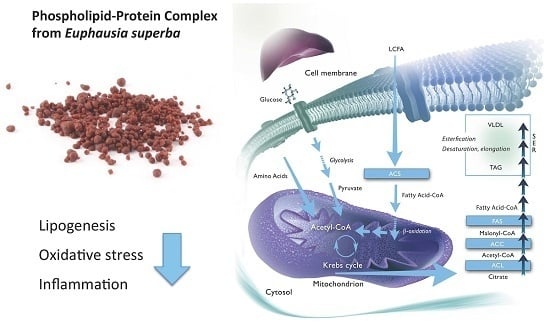
A Phospholipid-Protein Complex from Krill with Antioxidative and Immunomodulating Properties Reduced Plasma Triacylglycerol and Hepatic Lipogenesis in Rats
Abstract
:1. Introduction
2. Results
2.1. Animals and Diets

2.2. Plasma Lipids and Fatty Acid Composition

| Diets | |||
|---|---|---|---|
| Control | 11% PPC | ||
| SFAs | 30.25 ± 0.69 | 29.07 ± 1.39 | |
| C10:0 | 0.01 ± 0.00 | 0.01 ± 0.00 | |
| C12:0 | 0.03 ± 0.00 | 0.03 ± 0.00 | |
| C14:0 | 0.65 ± 0.21 | 0.76 ± 0.12 | |
| C16:0 | 19.57 ± 1.18 | 19.91 ± 0.86 | |
| C18:0 | 8.58 ± 1.87 | 6.87 ± 0.95 | |
| C20:0 | 0.07 ± 0.01 | 0.05 ± 0.01 ** | |
| C22:0 | 0.10 ± 0.02 | 0.08 ± 0.01 | |
| MUFAs | 26.69 ± 7.16 | 21.74 ± 2.91 | |
| C16:1n-7 | 3.09 ± 1.49 | 3.51 ± 1.27 | |
| C16:1n-9 | 0.36 ± 0.15 | 0.23 ± 0.04 | |
| C18:1n-7 | 3.51 ± 1.23 | 3.07 ± 0.54 | |
| C18:1n-9 (OA) | 18.72 ± 4.32 | 13.94 ± 1.56 * | |
| C20:1n-7 | 0.26 ± 0.09 | 0.24 ± 0.06 | |
| C20:1n-9 | 0.22 ±0.07 | 0.12 ± 0.03 ** | |
| C22:1n-7 | 0.04 ± 0.02 | 0.04 ± 0.01 | |
| C22:1n-9 | 0.01 ± 0.00 | 0.03 ±0.01 ** | |
| C24:1n-9 | 0.19 ± 0.06 | 0.21 ± 0.03 | |
| PUFAs | 42.92 ± 8.20 | 49.08 ± 5.72 | |
| n-9 PUFAs | 0.21 ± 0.03 | 0.09 ± 0.01 *** | |
| C20:3n-9 (MA) | 0.21 ± 0.03 | 0.09 ± 0.01 *** | |
| n-6 PUFAs | 38.10 ± 6.08 | 23.39 ± 2.51 *** | |
| C18:2n-6 (LA) | 18.97 ± 1.36 | 17.51 ± 1.84 | |
| C18:3n-6 (GLA) | 0.28 ± 0.05 | 0.10 ± 0.01 *** | |
| C20:3n-6 (DGLA) | 0.79 ± 0.25 | 0.55 ± 0.04 * | |
| C20:4n-6 (AA) | 16.97 ± 5.51 | 4.90 ± 0.93 *** | |
| C22:4n-6 | 0.44 ± 0.04 | 0.05 ± 0.01 *** | |
| C22:5n-6 (DPA) | 0.24 ± 0.05 | 0.06 ± 0.00 *** | |
| n-3 PUFAs | 4.62 ± 0.67 | 25.61 ± 1.75 *** | |
| C18:3n-3 (ALA) | 0.76 ± 0.09 | 1.41 ± 0.14 *** | |
| C18:4n-3 | 0.03 ± 0.01 | 0.51 ± 0.07 *** | |
| C20:4n-3 | 0.10 ± 0.01 | 0.54 ± 0.14 *** | |
| C20:5n-3 (EPA) | 0.54 ± 0.12 | 12.72 ± 1.41 *** | |
| C21:5n-3 (HPA) | 0.01 ± 0.00 | 0.28 ± 0.06 *** | |
| C22:5n-3 (DPA) | 0.62 ± 0.07 | 2.98 ± 0.41 *** | |
| C22:6n-3 (DHA) | 2.56 ± 0.53 | 7.17 ± 0.64 *** | |

2.3. Effect on Antioxidant Status
| Diets | |||||
|---|---|---|---|---|---|
| Symbol | Function | Control | 11% PPC | p-Value | |
| Srebf1 | Transcription factor | 1.00 ± 0.35 | 0.77 ± 0.08 | 0.189 | |
| Ppara | Transcription factor | 1.00 ± 0.34 | 1.65 ± 1.06 | 0.262 | |
| Cd36/Fat | Fatty acid import | 1.00 ± 0.23 | 1.27 ± 0.51 | 0.339 | |
| Cpt1a | β-oxidation | 1.00 ± 0.33 | 2.07 ± 1.22 | 0.092 | |
| Cpt2 | β-oxidation | 1.00 ± 0.33 | 2.05 ± 1.19 | 0.118 | |
| Cact/Slc25a20 | β-oxidation (transport) | 1.00 ± 0.19 | 1.53 ± 0.68 | 0.162 | |
