Design, Synthesis and Biological Evaluation of Novel Bromophenol Derivatives Incorporating Indolin-2-One Moiety as Potential Anticancer Agents
Abstract
:1. Introduction


2. Results and Discussion
2.1. Chemistry


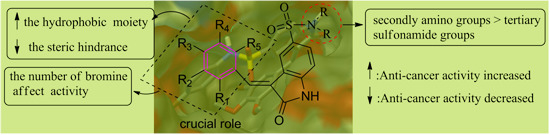
2.2. Anti-Cancer Activity
2.2.1. Antiproliferative Activity

| Compd | IC50 (μg/mL) a | ||||
|---|---|---|---|---|---|
| A549 | Bel7402 | HepG2 | Hct116 | Hela | |
| 4g | 6.6 ± 0.82 | 9.2 ± 0.84 | 13.2 ± 2.42 | 9.1 ± 0.13 | 7.4 ± 0.22 |
| 4h | 14.4 ± 1.86 | 12.3 ± 0.23 | 14.3 ± 0.86 | 9.8 ± 0.55 | 8.3 ± 0.67 |
| 4i | 9.9 ± 0.11 | 22.3 ± 1.11 | NA b | NA | NA |
| 5h | 10.1 ± 0.72 | 9.7 ± 2.35 | 11.2 ± 1.26 | 8.6 ± 0.26 | 18.0 ± 0.13 |
| 6d | 25.4 ± 0.82 | 18.6 ± 0.91 | NA | 5.6 ± 0.42 | 17.6 ± 0.89 |
| 6e | NA | NA | NA | 9.8 ± 0.20 | NA |
| 7a | 12.5 ± 0.19 | 7.9 ± 0.26 | 25.0 ± 0.18 | 6.1 ± 0.23 | 8.6 ± 0.14 |
| 7b | 12.5 ± 0.45 | 12.5 ± 0.39 | 14.2 ± 0.77 | 8.2 ± 0.54 | 9.3 ± 0.47 |
| TTEDM c | NA | 44.1 ± 1.19 | NA | 32.3 ± 2.03 | 23.5 ± 1.41 |
| Sunitinib d | 10.4± 0.54 | 3.2 ± 0.15 | 4.5 ± 0.23 | 2.8 ± 0.27 | 1.6 ± 0.08 |
2.2.2. Inhibitory Effect of Compound 4g on Cancer Cell Migration

3. Experimental Section
3.1. Chemistry
General Procedure for the Preparation of Derivatives (4–7)
3.2. Antiproliferative Activities Assays
3.2.1. Cell Culture and Proliferation Assay
3.2.2. Wound-Healing Assay
3.2.3. Statistical Analysis
4. Conclusions

Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.S.I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]; GLOBOCAN 2012 v1.0 2013; International Agency for Research on Cancer: Lyon, France, 2013. Available online: http://globocan.iarc.fr (accessed on 25 December 2014).
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their Bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef] [PubMed]
- Colon, M.; Guevara, P.; Gerwick, W.H.; Ballantine, D. 5'-Hydroxyisoavrainvilleol, a new diphenylmethane derivative from the tropical green-alga avrainvillea-nigricans. J. Nat. Prod. 1987, 50, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Shoeib, N.A.; Bibby, M.C.; Blunden, G.; Linley, P.A.; Swaine, D.J.; Wheelhouse, R.T.; Wright, C.W. In-vitro cytotoxic activities of the major bromophenols of the red alga Polysiphonia lanosa and some novel synthetic isomers. J. Nat. Prod. 2004, 67, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.J.; Fan, X.; Yan, X.J.; Tseng, C.K. Screening marine algae from China for their antitumor activities. J. Appl. Phycol. 2004, 16, 451–456. [Google Scholar] [CrossRef]
- Shi, D.Y.; Li, J.; Guo, S.J.; Su, H.; Fan, X. The antitumor effect of bromophenol derivatives in vitro and Leathesia nana extract in vivo. Chin. J. Oceanol. Limnol. 2009, 27, 277–282. [Google Scholar] [CrossRef]
- Eastwood, P.; Gonzalez, J.; Gomez, E.; Vidal, B.; Caturla, F.; Roca, R.; Balague, C.; Orellana, A. Indolin-2-one p38α inhibitors I: Design, profiling and crystallographic binding mode. Bioorg. Med. Chem. Lett. 2011, 21, 4130–4133. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Ma, L.; Huang, W.; Yu, X.; Zhang, Y.; Ji, H.; Tian, J. Synthesis and biological evaluation of 3-4-(amino/methylsulfonyl) phenyl methylene-indolin-2-one derivatives as novel COX-1/2 and 5-LOX inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 7349–7353. [Google Scholar] [CrossRef] [PubMed]
- Jeankumar, V.U.; Alokam, R.; Sridevi, J.P.; Suryadevara, P.; Matikonda, S.S.; Peddi, S.; Sahithi, S.; Alvala, M.; Yogeeswari, P.; Sriram, D. Discovery and structure optimization of a series of isatin derivatives as mycobacterium tuberculosis chorismate mutase inhibitors. Chem. Biol. Drug Des. 2014, 83, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Singh, B.; Vyas, B.; Silakari, O. Synthesis and biological activity of 4-aryl-3-benzoyl-5-phenylspiro pyrrolidine-2.3 “-indolin-2”-one derivatives as novel potent inhibitors of advanced glycation end product. Eur. J. Med. Chem. 2014, 79, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Tsuhako, A.L.; Co, E.W.; Aftab, D.T.; Bentzien, F.; Chen, J.; Cheng, W.; Engst, S.; Goon, L.; Klein, R.R.; et al. The design, synthesis, and biological evaluation of potent receptor tyrosine kinase inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 4979–4985. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-H.; Wu, W.-Y.; Cao, S.-L.; Liao, J.; Ma, L.; Gao, M.; Li, Z.-F.; Xu, X. Synthesis and antiproliferative evaluation of piperazine-1-carbothiohydrazide derivatives of indolin-2-one. Bioorg. Med. Chem. Lett. 2013, 23, 3304–3307. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Wang, L.-L.; Liu, M.-L.; Zhou, X.-B.; Fan, S.-Y.; Liu, H.-Y.; Zheng, Z.-B.; Li, S. Synthesis and antitumor activity of 5-1-(3-(dimethylamino)propyl)-5-halogenated-2-oxoindolin-(3Z)-ylideneme thyl-2,4-dimethyl-1H-pyrrole-3-carboxamides. Bioorg. Med. Chem. Lett. 2011, 21, 3062–3065. [Google Scholar] [CrossRef] [PubMed]
- Penthala, N.R.; Yerramreddy, T.R.; Madadi, N.R.; Crooks, P.A. Synthesis and in vitro evaluation of N-alkyl-3-hydroxy-3-(2-imino-3-methyl-5-oxoimidazolidin-4-yl)indolin-2-one analogs as potential anticancer agents. Bioorg. Med. Chem. Lett. 2010, 20, 4468–4471. [Google Scholar] [CrossRef] [PubMed]
- McComas, C.C.; Vu, A.T.; Mahaney, P.E.; Cohn, S.T.; Fensome, A.; Marella, M.A.; Nogle, L.; Trybulski, E.J.; Ye, F.; Zhang, P.; et al. Synthesis and activity of 1-(3-amino-1-phenylpropyl)indolin-2-ones: A new class of selective norepinephrine reuptake inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 4929–4931. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M. On the antioxidant activity of ortho- and meta-substituted indolin-2-one derivatives. Monatsh. Chem. 2014, 145, 291–299. [Google Scholar] [CrossRef]
- Prakash, C.R.; Raja, S. Synthesis, characterization and in vitro antimicrobial activity of some novel 5-substituted Schiff and Mannich base of isatin derivatives. J. Saudi Chem. Soc. 2013, 17, 337–344. [Google Scholar] [CrossRef]
- Praveen, C.; Ayyanar, A.; Perumal, P.T. Practical synthesis, anticonvulsant, and antimicrobial activity of N-allyl and N-propargyl di(indolyl)indolin-2-ones. Bioorg. Med. Chem. Lett. 2011, 21, 4072–4077. [Google Scholar] [CrossRef] [PubMed]
