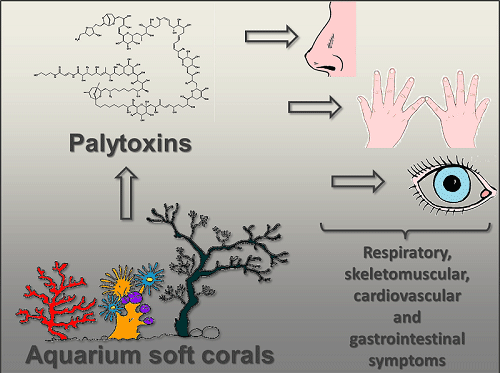
Palytoxin-Containing Aquarium Soft Corals as an Emerging Sanitary Problem
Abstract
:1. Introduction
1.1. Palytoxin: Producing Organisms
1.2. Palytoxin: Molecular Structure

1.3. Mechanism of Action
1.4. Human Risk Associated with Palytoxin Exposure
2. Human Poisonings Postulated to PLTX Exposure through Handling of Soft Corals
2.1. Exposure Routes
2.2. Human Poisonings Ascribed to Palytoxins-Contaminated Soft Corals: Direct Identification of PLTXs in the Corals
| Location, Year | Number of Patients | Corals | Signs and Symptoms | Treatment and Outcome | PLTXs Detection Method and Concentration | Reference |
|---|---|---|---|---|---|---|
| Virginia (USA), 2007 | 1 | Palythoa/Protopalythoa sp. | Foul odor. Difficult breathing, lightheadedness, chest pain, bronchoconstriction. | Anti-inflammatory corticosteroids and cough suppressant. Recovery after 1 month | Hemolysis neutralization assay (309 µg PLTX eq./g); HPLC (613 µg PLTX eq./g) | [54] |
| The Netherlands, 2014 * | 4 | Palythoa heliodiscus | Cough, dyspnea, chest pain, tachycardia, nausea. Leukocytosis with elevated neutrophils, CPK, CRP | Oxygen therapy, non-steroidal anti-inflammatory drugs. Recovery after more than 3 months | LC/MS (1018 µg PLTX/g wet coral; 46 µg 42-OH-PLTX/g wet coral) | [56] |
| Alaska (USA), 2014 | 3 | Palythoa heliodiscus | Dyspnea, scratchy throat, paresthesia, myalgia, spasms, ataxia, weakness, tremors, nausea, tachycardia, fever | Supportive therapy. Recovery within 2 days | HPLC, LC/MS (7.3 mg PLTX/g wet coral) | [57] |
| Oklahoma (USA), 1961 | 3 | Palythoa caribaeorum | Chills, nausea, headache | Recovery within 1 day | No experimental details (a compound identical to PLTX from P. toxica) | [1] |
| New York (USA), 2008 * | 1 | Palythoa sp. | Foul odor, shortness of breath, chest pain, sinus tachycardia | Inhaled albuterol. Recovery after 48 h | No analysis | [61] |
| The Netherlands, 2012 * | 4 | Zoanthids | Fever, hypotension, nausea, headache, shivering, muscle cramps. Leukocytosis, elevated CRP | Supportive therapy. Recovery within 48 h | No analysis | [62] |
| Switzerland, 2012 * | 3 | Palythoa sp. | Dyspnea, dry cough, nausea, headache, fever, chills, tachycardia, hypoxemia. Leukocytosis, slightly elevated LDH, CRP and procalcitonin. Restrictive ventilator pattern, diffuse bronchial swelling and secretion. | Treatment not reported. Recovery within 2 weeks | No analysis | [63] |
| New York (USA), 2013 * | 5 | Palythoa sp. | Shortness of breath, fever, dry cough, chills, myalgia, emesis. Leukocytosis, slightly elevated LDH, CPK, CKMB | Albuterol, levoflaxic, acetaminophen, hydration and supportive therapy. Recovery within 48 h | No analysis | [64,65] |
| Alaska, (USA), 2012–2014 | 9 | Zoanthids | Bitter metallic taste, fever, tremors, weakness, ataxia, cough, joint and muscle pain, pulmonary symptoms | Treatment not reported. Recovery within 24 h, but sometimes with pulmonary symptoms after 2 years | No analysis | [57] |
| New York (USA), 2015 * | 3 | Zoanthid corals | Fever, chills, myalgia, tachycardia, wheezes, hemoptysis, dyspnea, leukocytosis, bibasilar opacities | Albuterol, acetaminophen, supplemental oxygen, prednisone. Complete recovery after 1 month | No analysis | [66] |
| Location, Year | Number of Patients | Corals | Signs and Symptoms | Treatment and Outcome | PLTXs Detection and Concentration | Reference |
|---|---|---|---|---|---|---|
| Hawaii (USA), 1962 | 1 | Palythoa toxica | Dizziness, nausea, headache, malaise, discomfort to the hands | Supportive pharmacological treatment. Recovery after 1 week | NMR (280 µg PLTX/g wet weight) | [1] |