| Acox1 | β-oxidation | 1.00 ± 0.09 | 1.65 ± 0.44 | 0.017 | |
| Hmgcs2 | Ketogenesis | 1.00 ± 0.23 | 1.38 ± 0.70 | 0.320 | |
| Fasn | Fatty acid synthesis | 1.00 ± 0.71 | 0.37 ± 0.26 | 0.103 | |
| Acaca | Fatty acid synthesis | 1.00 ± 0.53 | 0.71 ± 0.23 | 0.298 | |
| Elo1 | Fatty acid elongation | 1.00 ± 0.10 | 1.06 ± 0.27 | 0.690 | |
| Scd1 | Δ9 desaturation | 1.00 ± 0.61 | 0.94 ± 0.59 | 0.890 | |
| Fads1 | ∆5 desaturation of fatty acids | 1.00 ± 0.18 | 0.53 ± 0.22 | 0.011 | |
| Fads2 | Δ6 desaturation of fatty acids | 1.00 ± 0.25 | 0.62 ± 0.24 | 0.061 | |
| ApoB | Cholesterol import | 1.00 ± 0.10 | 1.06 ± 0.20 | 0.617 | |
| Ldlr | Cholesterol import | 1.00 ± 0.19 | 1.03 ± 0.37 | 0.898 | |
| Hmgcr | Cholesterol synthesis | 1.00 ± 0.23 | 1.07± 0.19 | 0.636 | |
| Cyp7a1 | Bile synthesis | 1.00 ± 0.43 | 1.91 ± 1.64 | 0.304 | |
| Soat 1/Acat | Cholesterol ester formation | 1.00 ± 0.14 | 0.98 ± 0.31 | 0.888 | |
| Gpam | TAG synthesis (glycerolipid synthesis) | 1.00 ± 0.20 | 0.88 ± 0.18 | 0.386 | |
| Dgat | TAG synthesis | 1.00 ± 0.17 | 1.24 ± 0.26 | 0.166 | |
| Mttp | Lipoprotein assembly | 1.00 ± 0.11 | 1.20 ± 0.34 | 0.301 | |
| Lipc | Hepatisk lipase | 1.00 ± 0.13 | 0.98 ± 0.22 | 0.850 | |
| Aadac | Triglycerid lipase activity | 1.00 ± 0.11 | 1.20 ± 0.19 | 0.112 | |
| Sod2 | Mitochondrial antioxidant defense system | 1.00 ± 0.14 | 1.37 ± 0.26 | 0.038 | |
2.4. Effect on Systemic Inflammation

2.5. Hepatic Fatty Acid Metabolism

| Diets | |||
|---|---|---|---|
| Control | 11% PPC | ||
| SFAs | 33.89 ± 0.86 | 29.31 ± 1.77 *** | |
| C14:0 | 0.47 ± 0.11 | 0.62 ± 0.10 * | |
| C16:0 | 18.42 ± 1.26 | 18.82 ± 0.91 | |
| C18:0 | 13.62 ± 1.91 | 8.47 ± 1.23 *** | |
| C20:0 | 0.05 ± 0.00 | 0.04 ± 0.00 *** | |
| C22:0 | 0.12 ± 0.02 | 0.07 ± 0.01 *** | |
| MUFAs | 20.06 ± 3.19 | 20.40 ± 1.71 | |
| C16:1n-7 | 2.39 ± 1.02 | 3.13 ± 0.85 | |
| C16:1n-9 | 0.25 ± 0.06 | 0.27 ± 0.04 | |
| C18:1n-7 | 4.08 ± 1.12 | 3.41 ± 0.69 | |
| C18:1n-9 (OA) | 12.52 ± 1.64 | 12.81 ± 0.87 | |
| C20:1n-7 | 0.11 ± 0.02 | 0.14 ± 0.01 ** | |
| C20:1n-9 | 0.19 ± 0.03 | 0.12 ± 0.01 *** | |
| C22:1n-7 | 0.01 ± 0.00 | 0.01 ± 0.00 | |
| C22:1n-9 | 0.05 ± 0.03 | 0.02 ± 0.00 | |
| C24:1n-9 | 0.17 ± 0.03 | 0.13 ± 0.02 * | |
| PUFAs | 45.91 ± 2.74 | 50.20 ± 1.30 * | |
| n-9 PUFAs | 0.17 ± 0.02 | 0.08 ± 0.00 *** | |
| C20:3n-9 (MA) | 0.17 ± 0.02 | 0.08 ± 0.00 *** | |
| n-6 PUFAs | 39.13 ± 2.74 | 22.13 ± 1.36 *** | |
| C18:2n-6 (LA) | 14.80 ± 1.87 | 15.72 ± 1.40 | |
| C18:3n-6 (GLA) | 0.20 ± 0.03 | 0.09 ± 0.01 *** | |
| C20:3n-6 (DGLA) | 1.04 ± 0.38 | 0.71 ± 0.09 | |
| C20:4n-6 (AA) | 21.96 ± 2.13 | 5.30 ± 0.91 *** | |
