White Shrimp Litopenaeus vannamei That Have Received Gracilaria tenuistipitata Extract Show Early Recovery of Immune Parameters after Ammonia Stressing
Abstract
:1. Introduction
2. Results
2.1. Immune Parameters of Shrimp Immersed in Seawater Containing G. tenuistipitata Extract (GTE) Prior to and after Ammonia Stressing
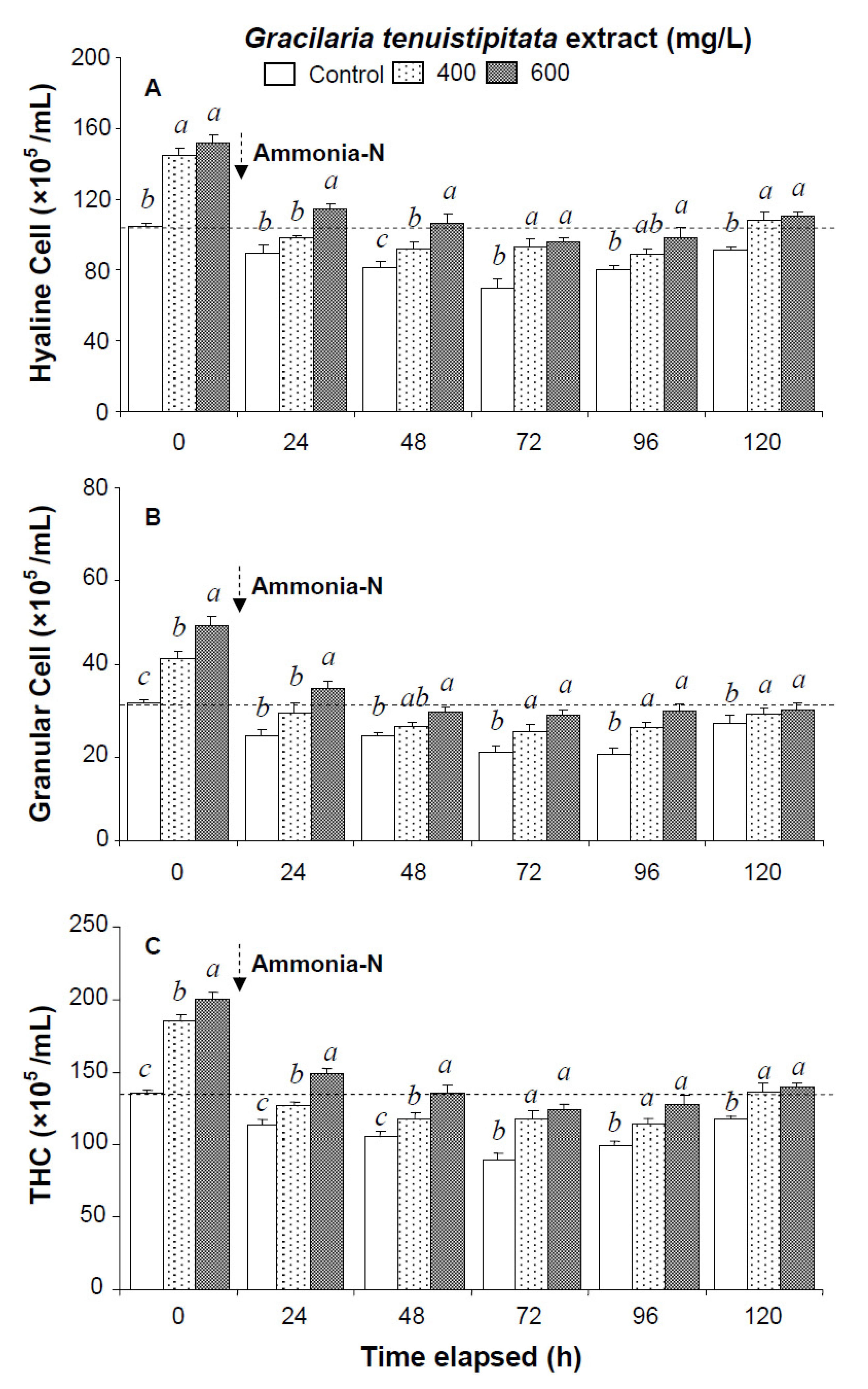


2.2. Transcript Levels of LGBP, PX, IB, ppA, proPO I, proPO II, α2-M, cytMnSOD, mtMnSOD, ecCuZnSOD, and HSP70 in Shrimp Immersed in Seawater Containing G. tenuistipitata Extract (GTE) Prior to and after Ammonia Stressing



3. Discussion
4. Materials and Methods
4.1. Preparation of G. tenuistipitata Extract (GTE)
4.2. Experimental Design for the Immersion Test
4.3. Immune Parameters of Shrimp Immersed in Seawater Containing G. tenuistipitata Extract (GTE) Prior to and after Ammonia Stressing
4.4. Measurements of Immune Parameters
4.5. Transcript Levels of LGBP, PX, IB, ppA, proPO I, proPO II, α2-M, cytMnSOD, mtMnSOD, ecCuZnSOD, and HSP70 in Shrimp Immersed in Seawater Containing G. tenuistipitata Extract (GTE) Prior to and after Ammonia Stressing
| Gene | Primer Name | Sequence 5′ to 3′ | Amplicon | Reference/GenBank |
|---|---|---|---|---|
| LGBP | Liva LGBP qPCR F | CGG CAA CCA GTA CGG AGG AAC | 115 bp | EU102286 |
| Liva LGBP qPCR R | GTG GAA ATC ATC GGC GAA GGA G | |||
| Peroxinectin | Liva PX qPCR F | ATC CAG CAG CCA GGT ATG | 147 bp | [12] |
| Liva PX qPCR R | CAG ACT CAT CAG ATC CAT TCC | |||
| Integrin β | Liva It β qPCR F | TTG GGC ATC GTG TTC GGA CTC | 184 bp | GQ889365 |
| Liva It β qPCR R | TGA AGG TGT TGG TCG CAG GTC | |||
| ppA | Liva ppA qPCR F | CTA GAG ACG TCG GTG TCA TCA CC | 151 bp | AY368151 |
| Liva ppA qPCR R | AAC TTG CCG TCC GAA GTG CG | |||
| proPO I | Liva proPO I qPCR F | ACG TCA CTT CCG GCA AGC GA | 156 bp | AY723296 |
| Liva proPO I qPCR R | CCT CCT TGT GAG CGT TGT CAG G | |||
| proPO II | Liva proPO II qPCR F | ACC ACT GGC ACT GGC ACC TCG TCT A | 161 bp | EU373096 |
| Liva proPO II qPCR R | TCG CCA GTT CTC GAG CTT CTG CAC | |||
| α2-macroglobulin | Liva A2M qPCR F | GCA CGT AAT CAA GAT CCG | 204 bp | DQ988330 |
| Liva A2M qPCR R | CCC ATC TCA TTA GCA CAA AC | |||
| cytMnSOD | Liva cytMnSOD qPCR F | TGA CGA GAG CTT TGG ATC ATT CC | 155 bp | DQ029053 |
| Liva cytMnSOD qPCR R | TGA TTT GCA AGG GAT CCT GGT T | |||
| mtMnSOD | Liva mtMnSOD qPCR F | CAG ACT TGC CCT ACG ATT AC | 216 bp | KP099968 |
| Liva mtMnSOD qPCR R | AGA TGG TGT GAT TGA TGT GAC | |||
| ecCuZnSOD | Liva CuZnSOD qPCR F | CGC GGG AGA CAC AGC TGA TTT C | 164 bp | HM371157 |
| Liva CuZnSOD qPCR R | GAA ATC CAG GGT GCC GGA GA | |||
| HSP70 | Liva Hsp70 qPCR F | CCT CCT ACG TCG CCT TCA CAG ACA | 233 bp | AY645906 |
| Liva Hsp70 qPCR R | GGG GTA GAA GGT CTT CTT GTC TCC C | |||
| EF1α | Liva EF1α qPCR F | ATG GTT GTC AAC TTT GCC CC | 500 bp | GU136229 |
| Liva EF1α qPCR R | TTG ACC TCC TTG ATC ACA CC |
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carroll, M.C.; Janeway, C.A., Jr. Innate immune recognition: Mechanism and pathways. Immunol. Rev. 2000, 173, 89–97. [Google Scholar]
