Indole Diterpenoids and Isocoumarin from the Fungus, Aspergillus flavus, Isolated from the Prawn, Penaeus vannamei
Abstract
:1. Introduction

2. Results and Discussion
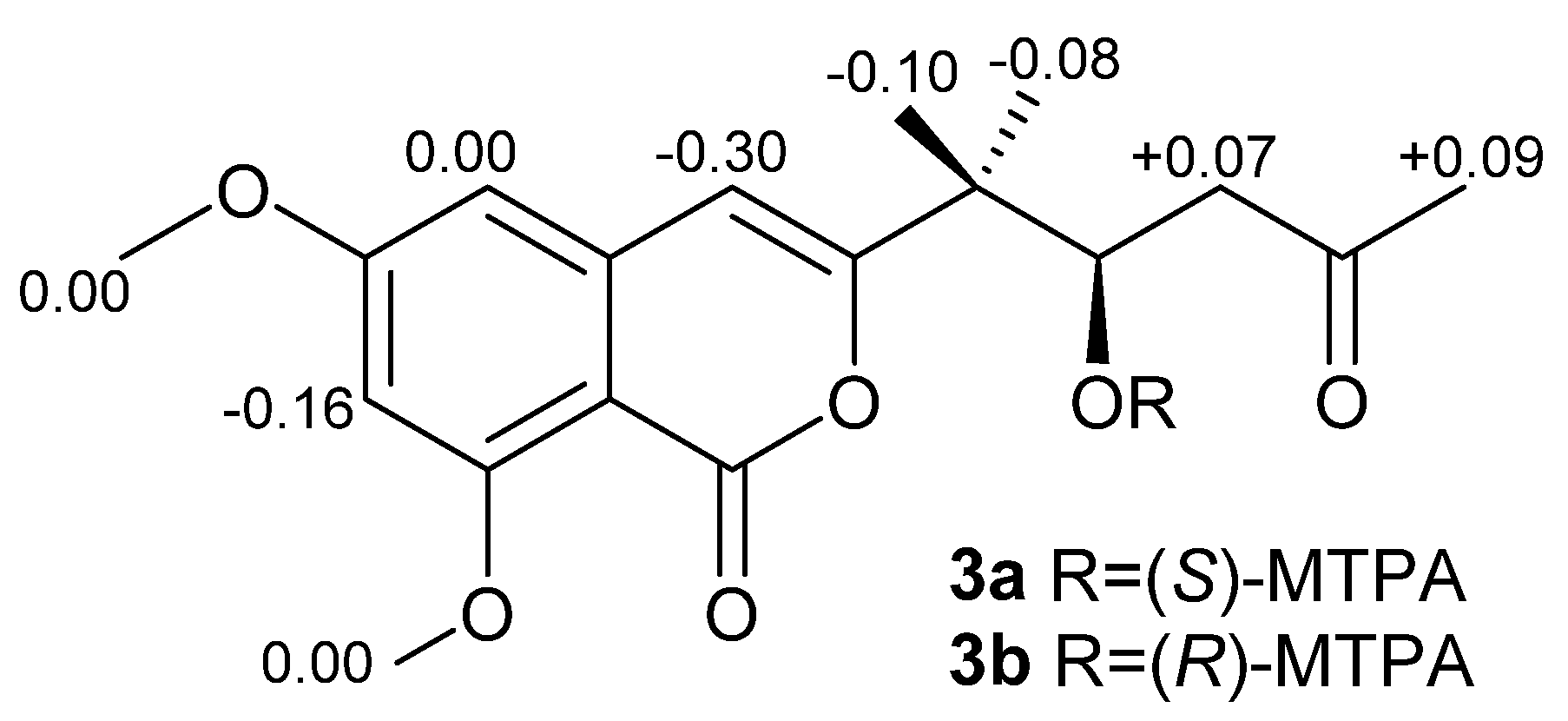
2.1. Structure Elucidation



2.2. Bioactivities the of Compounds Produced by Aspergillus flavus OUCMDZ-2205
3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation and Extraction
3.4. Purification and Identification
3.5. Supplementary Information Available
4. Conclusions
Supplementary Files
Acknowledgments
Conflicts of Interest
References
- Li, C.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. Thiersinines A and B: Novel antiinsectan indole diterpenoids from a new fungicolous Penicillium species (NRRL 28147). Org. Lett. 2002, 4, 3095–3098. [Google Scholar] [CrossRef]
- Gatenby, W.A.; Munday-Finch, S.C.; Wilkins, A.L.; Miles, C.O. Terpendole M, a novel indole-diterpenoid isolated from Lolium perenne infected with the endophytic fungus Neotyphodium lolii. J. Agric. Food Chem. 1999, 47, 1092–1097. [Google Scholar] [CrossRef]
- Li, C.; Gloer, J.B.; Wicklow, D.T. Thiersindoles A–C: New indole diterpenoids from Penicillium thiersii. J. Nat. Prod. 2003, 66, 1232–1235. [Google Scholar] [CrossRef]
- Laakso, J.A.; Gloer, J.B. Radarins A–D: New antiinsectan and cytotoxic indole diterpenoids from the sclerotia of Aspergillus sulphureus. J. Org. Chem. 1992, 57, 138–141. [Google Scholar]
- Gallagher, R.T.; Hawkes, A.D.; Steyn, P.S.; Vleggaar, R. Tremorgenic neurotoxins from perennial ryegrass causing ryegrass staggers disorder of livestock: Structure elucidation of lolitrem B. J. Chem. Soc. Chem. Commun. 1984, 9, 614–616. [Google Scholar]
- Fan, Y.Q.; Wang, Y.; Liu, P.P.; Fu, P.; Zhu, T.H.; Wang, W.; Zhu, W.M. Indole-diterpenoids with anti-H1N1 activity from the aciduric fungus Penicillium camemberti OUCMDZ-1492. J. Nat. Prod. 2013, 76, 1328–1336. [Google Scholar] [CrossRef]
- Mark, R.; Tepaske, J.B.; Gloer, D.T. Aflavarin and β-Aflatrem: New anti-insectan metabolites from the sclerotia of Aspergillus flavus. J. Nat. Prod. 1992, 55, 1080–1086. [Google Scholar] [CrossRef]
- Rex, T.G.; Janet, F.; Jon, C. Paspalinine, a tremorgenic metabolite from Claviceps paspali stevens et hall. Tetrahedron Lett. 1980, 21, 235–238. [Google Scholar]