| C22:4n-6 | 0.44 ± 0.06 | 0.07 ± 0.01 *** | |
| C22:5n-6 (DPA) | 0.29 ± 0.09 | 0.05 ± 0.01 *** | |
| n-3 PUFAs | 6.61 ± 0.61 | 27.98 ± 2.16 *** | |
| C18:3n-3 (ALA) | 0.42 ± 0.10 | 1.47 ± 0.29 *** | |
| C18:4n-3 | 0.02 ± 0.01 | 0.26 ± 0.08 *** | |
| C20:4n-3 | 0.09 ± 0.01 | 0.68 ± 0.18 *** | |
| C20:5n-3 (EPA) | 0.37 ± 0.05 | 10.82 ± 0.94 *** | |
| C21:5n-3 (HPA) | 0.00 ± 0.00 | 0.36 ± 0.07 *** | |
| C22:5n-3 (DPA) | 0.73 ± 0.09 | 4.54 ± 0.68 *** | |
| C22:6n-3 (DHA) | 4.98 ± 0.67 | 9.85 ± 0.97 *** | |

2.6. Cholesterol Synthesis, Degradation, and Esterification
3. Discussion
4. Materials and Methods
4.1. Animals and Dietary Interventions
| Components 1 | Diets | |||
|---|---|---|---|---|
| Control | 3% PPC | 6% PPC | 11% PPC | |
| Lard | 8.0 | 6.5 | 5.1 | 3.2 |
| Soy oil | 2.0 | 2.0 | 2.0 | 2.0 |
| Casein | 23.0 | 21.5 | 20.1 | 18.1 |
| PCC 2 | - | 3.3 | 6.4 | 10.9 |
| Lipids from PPC 3 | - | 1.5 | 2.9 | 4.8 |
| Protein from PPC | - | 1.5 | 2.9 | 4.9 |
4.2. Sampling Protocol
| Diets | ||||
|---|---|---|---|---|
| Fatty acids 1 | Control | 3% PPC | 6% PPC | 11% PPC |
| SFAs | 30.9 | 30.5 | 27.9 | 24.7 |
| C14:0 | 1.0 | 1.9 | 2.4 | 3.4 |
| C16:0 | 19.5 | 19.3 | 18.1 | 16.4 |
| C18:0 | 10.2 | 9.0 | 7.3 | 4.8 |
| C20:0 | 0.1 | 0.2 | 0.1 | 0.1 |
| C22:0 | 0.1 | 0.1 | <0.1 | <0.1 |
| MUFAs | 35.8 | 33.4 | 29.5 | 23.7 |
| C16:1n-7 | 1.2 | 1.4 | 1.5 | 1.6 |
| C18:1 (n-9) + (n-7) + (n-5) | 34.2 | 31.4 | 27.5 | 21.2 |
| C20:1 (n-9) + (n-7) | 0.4 | 0.5 | 0.4 | 0.4 |
| C22:1 (n-9) + (n-7) | <0.1 | 0.1 | 0.1 | 0.1 |
| C24:1n-9 | <0.1 | <0.1 | <0.1 | <0.1 |
| PUFAs | 32.7 | 32.6 | 33.4 | 33.2 |
| n-6 PUFAs | 30.3 | 26.1 | 23.6 | 17.7 |
| C18:2n-6 (LA) | 29.5 | 25.4 | 23.2 | 17.4 |
| C18:3n-6 | <0.1 | <0.1 | <0.1 | <0.1 |
| C20:2n-6 | 0.5 | 0.4 | 0.3 | 0.2 |
| C20:3n-6 | 0.1 | 0.1 | <0.1 | <0.1 |
| C20:4n-6 (AA) | 0.1 | 0.1 | 0.1 | 0.1 |
| C22:4n-6 | 0.1 | 0.1 | <0.1 | <0.1 |
| n-3 PUFAs | 2.4 | 6.5 | 9.7 | 15.4 |
| C18:3n-3 (ALA) | 2.3 | 2.5 | 2.8 | 2.9 |
| C18:4n-3 | <0.1 | 0.9 | 1.7 | 2.9 |
| C20:3n-3 | 0.1 | 0.1 | <0.1 | 0.1 |
| C20:4n-3 | <0.1 | <0.1 | <0.1 | 0.1 |
| C20:5n-3 (EPA) | <0.1 | 1.8 | 3.2 | 5.7 |
| C21:5n-3 (HPA) | <0.1 | 0.1 | 0.1 | 0.2 |
| C22:5n-3 (DPA) | <0.1 | 0.1 | <0.1 | 0.1 |
| C22:6n-3 (DHA) | <0.1 | 1.0 | 1.9 | 3.4 |
| n-6 PUFAs:n-3 PUFAs | 12.6:1 | 4.0:1 | 2.4:1 | 1.2:1 |
4.3. Quantification of Plasma Parameters
| Amino acid 1 | Diets | |||
|---|---|---|---|---|
| Control | 3% PPC | 6% PPC | 11% PPC | |
| Aspartic acid | 1.32 | 1.58 | 1.70 | 1.74 |
| Glutaminic acid | 4.18 | 4.68 | 4.65 | 4.40 |
| Hydroksyproline | <0.10 | <0.01 | <0.01 | <0.01 |
