Morphological and Proteomic Analyses Reveal that Unsaturated Guluronate Oligosaccharide Modulates Multiple Functional Pathways in Murine Macrophage RAW264.7 Cells
Abstract
:1. Introduction
2. Results
2.1. Preparation and Structural Analysis of GOS


| m/z | Charge State | Ion Format | Corresponding DP (a) | MW (b) |
|---|---|---|---|---|
| 351 | 1 | [M − H]− | 2 | 352 |
| 263 | 2 | [M − 2H]2− | 3 | 528 |
| 274 | 2 | [M + Na − 3H]2− | 3 | 528 |
| 283 | 2 | [M + K − 3H]2− | 3 | 528 |
| 571 | 1 | [M + 2Na − 3H]− | 3 | 528 |
| 362 | 2 | [M + Na − 3H]2− | 4 | 704 |
| 373 | 2 | [M + 2Na − 4H]2− | 4 | 704 |
| 382 | 2 | [M + 2Na − 4H + H2O]2− | 4 | 704 |
| 439 | 2 | [M − 2H]2− | 5 | 880 |
| 450 | 2 | [M + Na − 3H]2− | 5 | 880 |
| 461 | 2 | [M + 2Na − 4H]2− | 5 | 880 |
| 472 | 2 | [M + 3Na − 5H]2− | 5 | 880 |
| 481 | 2 | [M + 3Na-5H + H2O]2− | 5 | 880 |
| 527 | 2 | [M − 2H]2− | 6 | 1056 |
| 538 | 2 | [M + Na − 3H]2− | 6 | 1056 |
| 549 | 2 | [M + 2Na − 4H]2− | 6 | 1056 |
| 560 | 2 | [M + 3Na − 5H]2− | 6 | 1056 |
| 571 | 2 | [M + 4Na − 6H]2− | 6 | 1056 |
| 670 | 2 | [M + 5Na − 7H]2− | 7 | 1232 |
| 679 | 2 | [M + 5Na − 7H + H2O]2− | 7 | 1232 |
| 490 | 3 | [M + 3Na − 6H]3− | 8 | 1407 |
| 497 | 3 | [M + 4Na − 7H]3− | 8 | 1406 |
2.2. Comparison of Protein Expression Patterns between GOS-Treated and Control Cells

| Spot No. | Protein ID | Symbol | Accession Number | MW (kD)/pI | Peptides Matched (a) | Cov (%) (b) | Protein Score | Expr Level (c) | Reported Function |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 60S acidic ribosomal protein P2 | RPLP2 | P99027 | 11.65/4.38 | 3(2) | 31 | 168 | +3.3 ± 0.3 | Translation |
| 2 | Annexin A5 | ANXA5 | P48036 | 35.75/4.82 | 7(6) | 30 | 244 | +2.7 ± 0.2 | Inflammation |
| 3 | Cofilin-1 | CFL1 | P18760 | 18.56/8.22 | 4(3) | 26 | 256 | −2.1 ± 0.2 | Cell cytoskeleton |
| 4 | Cofilin-2 | CFL2 | P45591 | 18.71/7.66 | 1(1) | 6 | 73 | +2.4 ± 0.2 | Cell cytoskeleton |
| 5 | Cu/Zn-superoxide dismutase | SOD1 | P08228 | 15.94/6.02 | 4(4) | 24 | 315 | +2.1 ± 0.2 | Antioxidant |
| 6 | Fructose-bisphosphate aldolase | ALDOART1 | Q9CPQ9 | 39.35/8.30 | 5(4) | 18 | 296 | −5.9 ± 0.7 | Metabolic process |
| 7 | Galectin-1 | LGALS1 | P16045 | 14.86/5.28 | 6(6) | 43 | 426 | +2.3 ± 0.3 | Signal transduction |
| 8 | GTP-binding nuclear protein | RAN | P62826 | 24.42/7.01 | 6(5) | 21 | 324 | −2.2 ± 0.2 | Inflammation |
| 9 | Lactoylglutathione lyase | GLO1 | Q9CPU0 | 20.81/5.24 | 2(2) | 8 | 65 | +2.4 ± 0.2 | Signal transduction |
2.3. Western Blot Analysis for Validation of Differentially Expressed Proteins
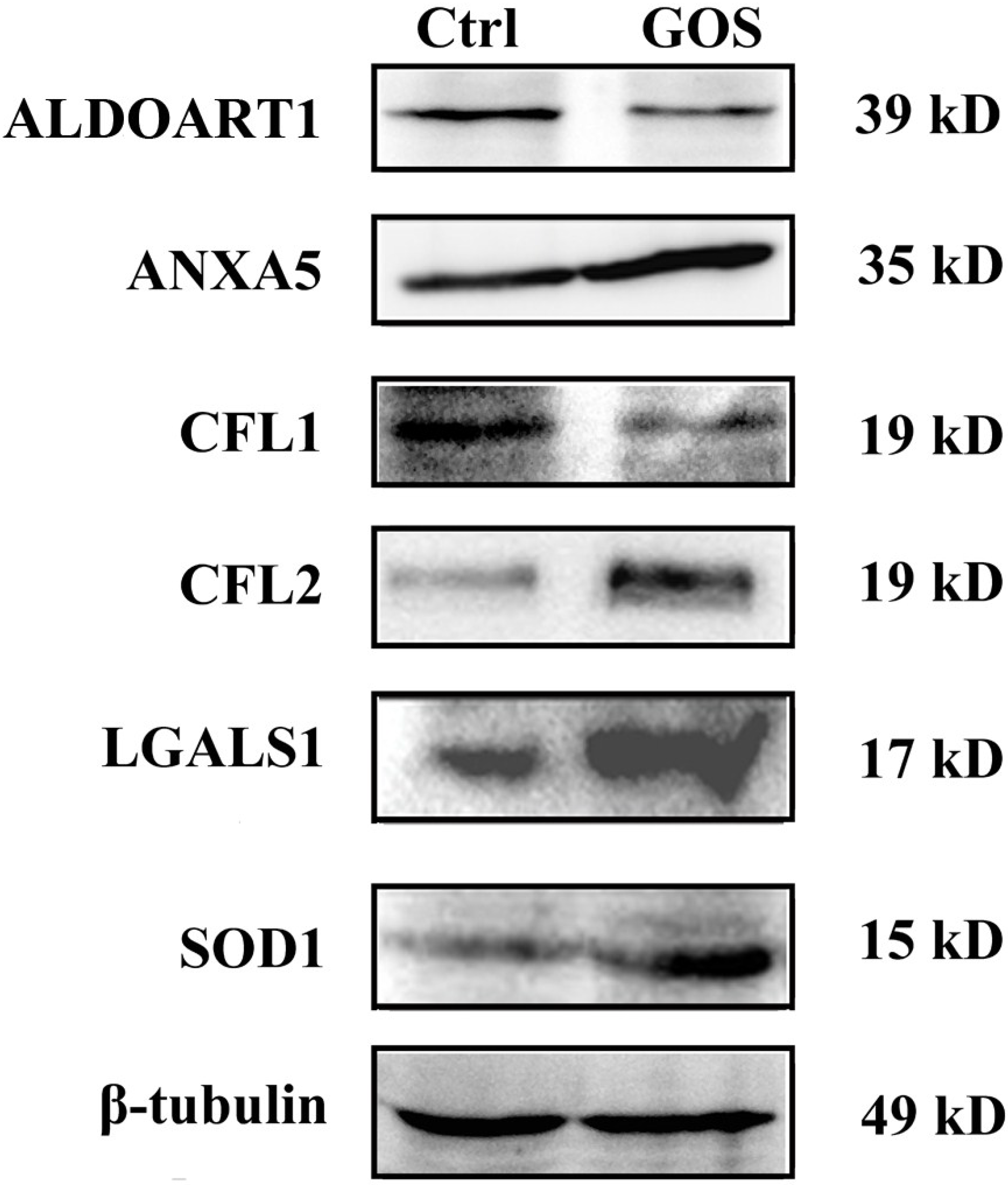
2.4. Effects of GOS on the Morphology and Actin Cytoskeleton Organization of RAW264.7 Cells


2.5. Effects of GOS on Lipopolysaccharide-Activated Morphological Changes in RAW264.7 Cells

3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of GOS
4.3. Mass Spectrometry Analysis
4.4. Cell Culture
4.5. Protein Extraction and 2-DE
4.6. Protein Identification by MS