- Zhang, C.W.; Jin, L.; Mondie, B.; Mitchell, S.S.; Castelhano, A.L.; Cai, W.Z.; Bergenhem, N. Leporin B: A novel hexokinase II gene inducing agent from an unidentified fungus. Bioorg. Med. Chem. Lett. 2003, 13, 1433–1435. [Google Scholar] [CrossRef]
- Holzapfel, C.W. The isolation and structure of cyclopiazonic acid, a toxic metabolite of penicillium cyclopium westling. Tetrahedron 1968, 24, 2101–2119. [Google Scholar] [CrossRef]
- Lin, A.Q.; Du, L.; Fang, Y.C.; Wang, F.Z.; Zhu, T.J.; Gu, Q.Q.; Zhu, W.M. iso-α-Cyclopiazonic acid, a new natural product isolated from the marine-derived fungus Aspergillus flavus CF-3. Chem. Nat. Compd. 2009, 45, 677–680. [Google Scholar] [CrossRef]
- Springer, J.P.; Bűchi, G.; Kobbe, B.; Demain, A.L.; Clardy, J. The structure of ditryptophenaline—A new metabolite of Aspergillus flavus. Tetrahedron Lett. 1977, 18, 2403–2406. [Google Scholar] [CrossRef]
- Cox, R.H.; Cole, R.J. Carbon-13 nuclear magnetic resonance studies of fungal metabolites, aflatoxins, and sterigmatocystins. J. Org. Chem. 1977, 42, 112–114. [Google Scholar] [CrossRef]
- Sugijanto, N.E.; Diesel, A.; Ebel, R.; Indrayanto, G.; Zaini, N.C. Chemical constituents of the endophytic fungus Lecythophora sp. isolated from Alyxia reinwardtii. Nat. Prod. Commun. 2009, 4, 1485–1488. [Google Scholar]
- Basappa, S.C.; Sreenivasamurthy, V.; Parpia, H. Aflatoxin and kojic acid production by resting cells of Aspergillus flavus Link. J. Gen. Microbiol. 1970, 61, 81–86. [Google Scholar] [CrossRef]
- Zheng, J.K.; Zhu, H.J.; Hong, K.; Wang, Y.; Liu, P.P.; Wang, X.; Peng, X. P.; Zhu, W.M. Novel cyclic hexapeptides from marine-derived fungus, Aspergillus sclerotiorum PT06-1. Org. Lett. 2009, 11, 5262–5265. [Google Scholar] [CrossRef]
- Frisch, G.W.; Trucks, H.B.; Schlegel, G.E.; Scuseria, M.A.; Robb, J.R.; Cheeseman, J.A.; Montgomery, T., Jr.; Vreven, K.N.; Kudin, J.C.; Burant, J M.; et al. Pople, Gaussian 03, Revision E. 01, Suite of Programs for ab Initio Calculation; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Cammi, R.; Tomasi, J. Remarks on the use of the apparent surface charges (ASC) methods in solvation problems: Iterative versus matrix-inversion procedures and the renormalization of the apparent charges. J. Comput. Chem. 1995, 16, 1449–1458. [Google Scholar] [CrossRef]