| Germany, 2008 * | 1 | Palythoa sp. and Parazoanthus sp. | Shivering, myalgia, weakness of the extremities, speech disturbance. Swelling and erythema at cut finger, numbness, and paresthesias of the arm. Slightly elevated CPK, LDH, CRP. Abnormal ECG | Infusion of intra-venous physiological fluids. Recovery after 48 h | Hemolysis neutralization assay (2–3 mg PLTX eq./g wet weight) | [58] |
| California (USA), 2009 * | 1 | Zoanthid corals | Metallic taste, perioral paresthesia, hives on torso and extremities, edema and erythema at hands. Urticarial rash on arms, things, abdomen, and back. | Intravenous diphenhydramine, methylprednisoline and lorazepam. Recovery after 24 h | No analysis | [67] |
| Georgia (USA), 2006 | 1 | Palythoa sp. | Chest pain, lightheadedness, weakness, and numbness of the left arm, tachycardia. Elevated CPK | Supportive treatment. Recovery after 48 h | Patient serum: haemolytic activity, no neutralization by anti-PLTX antibody; no PLTX-like compound detection by HPLC, LC/MS | [54] |
| Location, Year | Number of Patients | Corals | Signs and Symptoms | Treatment and Outcome | PLTXs Detection and Concentration | Reference |
|---|---|---|---|---|---|---|
| N.D. | 2 | Zoanthids | Ocular irritation and redness, bitter metallic taste, eye pain photophobia, blurry vision, purulent discharge from eyes, bilateral punctate epithelial erosion, conjuctival hyperemia | Moxifloxacin, artificial tears, topical prednisolone acetate, fluorometholone, moxifloxacin, cyclosporine drops | Not performed | [59] |
| Switzerland, 2015 * | 1 | Zoanthids | Eyes burning, dyspnea, nausea, shivering, conjunctival injection, superficial punctuate epitheliopathy, multiple corneal Descemet’s folds, corneal erosion. Leukocytosis, elevated CRP, CPK, LDH | Intravenous infusion of balanced crystalloid solution, Diphoterine®, topical antibiotics and steroid, amniotic membrane transplantation, sclera contact lenses (4 months). Recovery within several weeks | Not performed | [60] |
2.2.1. Inhalational Exposure to Vapors from Palytoxins-Contaminated Soft Corals
2.2.2. Cutaneous Exposure to Palytoxins-Contaminated Soft Corals
2.3. Human Poisonings Ascribed to Palytoxins-Contaminated Soft Corals: No Direct Identification of PLTXs in the Corals
2.3.1. Inhalational Exposure to Dust or Vapors from Soft Corals
2.3.2. Cutaneous Exposure to Soft Corals
2.3.3. Ocular Exposure to Soft Corals
2.4. Pharmacological Treatments
3. Palytoxins in Soft Corals
3.1. Palytoxin Analogs Identified in Soft Corals
| Genus | Species | Toxin | Coral Origin | Detection Method | References |
|---|---|---|---|---|---|
| Palythoa | toxica | PLTX | Coral reef; home aquarium * | NMR | [1] |
| 42S-OH-50S-PLTX | Coral reef; home aquarium * | LC/MS, NMR | [14] | ||
| tuberculosa | PLTX | Coral reef | Mouse bioassay, HPLC, HPTLC, UV detection | [73,74,75] | |
| 42S-OH-50R-PLTX | Coral reef | HR LC/MS, NMR | [15] | ||
| Deoxy-PLTX | Coral reef | HPLC | [73] | ||
| Homo-PLTX | |||||
| Bis-homo-PLTX | |||||
| Neo-PLTX | |||||
| vestitus | PLTX | Coral reef | N.D. | [69] | |
| margaritae | PLTX | Coral reef | HPLC, NMR | [68] | |
| mammillosa | PLTX | Coral reef | Ion exchange chromatography, haemolysis neutralization assay, HPLC | [3,70] | |
| caribaeorum | PLTX | Coral reef | Ion exchange chromatography, haemolysis neutralization assay, HPLC | [3,9,70,71,72] | |
| heliodiscus | PLTX | Home aquarium | HPLC, ESI-LC/MS | [55] | |
| Deoxy-PLTX | |||||
| 42-OH-PLTX ** | Home aquarium | LC/MS | [56] | ||
| caesia | PLTX | Coral reef | Haemolysis neutralization assay, HPLC | [72] | |
| N.D. | PLTX | Home aquarium | Haemolysis neutralization assay | [58] | |
| Zoanthus | sociatus | PLTX | Coral reef | Haemolysis neutralization assay, HPLC | [3] |