- Rowley, A.F.; Powell, A. Invertebrate immune systems specific, quasi-specific, or nonspecific? J. Immunol. 2007, 179, 7209–7214. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Wang, J.X. Pattern recognition receptors acting in innate immune system of shrimp against pathogen infections. Fish Shellfish Immunol. 2013, 34, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Cerenius, L.; Lee, B.L.; Söderhäll, K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008, 29, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Jiravanichpaisal, P.; Lee, B.L.; Söderhäll, K. Cell-mediated immunity in arthropods: Hematopoiesis, coagulation, melanization and opsonization. Immunobiology 2006, 211, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Liu, C.H.; Tsai, C.T.; Chen, J.C. Molecular cloning and characterisation of a pattern recognition molecule, lipopolysaccharide- and β-1,3-glucan binding protein (LGBP) from the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2005, 18, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Vaseeharan, B.; Chen, J.C. Identification and phylogenetic analysis on lipopolysaccharide and β-1,3-glucan binding protein (LGBP) of kuruma shrimp Marsupenaeus japonicus. Dev. Comp. Immunol. 2008, 32, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Tassanakajon, A.; Somboonwiwat, K.; Supungul, P.; Tang, S. Discovery of immune molecules and their crucial functions in shrimp immunity. Fish Shellfish Immunol. 2013, 34, 954–967. [Google Scholar] [CrossRef] [PubMed]
- Cerenius, L.; Söderhäll, K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004, 198, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiravanichpaisal, P.; Cerenius, L.; Söderhäll, I.; Söderhäll, K. Phenoloxidase is an important component of the defense against Aeromonas hydrophila infection in a crustacean, Pacifastacus leniusculus. J. Biol. Chem. 2007, 282, 33593–33598. [Google Scholar] [CrossRef] [PubMed]
- Amparyup, P.; Charoensapsri, W.; Tassanakajon, A. Prophenoloxidase system and its role in shrimp immune response against major pathogens. Fish Shellfish Immunol. 2013, 34, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Cheng, W.; Kuo, C.M.; Chen, J.C. Molecular cloning and characterisation of a cell adhesion molecule, peroxinectin from the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2004, 17, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chen, J.C.; Chen, Y.Y.; Liu, C.H.; Cheng, W.; Hsu, C.H.; Tsui, W.C. Characterization of white shrimp Litopenaeus vannamei integrin β and its role in immunomodulation by dsRNA-mediated gene silencing. Dev. Comp. Immunol. 2013, 40, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Bayne, C.J. Phagocytosis and non-self recognition in invertebrates-phagocytosis appears to be an ancient line of defense. Bioscience 1990, 40, 723–731. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, J.C.; Morni, W.Z.; Putra, D.F.; Huang, C.L.; Li, C.C.; Hsieh, J.F. Vaccination enhances early immune responses in white shrimp Litopenaeus vannamei after secondary exposure to Vibrio alginolyticus. PLoS ONE 2013, 8, e69722. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Cedeño, R.; Rodríguez, J.; van der Knapp, W.P.W.; Mialhe, E.; Bachère, E. Measurement of reactive oxygen intermediate production in haemocytes of the penaeid shrimp, Penaeus vannamei. Aquaculture 2000, 191, 89–107. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical and superoxide dismutase. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Gómez-Anduro, G.A.; Barillas-Mury, C.V.; Peregrino-Uriarte, A.B.; Gupta, L.; Gollas-Galván, T.; Hernández-López, J.; Yepiz-Plascencia, G. The cytosolic manganese superoxide dismutase from the shrimp Litopenaeus vannamei: Molecular cloning and expression. Dev. Comp. Immunol. 2006, 30, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chen, J.; Jiang, D.; Liao, S.; Wang, A. Transcriptional regulation of extracellular copper zinc superoxide dismutase from white shrimp Litopenaeus vannamei following Vibrio alginolyticus and WSSV infection. Fish Shellfish Immunol. 2011, 30, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, H.; Vriz, S.; Angelier, N. Molecular characterization of a heat shock cognate cDNA of zeabrafish, hsc70, and developmental expression of the corresponding transcripts. Dev. Comp. Immunol. 1997, 21, 223–233. [Google Scholar]
- FAO. 2011 FAO Yearbook. In Fishery and Aquaculture Statistics; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; p. 105. [Google Scholar]
- Chen, J.C.; Liu, P.C.; Lin, Y.T. Super intensive culture of red-tailed shrimp Penaeus penicillatus. J. World Aquac. Soc. 1988, 19, 127–131. [Google Scholar] [CrossRef]
- Chen, J.C.; Liu, P.C.; Lin, Y.T.; Lee, C.K. Highly intensive culture study of tiger prawn Penaeus monodon in Taiwan. In Aquaculture-A Biotechnology in Progress; de Pauw, D., Jaspers, E., Ackeforos, H., Wilkins, N., Eds.; European Aquaculture Society: Bredene, Belgium, 1989; pp. 377–382. [Google Scholar]
- Lin, Y.C.; Chen, J.C. Acute toxicity of ammonia on Litopenaeus vannamei Boone juveniles at different salinity levels. J. Exp. Mar. Biol. Ecol. 2001, 259, 109–119. [Google Scholar] [CrossRef]
- Chen, J.C.; Lin, C.Y. Effects of ammonia on growth and molting of Penaeus monodon juveniles. Comp. Biochem. Physiol. C Comp. Pharmacol. 1992, 101, 449–452. [Google Scholar] [CrossRef]
- Chen, J.C.; Chen, C.T. Changes of osmotic and electrolyte concentrations in the haemolymph of Penaeus japonicus exposed to ambient ammonia. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1996, 114, 35–38. [Google Scholar] [CrossRef]
- Liu, C.H.; Chen, J.C. Effect of ammonia on the immune response of white shrimp Litopenaeus vannamei and its susceptibility to Vibrio alginolyticus. Fish Shellfish Immunol. 2004, 16, 321–334. [Google Scholar] [CrossRef]