- Gross, E.K.U.; Dobson, J.F.; Petersilka, M. Density functional theory of time-dependent phenomena. In Density Functional Theory II; Nalewajski, R.F., Ed.; Springer-Verlag: Berlin, Germany, 1996; Volume 181, p. 81. [Google Scholar]
- Casida, M.E. Recent Advances in Density Functional Methods, Part I; Chong, D.P., Ed.; World Scientific: Singapore, Singapore, 1995; pp. 155–192. [Google Scholar]
- Gross, E.K.U.; Kohn, W. Time-dependent density functional theory. In Density Functional Theory of Many-Fermion Systems; Advances in Quantum Chemistry; Elsevier: Amsterdam, The Netherlands, 1990; Volume 21, pp. 255–291. [Google Scholar]
- Runge, E.; Gross, E.K.U. Density-functional theory for time-dependent systems. Phys. Rev. Lett. 1984, 52, 997–1000. [Google Scholar] [CrossRef]
- Ellestad, G.A.; Lovell, F.M.; Perkinson, N.A.; Hargreaves, R.T.; McGahren, W.J. New zearalenone related macrolides and isocoumarins from an unidentified fungus. J. Org. Chem. 1978, 43, 2339–2343. [Google Scholar]
- Lai, S.; Shizuri, Y.; Yamamura, S.; Kawai, K.; Furukawa, H. Three new phenolic metabolites from Penicillium species. Heterocycles 1991, 32, 297–305. [Google Scholar] [CrossRef]
- Watanabe, A.; Ono, Y.; Fujii, I.; Sankawa, U.; Mayorga, M.E.; Timberlake, W.E.; Yutaka Ebizuka, Y. Aspergillus fumigatus alb1 encodes naphthopyrone synthase when expressed in Aspergillus oryzae. Tetrahydron Lett. 1998, 39, 7733–7736. [Google Scholar]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Tepaske, M.R.; Gloer, J.B. Leporin A: An antiinsectan N-alkoxypyridone from the sclerotia of Aspergillus leporis. Tetrahedron Lett. 1991, 32, 5687–5690. [Google Scholar] [CrossRef]
- Nakadate, S.; Nozawa, K.; Yaguchi, T. Two new eujindoles from Eupenicillium javanicum. Heterocycles 2011, 83, 1867–1871. [Google Scholar] [CrossRef]
- Ogata, M.; Ueda, J.; Hoshi, M.; Hashimoto, J.; Nakashima, T.; Anzai, K.; Takagi, M.; Shin-ya, K. A novel indole-diterpenoid, JBIR-03 with anti-MRSA activity from Dichotomomyces cejpii var. cejpii NBRC 103559. J. Antibiot. 2007, 60, 645–648. [Google Scholar] [CrossRef]
- Clardy, J.; Springer, J.P. Paspaline and paspalicine two indole mevalonate metabolites from Claviceps paspali. Tetrahedron Lett. 1980, 21, 231–234. [Google Scholar] [CrossRef]