| Serine | 1.17 | 1.25 | 1.27 | 1.24 |
| Glycine | 0.41 | 0.44 | 0.49 | 0.53 |
| Histidine | 0.59 | 0.61 | 0.60 | 0.58 |
| Arginine | 0.69 | 0.78 | 0.82 | 0.83 |
| Threonine | 0.87 | 0.91 | 0.96 | 0.96 |
| Alanine | 0.62 | 0.70 | 0.77 | 0.80 |
| Proline | 2.20 | 2.28 | 2.23 | 2.13 |
| Tyrosine | 0.83 | 0.96 | 0.98 | 0.98 |
| Valine | 1.33 | 1.43 | 1.44 | 1.40 |
| Methionine | 0.52 | 0.58 | 0.60 | 0.61 |
| Isoleucine | 1.09 | 1.17 | 1.21 | 1.21 |
| Leucine | 1.93 | 2.07 | 2.14 | 2.11 |
| Phenylalanine | 1.04 | 1.13 | 1.17 | 1.16 |
| Lysine | 1.62 | 1.86 | 1.80 | 1.77 |
| Total amino acids detected | 19.6 | 21.5 | 21.9 | 21.5 |
4.4. Hepatic Enzyme Activities and Fatty Acid Composition
4.5. Gene Expression Analysis
4.6. Total Antioxidant Capacity and Inflammatory Markers
4.7. Statistical Analysis
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Connor, W.E. Importance of n-3 fatty acids in health and disease. Am. J. Clin. Nutr. 2000, 71, 171S–175S. [Google Scholar] [PubMed]
- He, K.; Song, Y.; Daviglus, M.L.; Liu, K.; van Horn, L.; Dyer, A.R.; Greenland, P. Accumulated evidence on fish consumption and coronary heart disease mortality: A meta-analysis of cohort studies. Circulation 2004, 109, 2705–2711. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Bronner, L.; Willett, W.C.; Stampfer, M.J.; Rexrode, K.M.; Albert, C.M.; Hunter, D.; Manson, J.E. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA 2002, 287, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J.; Nutrition, C. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2003, 23, e20–e30. [Google Scholar] [CrossRef] [PubMed]
- Skeaff, C.M.; Miller, J. Dietary fat and coronary heart disease: Summary of evidence from prospective cohort and randomised controlled trials. Ann. Nutr. Metab. 2009, 55, 173–201. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar]
- Nestel, P.; Clifton, P.; Colquhoun, D.; Noakes, M.; Mori, T.A.; Sullivan, D.; Thomas, B. Indications for Omega-3 Long Chain Polyunsaturated Fatty Acid in the Prevention and Treatment of Cardiovascular Disease. Heart Lung Circ. 2015. [Google Scholar] [CrossRef] [PubMed]
- Hartweg, J.; Perera, R.; Montori, V.; Dinneen, S.; Neil, H.A.; Farmer, A. Omega-3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef]
- Harris, W.S. n-3 fatty acids and serum lipoproteins: Human studies. Am. J. Clin. Nutr. 1997, 65, 1645S–1654S. [Google Scholar] [PubMed]
- Leslie, M.A.; Cohen, D.J.; Liddle, D.M.; Robinson, L.E.; Ma, D.W. A review of the effect of omega-3 polyunsaturated fatty acids on blood triacylglycerol levels in normolipidemic and borderline hyperlipidemic individuals. Lipids Health Dis. 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Knapp, H.R. Dietary fatty acids in human thrombosis and hemostasis. Am. J. Clin. Nutr. 1997, 65, 1687S–1698S. [Google Scholar] [PubMed]