- Hou, W.Y.; Chen, J.C. The immunostimulatory effect of hot-water extract of Gracilaria tenuistipitata on the white shrimp Litopenaeus vannamei and its resistance against Vibrio alginolyticus. Fish Shellfish Immunol. 2005, 19, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.T.; Chen, J.C. White shrimp Litopenaeus vannamei that received the hot-water extract of Gracilaria teniustisitata showed earlier recovery in immunity after a Vibrio algnolyticus injection. Fish Shellfish Immunol. 2009, 26, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Sirirustananun, N.; Chen, J.C.; Lin, Y.C.; Yeh, S.T.; Liou, C.H.; Sim, S.S.; Chiew, S.L. Dietary administration of a Gracilaria tenuistipitata extract enhances the immune response and resistance against Vibrio alginolyticus and white spot syndrome virus in the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2011, 31, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.T.; Lin, Y.C.; Huang, C.L.; Chen, J.C. White shrimp Litopenaeus vannamei that received the hot-water extract of Gracilaria tenuistipitata showed protective innate immunity and up-regulation of gene expressions after low-salinity stress. Fish Shellfish Immunol. 2010, 28, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.T.; Li, C.C.; Tsuei, W.J.; Chen, J.C. The protective immunity of white shrimp Litopenaeus vannamei that had been immersed in the hot-water extract of Gracilaria tenuistipitata and subjected to combined stresses of Vibrio alginolyticus injection and temperature change. Fish Shellfish Immunol. 2010, 29, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Yeh, S.T.; Li, C.C.; Chen, L.L.; Cheng, A.C.; Chen, J.C. An immersion of Gracilaria tenuistipitata extract improves the immunity and survival of white shrimp Litopenaeus vannamei challenged with white spot syndrome virus. Fish Shellfish Immunol. 2011, 31, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Yue, F.; Pan, L.; Xie, P.; Zheng, D.; Li, J. Immune response and expression of immune related genes in swimming crab Portunus trituberculatus exposed to elevated ambient ammonia-N stress. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 157, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chen, J.C.; Lin, Y.Y.; Putra, D.F.; Kitikiew, S.; Li, C.C.; Hsieh, J.F.; Liou, C.H.; Yeh, S.T. Shrimp that have received carrageenan via immersion and diet exhibit immunocompetence in phagocytois despite a post-plateau in immune parameters. Fish Shellfish Immunol. 2014, 36, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Kitikiew, S.; Chen, J.C.; Putra, D.F.; Lin, Y.C.; Yeh, S.T.; Liou, C.H. Fucoidan effectively provokes the innate immunity of white shrimp Litopenaeus vannamei and its resistance against experimental Virio alginolyticus infection. Fish Shellfish Immunol. 2013, 34, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.J.; Söderhäll, K.; Hamiliton, M. β-1,3-Glucan induced cellular defense reaction in the shore crab, Cracinus maenas. Comp. Biochem. Physiol. A Physiol. 1984, 77, 636–639. [Google Scholar] [CrossRef]
- Le Moullac, G.; Haffner, P. Environmental factors affecting immune responses in Crustacea. Aquaculture 2000, 191, 121–131. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Sim, S.S.; Chiew, S.L.; Yeh, S.T.; Liou, C.H.; Chen, J.C. Dietary administration of a Gracilaria tenuistipitata extract produces protective immunity of white shrimp Litopenaeus vannamei in response to ammonia stress. Aquaculture 2012, 370–371, 26–31. [Google Scholar]