- Singh, S.B.; Ondeyka, J.G.; Jayasuriya, H.; Zink, D.L.; Ha, S.N.; Dahl, R.A.; Greene, J.; Kim, J.A.; Smith, M.M.; Shoop, W.; et al. Nodulisporic acids D-F: Structure, biological activities, and biogenetic relationships. J. Nat. Prod. 2004, 67, 1496–1506. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Zaika, L.L. Spices and herbs: Their antimicrobial activity and its determination. J. Food Saf. 1988, 9, 97–118. [Google Scholar] [CrossRef]
- Li, J.S.; Kleeff, J.; Guo, J.C.; Fischer, L.; Giese, N.; Büchler, M.W.; Friess, H. Effects of STI571 (gleevec) on pancreatic cancer cell growth. Mol. Cancer 2003, 2, 32. [Google Scholar] [CrossRef]
- Somberg, R.; Pferdehirt, B.; Kupcho, K. A universal kinase aassay for a world of kinases. Promega Notes 2003, 83, 14–17. [Google Scholar]
- Grassauer, A.; Weinmuellner, R.; Meier, C.; Pretsch, A.; Prieschl-Grassauer, E.; Unger, H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol. J. 2008, 5, 107. [Google Scholar] [CrossRef]
- Mehta, N.N.; Sheetz, M.; Price, K. Selective PKC beta inhibition with ruboxistaurin and endothelial function in type-2 diabetes mellitus. Cardiovasc. Drugs Ther. 2009, 23, 17–24. [Google Scholar] [CrossRef]
- Mochly-Rosen, D.; Das, K.; Grimes, K.V. Protein kinase C, an elusive therapeutic target? Nat. Rev. Drug Discov. 2012, 11, 937–957. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sun, K.; Li, Y.; Guo, L.; Wang, Y.; Liu, P.; Zhu, W. Indole Diterpenoids and Isocoumarin from the Fungus, Aspergillus flavus, Isolated from the Prawn, Penaeus vannamei. Mar. Drugs 2014, 12, 3970-3981. https://doi.org/10.3390/md12073970
Sun K, Li Y, Guo L, Wang Y, Liu P, Zhu W. Indole Diterpenoids and Isocoumarin from the Fungus, Aspergillus flavus, Isolated from the Prawn, Penaeus vannamei. Marine Drugs. 2014; 12(7):3970-3981. https://doi.org/10.3390/md12073970
Chicago/Turabian StyleSun, Kunlai, Ye Li, Lei Guo, Yi Wang, Peipei Liu, and Weiming Zhu. 2014. "Indole Diterpenoids and Isocoumarin from the Fungus, Aspergillus flavus, Isolated from the Prawn, Penaeus vannamei" Marine Drugs 12, no. 7: 3970-3981. https://doi.org/10.3390/md12073970
APA StyleSun, K., Li, Y., Guo, L., Wang, Y., Liu, P., & Zhu, W. (2014). Indole Diterpenoids and Isocoumarin from the Fungus, Aspergillus flavus, Isolated from the Prawn, Penaeus vannamei. Marine Drugs, 12(7), 3970-3981. https://doi.org/10.3390/md12073970