- Hornstra, G. Influence of dietary fat type on arterial thrombosis tendency. J. Nutr. Health Aging 2001, 5, 160–166. [Google Scholar] [PubMed]
- Geleijnse, J.M.; Giltay, E.J.; Grobbee, D.E.; Donders, A.R.; Kok, F.J. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J. Hypertens 2002, 20, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Nodari, S.; Metra, M.; Milesi, G.; Manerba, A.; Cesana, B.M.; Gheorghiade, M.; Dei Cas, L. The role of n-3 PUFAs in preventing the arrhythmic risk in patients with idiopathic dilated cardiomyopathy. Cardiovasc. Drugs Ther. 2009, 23, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [PubMed]
- Lopez-Garcia, E.; Schulze, M.B.; Manson, J.E.; Meigs, J.B.; Albert, C.M.; Rifai, N.; Willett, W.C.; Hu, F.B. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J. Nutr. 2004, 134, 1806–1811. [Google Scholar] [PubMed]
- Madsen, T.; Skou, H.A.; Hansen, V.E.; Fog, L.; Christensen, J.H.; Toft, E.; Schmidt, E.B. C-reactive protein, dietary n-3 fatty acids, and the extent of coronary artery disease. Am. J. Cardiol. 2001, 88, 1139–1142. [Google Scholar] [CrossRef]
- Aksnes, A. Feed Ingredients. The impacts of nitrogen extrectives in aqua feed ingredients. Int. Aqua Feed 2005, 8, 28–30. [Google Scholar]
- Ngo, D.H.; Vo, T.S.; Ngo, D.N.; Wijesekara, I.; Kim, S.K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Khora, S.S. Marine fish-derived bioactive peptides and proteins for human therapeutics. Int. J. Pharm. Pharm. Sci. 2013, 5, 31–37. [Google Scholar]
- Pilon, G.; Ruzzin, J.; Rioux, L.E.; Lavigne, C.; White, P.J.; Froyland, L.; Jacques, H.; Bryl, P.; Beaulieu, L.; Marette, A. Differential effects of various fish proteins in altering body weight, adiposity, inflammatory status, and insulin sensitivity in high-fat-fed rats. Metabolism 2011, 60, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, R.; Fukunaga, K.; Arai, H.; Kanda, S.; Nishiyama, T.; Yoshida, M. Fish protein decreases serum cholesterol in rats by inhibition of cholesterol and bile acid absorption. J. Food Sci. 2011, 76, H116–H121. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Bettzieche, A.; Hirche, F.; Brandsch, C.; Stangl, G.I.; Eder, K. Dietary fish protein alters blood lipid concentrations and hepatic genes involved in cholesterol homeostasis in the rat model. Br. J. Nutr. 2006, 96, 674–682. [Google Scholar] [PubMed]