- Yu, B.P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994, 74, 139–162. [Google Scholar] [PubMed]
- Liu, C.H.; Cheng, W.; Chen, J.C. The peroxinectin of white shrimp Litopenaeus vannamei is synthesised in the semi-granular and granular cells, and its transcription is up-regulated with Vibrio alginolyticus infection. Fish Shellfish Immunol. 2005, 18, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Chang, P.S.; Chen, H.Y. Differential time-series expression of immune-related genes of Pacific white shrimp Litopenaeus vannamei in response to dietary inclusion of β-1,3-glucan. Fish Shellfish Immunol. 2008, 24, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Amparyup, P.; Sutthangkul, J.; Charoensapsri, W.; Tassanakajon, A. Pattern recognition protein to lipopolysaccharide and β-1,3-glucan and activates shrimp prophenoloxidase system. J. Biol. Chem. 2012, 287, 10060–10069. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chen, J.C.; Kong, H.Y.; Kuo, Y.I.; Lin, Y.C.; Chang, Y.H.; Huang, C.H. Lipopolysaccharide and β-1,3-glucan binds to seaweed polysaccharide and activates prophenoloxidase system in white shrimp. 2015; Unpublished work. [Google Scholar]
- Lin, Y.C.; Tayag, C.M.; Huang, C.L.; Tsui, W.C.; Chen, J.C. White shrimp Litopenaeus vannamei that had received the hot-water extract of Spirulina platensis showed earlier recovery in immunity and up-regulation of gene expressions after pH stress. Fish Shellfish Immunol. 2010, 29, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.Y.; Liu, K.F.; Liao, I.C.; Song, Y.L. Cloning and molecular characterization of heat shock cognate 70 from tiger shrimp (Penaeus monodon). Cell Stress Chaperones 2004, 9, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, F.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Terho, T.T.; Hartiala, K. Methods for determination of the sulfate content of glycosaminoglycans. Anal. Biochem. 1971, 41, 471–476. [Google Scholar] [CrossRef]
- Chao, K.P.; Su, Y.C.; Chen, S.C. Chemical composition and potential for utilization of the Rhizoclonium sp. J. Appl. Phycol. 1999, 11, 525–533. [Google Scholar] [CrossRef]
- Hayashi, T.; Hayashi, K. Calcium spirulina, an inhibitor of enveloped virus replication, from a blue green alga Spirulina platensis. J. Nat. Prod. 1996, 59, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.M.; Rankin, S.M.; Keeley, L.L. Characterization of the molt stages in Penaeus vannamei: Setogenesis and hemolymph levels of total protein ecdysteroids, and glucose. Biol. Bull. 1988, 175, 85–192. [Google Scholar] [CrossRef]
- Söderhäll, K.; Unestam, T. Activation of serum prophenoloxidase in arthropod immunity: The specificity of cell wall glucan activation and activation by purified fungal glycoprotein of crayfish phenoloxidase. Can. J. Microbiol. 1979, 25, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Hernández-López, J.; Gollas-Galván, T.; Vargas-Albores, F. Activation of the prophenoloxidase system of the brown shrimp (Penaeus californiensis Holmes). Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1996, 113, 61–66. [Google Scholar] [CrossRef]