| soladeri | PLTX | ||||
| pulchellus | PLTX | Haemolysis neutralization assay | [9] |
3.2. Toxicity of Palytoxin Analogs Identified in Soft Corals
4. Discussion
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ALT | alanine-aminotransferase |
| AST | aspartate-aminotransferase |
| CKMB | creatine kinase MB isoenzyme |
| CL | confidence limits |
| CPK | creatinine phosphor-kinase |
| CRP | C-reactive protein |
| ECG | electrocardiogram |
| ESI-MS | electrospray ionization-mass spectrometry |
| HPLC | high performance liquid chromatography |
| LC/MS | liquid chromatography associated with mass spectrometry |
| LD50 | dose giving 50% of lethality |
| LDH | lactate dehydrogenase |
| NCE | Na+/Ca2+ exchanger |
| NHE | Na+/H+ exchanger |
| NMR | nuclear magnetic resonance |
| Ost-D | ostreocin-D |
| OUA | ouabain |
| OVTX | ovatoxin |
| PLTX | palytoxin |
References
- Moore, R.E.; Helfrich, P.; Patterson, G.M.L. The deadly seaweed of Hana. Oceanus 1982, 25, 54–63. [Google Scholar]
- Tubaro, A.; Durando, P.; del Favero, G.; Ansaldi, F.; Icardi, G.; Deeds, J.R.; Sosa, S. Case definitions for human poisonings postulated to palytoxins exposure. Toxicon 2011, 57, 478–495. [Google Scholar] [CrossRef] [PubMed]
- Gleibs, S.; Mebs, D.; Werding, B. Studies on the origin and distribution of palytoxin in a caribbean coral reef. Toxicon 1995, 33, 1531–1537. [Google Scholar] [CrossRef]
- Usami, M.; Satake, M.; Ishida, S.; Inoue, A.; Kan, Y.; Yasumoto, T. Palytoxin analogs from the dinoflagellate Ostreopsis-siamensis. J. Am. Chem. Soc. 1995, 117, 5389–5390. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; Fattorusso, C.; Forino, M.; Tartaglione, L.; Yasumoto, T.; Battocchi, C.; Giacobbe, M.; Amorim, A.; et al. Investigation of toxin profile of mediterranean and atlantic strains of Ostreopsis cf. siamensis (Dinophyceae) by liquid chromatography-high resolution mass spectrometry. Harmful Algae 2013, 23, 19–27. [Google Scholar] [CrossRef]
- Lenoir, S.; Ten-Hage, L.; Turquet, J.; Quod, J.P.; Bernard, C.; Hennion, M.C. First evidence of palytoxin analogues from an Ostreopsis mascarenensis (Dinophyceae) benthic bloom in southwestern indian ocean. J. Phycol. 2004, 40, 1042–1051. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; Grauso, L.; Tartaglione, L.; Guerrini, F.; Pezzolesi, L.; Pistocchi, R.; et al. Isolation and structure elucidation of ovatoxin-a, the major toxin produced by Ostreopsis ovata. J. Am. Chem. Soc. 2012, 134, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Frolova, G.M.; Kuznetsova, T.A.; Mikhailov, V.V.; Elyakov, G.B. An enzyme linked immunosorbent assay for detecting palytoxin-producing bacteria. Bioorg. Chem. 2000, 26, 315–320. [Google Scholar] [CrossRef]
- Seemann, P.; Gernert, C.; Schmitt, S.; Mebs, D.; Hentschel, U. Detection of hemolytic bacteria from Palythoa caribaeorum (Cnidaria, Zoantharia) using a novel palytoxin-screening assay. Antonie Van Leeuwenhoek J. 2009, 96, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Kerbrat, A.S.; Amzil, Z.; Pawlowiez, R.; Golubic, S.; Sibat, M.; Darius, H.T.; Chinain, M.; Laurent, D. First evidence of palytoxin and 42-hydroxy-palytoxin in the marine cyanobacterium Trichodesmium. Mar. Drugs 2011, 9, 543–560. [Google Scholar] [CrossRef] [PubMed]
- Uemura, D.; Ueda, K.; Hirata, Y.; Naoki, H.; Iwashita, T. Structure of palytoxin. Tetrahedron 1981, 22, 2781–2784. [Google Scholar] [CrossRef]
- Moore, R.E.; Bartolini, G. Structure of palytoxin. J. Am. Chem. Soc. 1981, 103, 2491–2494. [Google Scholar] [CrossRef]