- Li, P.K.; Xiao, Z.L.; Hu, Z.G.; Pandit, B.; Sun, Y.J.; Sackett, D.L.; Werbovetz, K.; Lewis, A.; Johnsamuel, J. Conformationally restricted analogs of Combretastatin A-4 derived from SU5416. Bioorg. Med. Chem. Lett. 2005, 15, 5382–5385. [Google Scholar] [CrossRef] [PubMed]
- Dweedar, H.E.; Mahrous, H.; Ibrahim, H.S.; Abdel-Aziz, H.A. Analogue-based design, synthesis and biological evaluation of 3-substituted-(methylenehydrazono)indolin-2-ones as anticanceragents. Eur. J. Med. Chem. 2014, 78, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.; Jonker, D.J.; Laurie, S.A.; Call, J.A.; Diab, S.G.; Goss, G.; McWilliam, M.; Wang, E.; Chao, R.; Eckhardt, S.G.; et al. Sunitinib (SU) in combination with pemetrexed (P) in patients (pts) with advanced solid malignancies: A phase I dose escalation study. J. Clin. Oncol. 2008, 26, 3566. [Google Scholar] [CrossRef]
- Sattler, M.; Pride, Y.B.; Ma, P.; Gramlich, J.L.; Chu, S.C.; Quinnan, L.A.; Shirazian, S.; Liang, C.X.; Podar, K.; Christensen, J.G.; et al. A novel small molecule met inhibitor induces apoptosis in cells transformed by the oncogenic TPR-MET tyrosine kinase. Cancer Res. 2003, 63, 5462–5469. [Google Scholar] [PubMed]
- Christensen, J.G.; Schreck, R.; Burrows, J.; Kuruganti, P.; Chan, E.; Le, P.; Chen, J.; Wang, X.Y.; Ruslim, L.; Blake, R.; et al. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003, 63, 7345–7355. [Google Scholar] [PubMed]
- Wang, S.; Zhao, Y.; Zhang, G.; Lv, Y.; Zhang, N.; Gong, P. Design, synthesis and biological evaluation of novel 4-thiazolidinones containing indolin-2-one moiety as potential antitumor agent. Eur. J. Med. Chem. 2011, 46, 3509–3518. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, Y.; Zhu, W.; Liu, Y.; Guo, K.; Gong, P. Synthesis and anticancer activity of indolin-2-one derivatives bearing the 4-thiazolidinone moiety. Arch. Pharm. 2012, 345, 73–80. [Google Scholar] [CrossRef]
- Singh, P.; Kaur, M.; Holzer, W. Synthesis and evaluation of indole, pyrazole, chromone and pyrimidine based conjugates for tumor growth inhibitory activities—Development of highly efficacious cytotoxic agents. Eur. J. Med. Chem. 2010, 45, 4968–4982. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Guo, S.J.; Shi, D.Y.; Guo, C.; Wang, T. Discovery of novel bromophenol 3,4-dibromo-5-(2-bromo-3,4-dihydroxy-6-(isobutoxymethyl)benzyl)benzene-1,2-diol as protein tyrosine phosphatase 1B inhibitor and its anti-diabetic properties in C57BL/KsJ-db/db mice. Eur. J. Med. Chem. 2013, 64, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.C.; Shi, D.Y.; Hu, Z.Q. Synthesis and protein tyrosine phosphatase 1B inhibition activities of two new synthetic bromophenols and their methoxy derivatives. Chin. J. Oceanol. Limn. 2011, 29, 1237–1242. [Google Scholar] [CrossRef]
- Guo, S.J.; Li, J.; Li, T.; Shi, D.Y.; Han, L.J. Synthesis of three bromophenols from red algae as PTP1B inhibitors. Chin. J. Oceanol. Limn. 2011, 29, 68–74. [Google Scholar] [CrossRef]
- Shi, D.Y.; Guo, S.J.; Jiang, B.; Guo, C.; Wang, T.; Zhang, L.Y.; Li, J.Y. HPN, a synthetic analogue of bromophenol from red alga rhodomela confervoides: Synthesis and anti-diabetic effects in C57BL/KsJ-db/db mice. Mar. Drugs 2013, 11, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Astin, J.W.; Batson, J.; Kadir, S.; Charlet, J.; Persad, R.A.; Gillatt, D.; Oxley, J.D.; Nobes, C.D. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat. Cell. Biol. 2010, 12, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-J.; Wang, S.-Y.; Jiang, B.; Wu, N.; Li, X.-Q.; Wang, B.-C.; Luo, J.; Yang, M.; Jin, S.-H.; Shi, D.-Y. Design, Synthesis and Biological Evaluation of Novel Bromophenol Derivatives Incorporating Indolin-2-One Moiety as Potential Anticancer Agents. Mar. Drugs 2015, 13, 806-823. https://doi.org/10.3390/md13020806
Wang L-J, Wang S-Y, Jiang B, Wu N, Li X-Q, Wang B-C, Luo J, Yang M, Jin S-H, Shi D-Y. Design, Synthesis and Biological Evaluation of Novel Bromophenol Derivatives Incorporating Indolin-2-One Moiety as Potential Anticancer Agents. Marine Drugs. 2015; 13(2):806-823. https://doi.org/10.3390/md13020806
Chicago/Turabian StyleWang, Li-Jun, Shuai-Yu Wang, Bo Jiang, Ning Wu, Xiang-Qian Li, Bao-Cheng Wang, Jiao Luo, Meng Yang, Shui-Hua Jin, and Da-Yong Shi. 2015. "Design, Synthesis and Biological Evaluation of Novel Bromophenol Derivatives Incorporating Indolin-2-One Moiety as Potential Anticancer Agents" Marine Drugs 13, no. 2: 806-823. https://doi.org/10.3390/md13020806
APA StyleWang, L.-J., Wang, S.-Y., Jiang, B., Wu, N., Li, X.-Q., Wang, B.-C., Luo, J., Yang, M., Jin, S.-H., & Shi, D.-Y. (2015). Design, Synthesis and Biological Evaluation of Novel Bromophenol Derivatives Incorporating Indolin-2-One Moiety as Potential Anticancer Agents. Marine Drugs, 13(2), 806-823. https://doi.org/10.3390/md13020806