- Bell, K.L.; Smith, V.J. In vitro superoxide production by hyaline cells of the shore crab Carcinus maenas (L.). Dev. Comp. Immunol. 1993, 17, 211–219. [Google Scholar] [CrossRef]
- Biagini, G.; Sala, D.; Zini, I. Diethyldithiocarbamate, a superoxide dismutase inhibitor, counteracts the maturation of ischemic-like lesions caused by endothelin-1 intrastriatal injection. Neurosci. Lett. 1995, 90, 212–216. [Google Scholar] [CrossRef]
- Soteo-Mundo, R.R.; Islas-Osuna, M.A.; de-la-Re-Vega, E.; Hernández-López, J.; Vargas-Albores, F.; Yepiz-Plascencia, G. cDNA cloning of the lysozyme of the white shrimp Penaeus vannamei. Fish Shellfish Immunol. 2003, 15, 325–331. [Google Scholar] [CrossRef]
- Ellis, A.E. Lysozyme assay. In Technique in Fish Immunology-1; Stolen, J.S., Fletcher, T.C., Anderson, D.P., Roberson, B.S., van Muiswinkel, W.B., Eds.; SOS Publications: Fair Haven, NJ, USA, 1990; pp. 101–103. [Google Scholar]
- Tayag, C.M.; Lin, Y.C.; Li, C.C.; Liou, C.H.; Chen, J.C. Administration of the hot-water extract of Spirulina platensis enhanced the immune response of white shrimp Litopenaeus vannamei and its resistance against Vibrio alginolyticus. Fish Shellfish Immunol. 2010, 28, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.N. A rapid and sensitivity method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Chen, J.C. Study on the oxyhemocyanin, deoxyhemocyanin, oxygen affinity and acid-base balance of Marsupenaeus japonicus following exposure to combined elevated nitrite and nitrate. Aquat. Toxicol. 2002, 61, 181–193. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, J.C.; Man, S.N.; Morni, W.Z.; Suhaili, A.S.N.; Cheng, S.Y.; Hsu, C.H. Modulation of innate immunity and gene expressions in white shrimp Litopenaeus vannamei following long-term starvation and re-feeding. Results Immunol. 2012, 2, 148–156. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-Y.; Chen, J.-C.; Lin, Y.-C.; Yeh, S.-T.; Huang, C.-L. White Shrimp Litopenaeus vannamei That Have Received Gracilaria tenuistipitata Extract Show Early Recovery of Immune Parameters after Ammonia Stressing. Mar. Drugs 2015, 13, 3606-3624. https://doi.org/10.3390/md13063606
Chen Y-Y, Chen J-C, Lin Y-C, Yeh S-T, Huang C-L. White Shrimp Litopenaeus vannamei That Have Received Gracilaria tenuistipitata Extract Show Early Recovery of Immune Parameters after Ammonia Stressing. Marine Drugs. 2015; 13(6):3606-3624. https://doi.org/10.3390/md13063606
Chicago/Turabian StyleChen, Yu-Yuan, Jiann-Chu Chen, Yong-Chin Lin, Su-Tuen Yeh, and Chien-Lun Huang. 2015. "White Shrimp Litopenaeus vannamei That Have Received Gracilaria tenuistipitata Extract Show Early Recovery of Immune Parameters after Ammonia Stressing" Marine Drugs 13, no. 6: 3606-3624. https://doi.org/10.3390/md13063606





