.; Sun, Y.-L.; Salim, A.A.; Chen, Z.-S.; Capon, R.J. Parguerenes: Marine red alga bromoditerpenes as inhibitors of P-glycoprotein (ABCB1) in multidrug resistant human cancer cells. Biochem. Pharmacol. 2013, 85, 1257–1268. [Google Scholar]
- Niemann, H.; Lin, W.; Müller, W.E.G.; Kubbutat, M.; Lai, D.; Proksch, P. Trimeric hemibastadin congener from the marine sponge Ianthella basta. J. Nat. Prod. 2013, 76, 121–125. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Stonik, V.A.; Fedorov, S.N. Marine Low Molecular Weight Natural Products as Potential Cancer Preventive Compounds. Mar. Drugs 2014, 12, 636-671. https://doi.org/10.3390/md12020636
Stonik VA, Fedorov SN. Marine Low Molecular Weight Natural Products as Potential Cancer Preventive Compounds. Marine Drugs. 2014; 12(2):636-671. https://doi.org/10.3390/md12020636
Chicago/Turabian StyleStonik, Valentin A., and Sergey N. Fedorov. 2014. "Marine Low Molecular Weight Natural Products as Potential Cancer Preventive Compounds" Marine Drugs 12, no. 2: 636-671. https://doi.org/10.3390/md12020636
APA StyleStonik, V. A., & Fedorov, S. N. (2014). Marine Low Molecular Weight Natural Products as Potential Cancer Preventive Compounds. Marine Drugs, 12(2), 636-671. https://doi.org/10.3390/md12020636