- Inuzuka, T.; Uemura, D.; Arimoto, H. The conformational features of palytoxin in aqueous solution. Tetrahedron 2008, 64, 7718–7723. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’aversano, C.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; Grauso, L.; Tartaglione, L.; Florio, C.; Lorenzon, P.; de Bortoli, M.; et al. Stereostructure and biological activity of 42-hydroxy-palytoxin: A new palytoxin analogue from hawaiian Palythoa subspecies. Chem. Res. Toxicol. 2009, 22, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; Forino, M.; Tartaglione, L.; Pelin, M.; Sosa, S.; Tubaro, A.; Chaloin, O.; Poli, M.; et al. Stereoisomers of 42-hydroxy palytoxin from hawaiian Palythoa toxica and P. tuberculosa: Stereostructure elucidation, detection, and biological activities. J. Nat. Prod. 2014, 77, 351–357. [Google Scholar] [PubMed]
- Ito, E.; Yasumoto, T. Toxicological studies on palytoxin and ostreocin-D administered to mice by three different routes. Toxicon 2009, 54, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Amzil, Z.; Sibat, M.; Chomerat, N.; Grossel, H.; Marco-Miralles, F.; Lemee, R.; Nezan, E.; Sechet, V. Ovatoxin-a and palytoxin accumulation in seafood in relation to Ostreopsis cf. ovata blooms on the french Mediterranean coast. Mar. Drugs 2012, 10, 477–496. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Castellano, V.; Scalco, E.; Serpe, L.; Zingone, A.; Soprano, V. New palytoxin-like molecules in Mediterranean Ostreopsis cf. ovata (Dinoflagellates) and in Palythoa tuberculosa detected by liquid chromatography-electrospray ionization time-of-flight mass spectrometry. Toxicon 2010, 56, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; Grauso, L.; Tartaglione, L.; Guerrini, F.; Pistocchi, R. Complex palytoxin-like profile of Ostreopsis ovata. Identification of four new ovatoxins by high-resolution liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2735–2744. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Iacovo, E.D.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Battocchi, C.; Crinelli, R.; Carloni, E.; Magnani, M.; et al. Unique toxin profile of a mediterranean Ostreopsis cf. ovata strain: HR LC-MSn characterization of ovatoxin-f, a new palytoxin congener. Chem. Res. Toxicol. 2012, 25, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Brissard, C.; Herrenknecht, C.; Sechet, V.; Herve, F.; Pisapia, F.; Harcouet, J.; Lemee, R.; Chomerat, N.; Hess, P.; Amzil, Z. Complex toxin profile of french mediterranean Ostreopsis cf. ovata strains, seafood accumulation and ovatoxins prepurification. Mar. Drugs 2014, 12, 2851–2876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brissard, C.; Herve, F.; Sibat, M.; Sechet, V.; Hess, P.; Amzil, Z.; Herrenknecht, C. Characterization of ovatoxin-h, a new ovatoxin analog, and evaluation of chromatographic columns for ovatoxin analysis and purification. J. Chromatogr. A 2015, 1388, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Altares, M.; Tartaglione, L.; Dell’Aversano, C.; Carnicer, O.; de la Iglesia, P.; Forino, M.; Diogene, J.; Ciminiello, P. The novel ovatoxin-g and isobaric palytoxin (so far referred to as putative palytoxin) from Ostreopsis cf. ovata (NW Mediterranean sea): Structural insights by LC-high resolution MSn. Anal. Bioanal. Chem. 2015, 407, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Pelin, M.; Forino, M.; Brovedani, V.; Tartaglione, L.; Dell’Aversano, C.; Pistocchi, R.; Poli, M.; Sosa, S.; Florio, C.; Ciminiello, P.; et al. Ovatoxin-a, A palytoxin analogue isolated from Ostreopsis cf. ovata Fukuyo: Cytotoxic activity and ELISA detection. Environ. Sci. Technol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Artigas, P.; Gadsby, D.C. Large diameter of palytoxin-induced Na/K pump channels and modulation of palytoxin interaction by Na/K pump ligands. J. Gen. Physiol. 