4.7. Western Blot Analysis
4.8. Cell Morphology and Actin Cytoskeleton Organization
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haug, A. Alginic acid. Isolation and fractionation with potassium chloride and manganous ions. Methods Carbohydr. Chem. 1965, 5, 69–72. [Google Scholar]
- Wan, L.; Heng, P.; Chan, L. Drug encapsulation in alginate microspheres by emulsification. J. Microencapsul. 1992, 9, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Endo, T.; Nakakita, R.; Murata, K.; Yonemoto, Y.; Okayama, K. Effect of depolymerized alginates on the growth of bifidobacteria. Biosci. Biotechnol. Biochem. 1992, 56, 355. [Google Scholar] [CrossRef] [PubMed]
- Kawada, A.; Hiura, N.; Tajima, S.; Takahara, H. Alginate oligosaccharides stimulate VEGF-mediated growth and migration of human endothelial cells. Arch. Dermatol. Res. 1999, 291, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Kawada, A.; Hiura, N.; Shiraiwa, M.; Tajima, S.; Hiruma, M.; Hara, K.; Ishibashi, A.; Takahara, H. Stimulation of human keratinocyte growth by alginate oligosaccharides, a possible co-factor for epidermal growth factor in cell culture. FEBS Lett. 1997, 408, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Xu, X.; Tamura, T.; Oda, T.; Muramatsu, T. Enzymatically depolymerized alginate oligomers that cause cytotoxic cytokine production in human mononuclear cells. Biosci. Biotechnol. Biochem. 2003, 67, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Kurachi, M.; Nakashima, T.; Kim, D.; Yamaguchi, K.; Oda, T.; Iwamoto, Y.; Muramatsu, T. Structure-activity relationship of alginate oligosaccharides in the induction of cytokine production from RAW264.7 cells. FEBS Lett. 2005, 579, 4423–4429. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kurachi, M.; Yamaguchi, K.; Oda, T. Induction of multiple cytokine secretion from RAW264.7 cells by alginate oligosaccharides. Biosci. Biotechnol. Biochem. 2007, 71, 238–241. [Google Scholar] [CrossRef] [PubMed]
- An, Q.D.; Zhang, G.L.; Wu, H.T.; Zhang, Z.C.; Zheng, G.S.; Luan, L.; Murata, Y.; Li, X. Alginate-deriving oligosaccharide production by alginase from newly isolated Flavobacterium sp. LXA and its potential application in protection against pathogens. J. Appl. Microbiol. 2009, 106, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Hiroki, T.; Takeshita, S.; Jiang, Z.; Kim, D.; Yamaguchi, K.; Oda, T. Comparative study on antioxidative and macrophage-stimulating activities of polyguluronic acid (PG) and polymannuronic acid (PM) prepared from alginate. Carbohydr. Res. 2012, 352, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Falkeborg, M.; Cheong, L.Z.; Gianfico, C.; Sztukiel, K.M.; Kristensen, K.; Glasius, M.; Xu, X.; Guo, Z. Alginate oligosaccharides: Enzymatic preparation and antioxidant property evaluation. Food Chem. 2014, 164, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Tusi, S.K.; Khalaj, L.; Ashabi, G.; Kiaei, M.; Khodagholi, F. Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials 2011, 32, 5438–5458. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Hirano, A.; Wada, H.; Takahashi, K.; Hattori, M. Alginic acid oligosaccharide suppresses Th2 development and IgE production by inducing IL-12 production. Int. Arch. Allergy Immunol. 2004, 133, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Venter, G.; Oerlemans, F.T.; Wijers, M.; Willemse, M.; Fransen, J.A.; Wieringa, B. Glucose controls morphodynamics of LPS-stimulated macrophages. PLoS ONE 2014, 9, e96786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallabhapurapu, S.; Karin, M. Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Bi, D.; Wu, X.; Wang, Q.; Wei, G.; Chi, L.; Jiang, Z.; Oda, T.; Wan, M. Unsaturated guluronate oligosaccharide enhances the antibacterial activities of macrophages. FASEB J. 2014, 28, 2645–2654. [Google Scholar] [CrossRef] [PubMed]
- Kurachi, M.; Nakashima, T.; Miyajima, C.; Iwamoto, Y.; Muramatsu, T.; Yamaguchi, K.; Oda, T. Comparison of the activities of various alginates to induce TNF-α secretion in RAW264.7 cells. J. Infect. Chemother. 2005, 11, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, X.; Wang, Q.; Cai, N.; Zhang, H.; Jiang, Z.; Wan, M.; Oda, T. Immunomodulatory effects of alginate oligosaccharides on murine macrophage RAW264. 7 cells and their structure-activity relationships. J. Agric. Food Chem. 2014, 62, 3168–3176. [Google Scholar] [CrossRef]
- Van den Bogaerdt, A.J.; El Ghalbzouri, A.; Hensbergen, P.J.; Reijnen, L.; Verkerk, M.; Kroon-Smits, M.; Middelkoop, E.; Ulrich, M.M. Differential expression of CRABP-II in fibroblasts derived from dermis and subcutaneous fat. Biochem. Biophys. Res. Commun. 2004, 315, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Aebersold, R.; Mann, M. Mass spectrometry-based proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Zhang, J.S.; Jiang, Y.; Zheng, Z.Y.; Zhan, X.B.; Lin, C.C. Structure of oligosaccharide F21 derived from exopolysaccharide WL-26 produced by Sphingomonas sp. ATCC 31555. Carbohydr. Polym. 2012, 90, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.E.; Park, H.S.; Kim, J.-A.; Nagappan, A.; Zhang, J.; Kang, S.; Won, C.; Cho, J.H.; Kim, E.H.; Kim, G.S. Anti-oxidant and anti-inflammatory effects of Fraxinus rhynchophylla on lipopolysaccharide (LPS)-induced murine Raw 264.7 cells. J. Biomed. Res. 2012, 13, 331–338. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, S.A.; Choi, Y.M.; Kwon, H.K.; Shim, W.; Lee, G.; Choi, S. Capric acid inhibits NO production and STAT3 activation during LPS-induced osteoclastogenesis. PLoS ONE 2011, 6, e27739. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kurachi, M.; Yamaguchi, K.; Oda, T. Stimulation of multiple cytokine production in mice by alginate oligosaccharides following intraperitoneal administration. Carbohydr. Res. 2007, 342, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Ruland, J. Return to homeostasis: Downregulation of NF-κB responses. Nat. Immunol. 