The Chemically Synthesized Ageladine A-Derivative LysoGlow84 Stains Lysosomes in Viable Mammalian Brain Cells and Specific Structures in the Marine Flatworm Macrostomum lignano
Abstract
:1. Introduction

2. Results and Discussion
2.1. Synthesis of LysoGlow84

2.2. Stability of LysoGlow84 in Solution
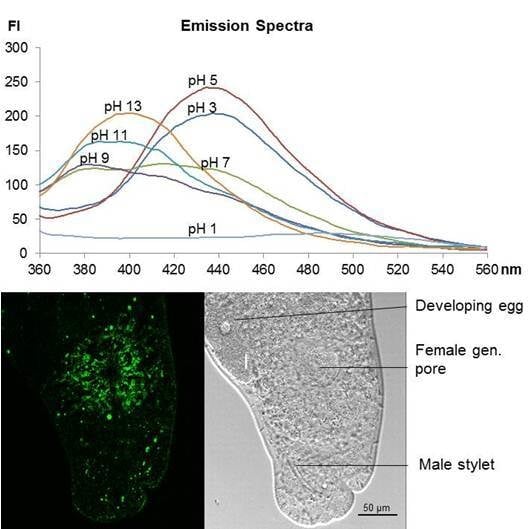
2.3. Fluorescence Spectra Vary with pH Values