- Suarez-Jimenez, G.M.; Burgos-Hernandez, A.; Ezquerra-Brauer, J.M. Bioactive peptides and depsipeptides with anticancer potential: Sources from marine animals. Mar. Drugs 2012, 10, 963–986. [Google Scholar] [CrossRef] [PubMed]
- Nazeer, R.A.; Sampath Kumar, N.S.; Jai Ganesh, R. In vitro and in vivo studies on the antioxidant activity of fish peptide isolated from the croaker (Otolithes ruber) muscle protein hydrolysate. Peptides 2012, 35, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Ryu, B.; Vo, T.S.; Himaya, S.W.; Wijesekara, I.; Kim, S.K. Free radical scavenging and angiotensin-I converting enzyme inhibitory peptides from Pacific cod (Gadus macrocephalus) skin gelatin. Int. J. Biol. Macromol. 2011, 49, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Sampath Kumar, N.S.; Nazeer, R.A.; Jaiganesh, R. Purification and identification of antioxidant peptides from the skin protein hydrolysate of two marine fishes, horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber). Amino Acids 2012, 42, 1641–1649. [Google Scholar] [CrossRef]
- Parolini, C.; Vik, R.; Busnelli, M.; Bjorndal, B.; Holm, S.; Brattelid, T.; Manzini, S.; Ganzetti, G.S.; Dellera, F.; Halvorsen, B.; et al. A salmon protein hydrolysate exerts lipid-independent anti-atherosclerotic activity in ApoE-deficient mice. PLoS ONE 2014, 9, e97598. [Google Scholar] [CrossRef] [PubMed]
- Tou, J.C.; Jaczynski, J.; Chen, Y.C. Krill for human consumption: Nutritional value and potential health benefits. Nutr. Rev. 2007, 65, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Tillander, V.; Bjørndal, B.; Burri, L.; Bohov, P.; Skorve, J.; Berge, R.K.; Alexson, S.E.H. Fish oil and krill oil supplementations differentially regulate lipid catabolic and synthetic pathways in mice. Nutr. Metab. 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Burri, L.; Berge, K.; Wibrand, K.; Berge, R.K.; Barger, J.L. Differential effects of krill oil and fish oil on the hepatic transcriptome in mice. Front. Nutrigenomics 2011, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Schneider, I.; Meyer, H.; Neubronner, J.; von Schacky, C.; Hahn, A. Incorporation of EPA and DHA into plasma phospholipids in response to different omega-3 fatty acid formulations—A comparative bioavailability study of fish oil vs. krill oil. Lipids Health Dis. 2011, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Kohler, A.; Sarkkinen, E.; Tapola, N.; Niskanen, T.; Bruheim, I. Bioavailability of fatty acids from krill oil, krill meal and fish oil in healthy subjects-a randomized, single-dose, cross-over trial. Lipids Health Dis. 2015, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Salem, N., Jr.; Kuratko, C.N. A reexamination of krill oil bioavailability studies. Lipids Health Dis. 2014, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xue, C.; Wang, Y.; Yang, B. Extraction of proteins with low fluoride level from Antarctic krill (Euphausia superba) and their composition analysis. J. Agric. Food Chem. 2011, 59, 6108–6112. [Google Scholar] [CrossRef] [PubMed]