2011, 12, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.A.; Campagna, L.; Molinero, L.L.; Cerliani, J.P.; Croci, D.O.; Ilarregui, J.M.; Fuertes, M.B.; Nojek, I.M.; Fededa, J.P.; Zwirner, N.W.; et al. Nuclear factor (NF)-κB controls expression of the immunoregulatory glycan-binding protein galectin-1. Mol. Immunol. 2011, 48, 1940–1949. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Toscano, M.A. Turning “sweet” on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009, 9, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.; Lee, C.; Lee, K.S.; Ham, C.S.; Seong, R.H.; Kim, S.S.; Jeon, S.H. CD7 expression and galectin-1-induced apoptosis of immature thymocytes are directly regulated by NF-κB upon T-cell activation. Biochem. Biophys. Res. Commun. 2008, 370, 149–153. [Google Scholar] [CrossRef] [PubMed]
- De Hemptinne, V.; Rondas, D.; Toepoel, M.; Vancompernolle, K. Phosphorylation on Thr-106 and NO-modification of glyoxalase I suppress the TNF-induced transcriptional activity of NF-kappaB. Mol. Cell. Biochem. 2009, 325, 169–178. [Google Scholar] [CrossRef]
- Barrionuevo, P.; Beigier-Bompadre, M.; Ilarregui, J.M.; Toscano, M.A.; Bianco, G.A.; Isturiz, M.A.; Rabinovich, G.A. A novel function for galectin-1 at the crossroad of innate and adaptive immunity: Galectin-1 regulates monocyte/macrophage physiology through a nonapoptotic ERK-dependent pathway. J. Immunol. 2007, 178, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Blackwood, R.A.; Ernst, J.D. Characterization of Ca2(+)-dependent phospholipid binding, vesicle aggregation and membrane fusion by annexins. Biochem. J. 1990, 266, 195–200. [Google Scholar] [PubMed]
- Kang, A.D.; Wong, P.M.; Chen, H.; Castagna, R.; Chung, S.W.; Sultzer, B.M. Restoration of lipopolysaccharide-mediated B-cell response after expression of a cDNA encoding a GTP-binding protein. Infect. Immun. 1996, 64, 4612–4617. [Google Scholar] [PubMed]
- Frey, B.; Munoz, L.E.; Pausch, F.; Sieber, R.; Franz, S.; Brachvogel, B.; Poschl, E.; Schneider, H.; Rodel, F.; Sauer, R.; et al. The immune reaction against allogeneic necrotic cells is reduced in Annexin A5 knock out mice whose macrophages display an anti-inflammatory phenotype. J. Cell. Mol. Med. 2009, 13, 1391–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Yuan, Q.; Sultzer, B.M.; Chung, S.W.; Wong, P.M. The involvement of Ran GTPase in lipopolysaccharide endotoxin-induced responses. J. Endotoxin Res. 2001, 7, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Scandalios, J.G. Oxygen Stress and Superoxide Dismutases. Plant Physiol. 1993, 101, 7–12. [Google Scholar] [PubMed]
- Fadeel, B.; Ahlin, A.; Henter, J.I.; Orrenius, S.; Hampton, M.B. Involvement of caspases in neutrophil apoptosis: regulation by reactive oxygen species. Blood 1998, 92, 4808–4818. [Google Scholar] [PubMed]
- Forman, H.J.; Torres, M. Redox signaling in macrophages. Mol. Aspects Med. 2001, 22, 189–216. [Google Scholar] [CrossRef] [PubMed]