2.4. Effects of LysoGlow84 on Cultured Primary Astrocytes

2.5. Staining of Living Individuals of the Flatworm Macrostomum Lignano with LysoGlow84 and LysoTracker® Red DND-99



3. Experimental Section
3.1. Chemicals
3.2. Synthesis and Chemical Characterization of LysoGlow84
3.3. Characterization of LysoGlow84 as Fluorescent Molecule
3.4. Cell Culture Experiments on Cultured Astrocytes
3.5. Experiments on Macrostomum Lignano
3.6. Fluorescence Microscopy
3.7. Presentation of Data
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haddock, S.H.; Moline, M.A.; Case, J.F. Bioluminescence in the sea. Ann. Rev. Mar. Sci. 2010, 2, 443–493. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.L.; James, H.R. Laboratory analysis of the selective absorption of light by seawater. J. Opt. Soc. Am. 1939, 29, 43–55. [Google Scholar] [CrossRef]
- Fujita, M.; Nakao, Y.; Matsunaga, S.; Seiki, M.; Itoj, Y.; Yamashita, J.; van Soest, R.W.M.; Fusetani, N. Ageladine A: An anti angiogenetic matrix metalloproteinase inhibitor from the marine sponge Agelas nakamurai. J. Am. Chem. Soc. 2003, 125, 15700–15701. [Google Scholar] [CrossRef] [PubMed]
- Bickmeyer, U.; Grube, A.; Klings, K.W.; Köck, M. Ageladine A, a pyrrole-imidazole alkaloid from marine sponges, is a pH sensitive membrane permeable dye. Biochem. Biophys. Res. Commun. 2008, 373, 419–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickmeyer, U. The alkaloid ageladine A, originally isolated from marine sponges, used for pH-sensitive imaging of transparent marine animals. Mar. Drugs 2012, 10, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickmeyer, U.; Heine, M.; Podbielski, I.; Münd, D.; Köck, M.; Karuso, P. Tracking of fast moving neuronal vesicles with ageladine A. Biochem. Biophys. Res. Commun. 2010, 402, 489–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shengule, S.R.; Karuso, P. Concise total synthesis of the marine natural product ageladine A. Org. Lett. 2006, 31, 4083–4084. [Google Scholar] [CrossRef]
- Meketa, M.L.; Weinreb, S.M. Total synthesis of ageladine A, an angiogenesis inhibitor from the marine sponge Agelas nakamurai. Org. Lett. 2006, 30, 1443–1446. [Google Scholar] [CrossRef]
- Ando, N.; Terashima, S. Synthesis of novel ageladine a analogs showing more potent matrix metalloproteinase (MMP)-12 inhibitory activity than the natural product. Bioorganic Med. Chem. Lett. 2009, 19, 5461–5463. [Google Scholar] [CrossRef]
- Appelqvist, H.; Waster, P.; Kagedal, K.; Ollinger, K. The lysosome: From waste bag to potential therapeutic target. J. Mol. Cell Biol. 2013, 5, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Ladurner, P.; Schärer, L.; Salvenmoser, W.; Rieger, R.M. A new model organism among the lower Bilateria and the use of digital microscopy intaxonomy of meiobenthic Platyhelminthes: Macrostomum lignano, n. sp. (Rhabditophora, Macrostomorpha). J. Zool. Syst. Evol. Res. 2005, 43, 114–126. [Google Scholar] [CrossRef]
- Rivera-Ingraham, G.A.; Bickmeyer, U.; Abele, D. The physiological response of the marine platyhelminth Macrostomum lignano to different environmental oxygen concentrations. J. Exp. Biol. 2013, 216, 2741–2751. [Google Scholar] [CrossRef] [PubMed]
- Tietje, K.; Rivera-Ingraham, G.; Petters, C.; Abele, D.; Dringen, R.; Bickmeyer, U. Reporter dyes demonstrate functional expression of multidrug resistance proteins in the marine flatworm Macrostomum lignano: The sponge-derived dye Ageladine A is not a substrate of these transporters. Mar. Drugs 2013, 11, 3951–3969. [Google Scholar] [CrossRef] [PubMed]
- Shengule, S.R.; Loa-Kum-Cheung, W.L.; Parish, C.R.; Blairvacq, M.; Meijer, L.; Nakao, Y.; Karuso, P. A one-pot synthesis and biological activity of ageladine A and analogues. J. Med. Chem. 2011, 54, 2492–2503. [Google Scholar] [CrossRef] [PubMed]
- Ando, N.; Terashima, S. Synthesis and matrix metalloproteinase (MMP)-12 inhibitory activity of ageladine A and its analogs. Bioorganic Med. Chem. Lett. 2007, 17, 4495–4499. [Google Scholar] [CrossRef]
- Ma, Y.; Nam, S.; Jove, R.; Yakushijin, K.; Horne, D.A. Synthesis and anticancer activities of ageladine A and structural analogs. Bioorganic Med. Chem. Lett. 2010, 20, 83–86. [Google Scholar] [CrossRef]
- Van den Eynde, J.J.; Delfosse, F.; Mayence, A.; Van Haverbeke, Y. 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone, a mild catalyst for the formation of carbon-nitrogen bonds. Tetrahedron 1995, 51, 6511–6516. [Google Scholar] [CrossRef]
- Vicente-Garcia, E.; Ramón, R.; Preciado, S.; Lavilla, R. Multicomponent reaction access to complex quinolines via oxidation of the Povarov adducts. Beilstein J. Org. Chem. 2011, 7, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Karuso, P.; Shengule, S.R. Synthesis of Ageladine a and Analogs Thereof. Patent WO 2009/152584 A1, 23 December 2009. [Google Scholar]
- Freundt, E.; Czapiga, M.; Lenardo, M. Photoconversion of LysoTracker® Red DND-99 to a green fluorescent molecule. Cell Res. 2007, 17, 956–958. [Google Scholar] [CrossRef] [PubMed]
- Thein, T.; Mariani, F. Use of LysoTracker to detect programmed cell death in embryos and differentiating embryonic stem cells. J. Vis. Exp. 2012, 68, 4254. [Google Scholar] [PubMed]
- Wiegand, U.K.; Duncan, R.R.; Greaves, J.; Chow, R.H.; Shipston, M.J.; Apps, D.K. Red, yellow, green go!—A novel tool for microscopic segregation of secretory vesicle pools according to their age. Biochem. Soc. Trans. 2003, 31, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L.; Ryther, J.H. Studies on marine planktonic diatoms. I. Cyclotella680 nana Hustedt and Detonula confervaceae (Cleve). Gran. Can. J. Microbiol. 1962, 8, 229–681. [Google Scholar] [CrossRef]
- Hamprecht, B.; Löffler, F. Primary glial cultures as a model for studying hormone action. Methods Enzymol. 1985, 109, 341–345. [Google Scholar] [PubMed]
- Tulpule, K.; Hohnholt, M.C.; Hirrlinger, J.; Dringen, R. Primary cultures of astrocytes and neurons as model systems to study the metabolism and metabolite export from brain cells. In Neuromethods 90: Brain Energy Metabolism; Hirrlinger, J., Waagepetersen, H., Eds.; Springer: New York, NY, USA, 2014; pp. 45–72. [Google Scholar]
- Scheiber, I.F.; Schmidt, M.M.; Dringen, R. Zinc prevents the copper-induced damage of cultured astrocytes. Neurochem. Int. 2010, 57, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R.; Gebhardt, R.; Hamprecht, B. Glycogen in astrocytes: Possible function as lactate supply for neighboring cells. Brain Res. 1993, 623, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Liddell, J.R.; Zwingmann, C.; Schmidt, M.M.; Thiessen, A.; Leibfritz, D.; Robinson, S.R.; Dringen, R. Sustained hydrogen peroxide stress decreases lactate production by cultured astrocytes. J. Neurosci. Res. 2009, 87, 2696–2708. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mordhorst, T.; Awal, S.; Jordan, S.; Petters, C.; Sartoris, L.; Dringen, R.; Bickmeyer, U. The Chemically Synthesized Ageladine A-Derivative LysoGlow84 Stains Lysosomes in Viable Mammalian Brain Cells and Specific Structures in the Marine Flatworm Macrostomum lignano. Mar. Drugs 2015, 13, 920-935. https://doi.org/10.3390/md13020920
Mordhorst T, Awal S, Jordan S, Petters C, Sartoris L, Dringen R, Bickmeyer U. The Chemically Synthesized Ageladine A-Derivative LysoGlow84 Stains Lysosomes in Viable Mammalian Brain Cells and Specific Structures in the Marine Flatworm Macrostomum lignano. Marine Drugs. 2015; 13(2):920-935. https://doi.org/10.3390/md13020920
Chicago/Turabian StyleMordhorst, Thorsten, Sushil Awal, Sebastian Jordan, Charlotte Petters, Linda Sartoris, Ralf Dringen, and Ulf Bickmeyer. 2015. "The Chemically Synthesized Ageladine A-Derivative LysoGlow84 Stains Lysosomes in Viable Mammalian Brain Cells and Specific Structures in the Marine Flatworm Macrostomum lignano" Marine Drugs 13, no. 2: 920-935. https://doi.org/10.3390/md13020920
APA StyleMordhorst, T., Awal, S., Jordan, S., Petters, C., Sartoris, L., Dringen, R., & Bickmeyer, U. (2015). The Chemically Synthesized Ageladine A-Derivative LysoGlow84 Stains Lysosomes in Viable Mammalian Brain Cells and Specific Structures in the Marine Flatworm Macrostomum lignano. Marine Drugs, 13(2), 920-935. https://doi.org/10.3390/md13020920