- Bjorndal, B.; Vik, R.; Brattelid, T.; Vigerust, N.F.; Burri, L.; Bohov, P.; Nygard, O.; Skorve, J.; Berge, R.K. Krill powder increases liver lipid catabolism and reduces glucose mobilization in tumor necrosis factor-alpha transgenic mice fed a high-fat diet. Metabolism 2012, 61, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Berge, K.; Piscitelli, F.; Hoem, N.; Silvestri, C.; Meyer, I.; Banni, S.; di Marzo, V. Chronic treatment with krill powder reduces plasma triglyceride and anandamide levels in mildly obese men. Lipids Health Dis. 2013, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Shimano, H.; Yahagi, N.; Ide, T.; Amemiya-Kudo, M.; Matsuzaka, T.; Nakakuki, M.; Tomita, S.; Okazaki, H.; Tamura, Y.; et al. Polyunsaturated fatty acids suppress sterol regulatory element-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements. J. Biol. Chem. 2002, 277, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Vigerust, N.F.; Bjorndal, B.; Bohov, P.; Brattelid, T.; Svardal, A.; Berge, R.K. Krill oil versus fish oil in modulation of inflammation and lipid metabolism in mice transgenic for TNF-alpha. Eur. J. Nutr. 2012, 52, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Debose-Boyd, R.A. Control of cholesterol synthesis through regulated ER-associated degradation of HMG CoA reductase. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Li, D.M.; Zhou, D.Y.; Zhu, B.W.; Chi, Y.L.; Sun, L.M.; Dong, X.P.; Qin, L.; Qiao, W.Z.; Murata, Y. Effects of krill oil intake on plasma cholesterol and glucose levels in rats fed a high-cholesterol diet. J. Sci. Food Agric. 2013, 93, 2669–2675. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Heo, S.J.; Yoon, W.J.; Kang, S.M.; Ahn, G.; Yi, T.H.; Jeon, Y.J. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-kappaB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur. J. Pharmacol. 2010, 649, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Assies, J.; Mocking, R.J.; Lok, A.; Ruhe, H.G.; Pouwer, F.; Schene, A.H. Effects of oxidative stress on fatty acid- and one-carbon-metabolism in psychiatric and cardiovascular disease comorbidity. Acta Psychiatr. Scand. 2014, 130, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [CrossRef] [PubMed]