- Marikovsky, M.; Ziv, V.; Nevo, N.; Harris-Cerruti, C.; Mahler, O. Cu/Zn superoxide dismutase plays important role in immune response. J. Immunol. 2003, 170, 2993–3001. [Google Scholar] [CrossRef] [PubMed]
- Pavlides, S.; Tsirigos, A.; Vera, I.; Flomenberg, N.; Frank, P.G.; Casimiro, M.C.; Wang, C.; Fortina, P.; Addya, S.; Pestell, R.G.; et al. Loss of stromal caveolin-1 leads to oxidative stress, mimics hypoxia and drives inflammation in the tumor microenvironment, conferring the “reverse Warburg effect”: A transcriptional informatics analysis with validation. Cell Cycle 2010, 9, 2201–2219. [Google Scholar] [CrossRef] [PubMed]
- Vats, D.; Mukundan, L.; Odegaard, J.I.; Zhang, L.; Smith, K.L.; Morel, C.R.; Wagner, R.A.; Greaves, D.R.; Murray, P.J.; Chawla, A. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 2006, 4, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Cramer, T.; Yamanishi, Y.; Clausen, B.E.; Forster, I.; Pawlinski, R.; Mackman, N.; Haase, V.H.; Jaenisch, R.; Corr, M.; Nizet, V.; et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 2003, 112, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Patron, N.J.; Rogers, M.B.; Keeling, P.J. Gene replacement of fructose-1,6-bisphosphate aldolase supports the hypothesis of a single photosynthetic ancestor of chromalveolates. Eukaryot. Cell 2004, 3, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, F.; Gurniak, C.B.; Fleischer, B.; Kirfel, G.; Witke, W. Immunological responses and actin dynamics in macrophages are controlled by N-cofilin but are independent from ADF. PLoS ONE 2012, 7, e36034. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, P.; Drubin, D.G. Cofilin promotes rapid actin filament turnover in vivo. Nature 1997, 388, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, B.W.; Bamburg, J.R. ADF/cofilin: A functional node in cell biology. Trends Cell Biol. 2010, 20, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Hotulainen, P.; Paunola, E.; Vartiainen, M.K.; Lappalainen, P. Actin-depolymerizing factor and cofilin-1 play overlapping roles in promoting rapid F-actin depolymerization in mammalian nonmuscle cells. Mol. Biol. Cell 2005, 16, 649–664. [Google Scholar] [CrossRef]
- Van Troys, M.; Huyck, L.; Leyman, S.; Dhaese, S.; Vandekerkhove, J.; Ampe, C. Ins and outs of ADF/cofilin activity and regulation. Eur. J. Cell Biol. 2008, 87, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Kaganovsky, E.; Rahimipour, S.; Ben-Aroya, N.; Okon, E.; Koch, Y. Two forms of gonadotropin-releasing hormone (GnRH) are expressed in human breast tissue and overexpressed in breast cancer: A putative mechanism for the antiproliferative effect of GnRH by down-regulation of acidic ribosomal phosphoproteins P1 and P2. Cancer Res. 2002, 62, 1036–1044. [Google Scholar] [PubMed]
- Vadiveloo, P.K.; Keramidaris, E.; Morrison, W.A.; Stewart, A.G. Lipopolysaccharide-induced cell cycle arrest in macrophages occurs independently of nitric oxide synthase II induction. Biochim. Biophys. Acta 2001, 1539, 140–146. [Google Scholar] [CrossRef]