- Jansson, S.T.K.; Ervik, J.R.; Grimsmo, L. Reduced Fluoride Content Phospholipids/Peptide Complex Meal. US Patent. Patent No. US 8,758,829 B2, 24 June 2014. [Google Scholar]
- Strand, E.; Bjorndal, B.; Nygard, O.; Burri, L.; Berge, C.; Bohov, P.; Christensen, B.J.; Berge, K.; Wergedahl, H.; Viste, A.; et al. Long-Term treatment with the pan-PPAR agonist tetradecylthioacetic acid or fish oil is associated with increased cardiac content of n-3 fatty acids in rat. Lipids Health Dis. 2012, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Berge, R.K.; Flatmark, T.; Osmundsen, H. Enhancement of long-chain acyl-CoA hydrolase activity in peroxisomes and mitochondria of rat liver by peroxisomal proliferators. Eur. J. Biochem. 1984, 141, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Skorve, J.; al-Shurbaji, A.; Asiedu, D.; Bjorkhem, I.; Berglund, L.; Berge, R.K. On the mechanism of the hypolipidemic effect of sulfur-substituted hexadecanedioic acid (3-thiadicarboxylic acid) in normolipidemic rats. J. Lipid Res. 1993, 34, 1177–1185. [Google Scholar] [PubMed]
- Vik, R.; Bjorndal, B.; Bohov, P.; Brattelid, T.; Svardal, A.; Nygard, O.K.; Nordrehaug, J.E.; Skorve, J.; Berge, R.K. Hypolipidemic effect of dietary water-soluble protein extract from chicken: impact on genes regulating hepatic lipid and bile acid metabolism. Eur. J. Nutr. 2015, 54, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Willumsen, N.; Hexeberg, S.; Skorve, J.; Lundquist, M.; Berge, R.K. Docosahexaenoic acid shows no triglyceride-lowering effects but increases the peroxisomal fatty acid oxidation in liver of rats. J. Lipid Res. 1993, 34, 13–22. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramsvik, M.S.; Bjørndal, B.; Bruheim, I.; Bohov, P.; Berge, R.K. A Phospholipid-Protein Complex from Krill with Antioxidative and Immunomodulating Properties Reduced Plasma Triacylglycerol and Hepatic Lipogenesis in Rats. Mar. Drugs 2015, 13, 4375-4397. https://doi.org/10.3390/md13074375
Ramsvik MS, Bjørndal B, Bruheim I, Bohov P, Berge RK. A Phospholipid-Protein Complex from Krill with Antioxidative and Immunomodulating Properties Reduced Plasma Triacylglycerol and Hepatic Lipogenesis in Rats. Marine Drugs. 2015; 13(7):4375-4397. https://doi.org/10.3390/md13074375
Chicago/Turabian StyleRamsvik, Marie S., Bodil Bjørndal, Inge Bruheim, Pavol Bohov, and Rolf K. Berge. 2015. "A Phospholipid-Protein Complex from Krill with Antioxidative and Immunomodulating Properties Reduced Plasma Triacylglycerol and Hepatic Lipogenesis in Rats" Marine Drugs 13, no. 7: 4375-4397. https://doi.org/10.3390/md13074375