- Xaus, J.; Comalada, M.; Valledor, A.F.; Lloberas, J.; Lopez-Soriano, F.; Argiles, J.M.; Bogdan, C.; Celada, A. LPS induces apoptosis in macrophages mostly through the autocrine production of TNF-α. Blood 2000, 95, 3823–3831. [Google Scholar] [PubMed]
- Rodriguez-Prados, J.C.; Traves, P.G.; Cuenca, J.; Rico, D.; Aragones, J.; Martin-Sanz, P.; Cascante, M.; Bosca, L. Substrate fate in activated macrophages: A comparison between innate, classic, and alternative activation. J. Immunol. 2010, 185, 605–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haug, A.; Larsen, B.; Smidsrød, O. A study of the constitution of alginic acid by partial acid hydrolysis. Acta Chem. Scand. 1966, 20, 183–190. [Google Scholar] [CrossRef]
- Linker, A.; Jones, R.S. A new polysaccharide resembling alginic acid isolated from pseudomonads. J. Biol. Chem. 1966, 241, 3845–3851. [Google Scholar] [PubMed]
- Xu, X.; Iwamoto, Y.; Kitamura, Y.; Oda, T.; Muramatsu, T. Root growth-promoting activity of unsaturated oligomeric uronates from alginate on carrot and rice plants. Biosci. Biotechnol. Biochem. 2003, 67, 2022–2025. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Lan, Z.; Sun, X.; Shi, L.; Liu, Q.; Ni, J. Proteomic analysis of lanthanum citrate-induced apoptosis in human cervical carcinoma SiHa cells. BioMetals 2010, 23, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Midha, M.K.; Verma, H.N.; Basu, A.; Rao, K.V.; Manivel, V. Characterizing virulence-specific perturbations in the mitochondrial function of macrophages infected with Mycobacterium tuberculosis. Sci. Rep. 2013, 3, 1328. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Bi, D.-C.; Li, C.; Fang, W.-S.; Zhou, R.; Li, S.-M.; Chi, L.-L.; Wan, M.; Shen, L.-M. Morphological and Proteomic Analyses Reveal that Unsaturated Guluronate Oligosaccharide Modulates Multiple Functional Pathways in Murine Macrophage RAW264.7 Cells. Mar. Drugs 2015, 13, 1798-1818. https://doi.org/10.3390/md13041798
Xu X, Bi D-C, Li C, Fang W-S, Zhou R, Li S-M, Chi L-L, Wan M, Shen L-M. Morphological and Proteomic Analyses Reveal that Unsaturated Guluronate Oligosaccharide Modulates Multiple Functional Pathways in Murine Macrophage RAW264.7 Cells. Marine Drugs. 2015; 13(4):1798-1818. https://doi.org/10.3390/md13041798
Chicago/Turabian StyleXu, Xu, De-Cheng Bi, Chao Li, Wei-Shan Fang, Rui Zhou, Shui-Ming Li, Lian-Li Chi, Min Wan, and Li-Ming Shen. 2015. "Morphological and Proteomic Analyses Reveal that Unsaturated Guluronate Oligosaccharide Modulates Multiple Functional Pathways in Murine Macrophage RAW264.7 Cells" Marine Drugs 13, no. 4: 1798-1818. https://doi.org/10.3390/md13041798
APA StyleXu, X., Bi, D.-C., Li, C., Fang, W.-S., Zhou, R., Li, S.-M., Chi, L.-L., Wan, M., & Shen, L.-M. (2015). Morphological and Proteomic Analyses Reveal that Unsaturated Guluronate Oligosaccharide Modulates Multiple Functional Pathways in Murine Macrophage RAW264.7 Cells. Marine Drugs, 13(4), 1798-1818. https://doi.org/10.3390/md13041798