2004, 123, 357–376. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Almeida, A.C.; Infantosi, A.F.; Teixeira, H.Z.; Duarte, M.A. Model and simulation of Na+/K+ pump phosphorylation in the presence of palytoxin. Comput. Biol. Chem. 2008, 32, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Habermann, E.; Chhatwal, G.S. Ouabain inhibits the increase due to palytoxin of cation permeability of erythrocytes. Naunyn Schmiedebergs Arch. Pharmacol. 1982, 319, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Pelin, M.; Zanette, C.; De Bortoli, M.; Sosa, S.; Loggia, R.D.; Tubaro, A.; Florio, C. Effects of the marine toxin palytoxin on human skin keratinocytes: Role of ionic imbalance. Toxicology 2011, 282, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Pelin, M.; Sosa, S.; Della Loggia, R.; Poli, M.; Tubaro, A.; Decorti, G.; Florio, C. The cytotoxic effect of palytoxin on Caco-2 cells hinders their use for in vitro absorption studies. Food Chem. Toxicol. 2012, 50, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Schilling, W.P.; Snyder, D.; Sinkins, W.G.; Estacion, M. Palytoxin-induced cell death cascade in bovine aortic endothelial cells. Am. J. Physiol. Cell Physiol. 2006, 291, C657–C667. [Google Scholar] [CrossRef] [PubMed]
- Vale-Gonzalez, C.; Pazos, M.J.; Alfonso, A.; Vieytes, M.R.; Botana, L.M. Study of the neuronal effects of ouabain and palytoxin and their binding to Na, K-ATPases using an optical biosensor. Toxicon 2007, 50, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Pelin, M.; Boscolo, S.; Poli, M.; Sosa, S.; Tubaro, A.; Florio, C. Characterization of palytoxin binding to HaCaT cells using a monoclonal anti-palytoxin antibody. Mar. Drugs 2013, 11, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Rossini, G.P.; Bigiani, A. Palytoxin action on the Na+, K+-ATPase and the disruption of ion equilibria in biological systems. Toxicon 2011, 57, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Pelin, M.; Ponti, C.; Sosa, S.; Gibellini, D.; Florio, C.; Tubaro, A. Oxidative stress induced by palytoxin in human keratinocytes is mediated by a H+-dependent mitochondrial pathway. Toxicol. Appl. Pharm. 2013, 266, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pelin, M.; Sosa, S.; Pacor, S.; Tubaro, A.; Florio, C. The marine toxin palytoxin induces necrotic death in HaCaT cells through a rapid mitochondrial damage. Toxicol. Lett. 2014, 229, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, D.W.; Minden, A.; Sanchez, I.; Wattenberg, E.V. Regulation of a c-Jun amino-terminal kinase stress-activated protein kinase cascade by a sodium-dependent signal transduction pathway. J. Biol. Chem. 1997, 272, 23905–23911. [Google Scholar] [CrossRef] [PubMed]
- Wattenberg, E.V.; Byron, K.L.; Villereal, M.L.; Fujiki, H.; Rosner, M.R. Sodium as a mediator of non-phorbol tumor promoter action—Down-modulation of the epidermal growth-factor receptor by palytoxin. J. Biol. Chem. 1989, 264, 14668–14673. [Google Scholar] [PubMed]
- Perez-Gomez, A.; Novelli, A.; Fernandez-Sanchez, M.T. Na+/K+-ATPase inhibitor palytoxin enhances vulnerability of cultured cerebellar neurons to domoic acid via sodium-dependent mechanisms. J. Neurochem. 2010, 114, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Ares, I.R.; Louzao, M.C.; Vieytes, A.R.; Yasumoto, T.; Botana, L.M. Actin cytoskeleton of rabbit intestinal cells is a target for potent marine phycotoxins. J. Exp. Biol. 2005, 208, 4345–4354. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, R.E.; Deshpande, S.S.; Adler, M. Cytotoxic actions of palytoxin on aortic smooth muscle cells in culture. J. Appl. Toxicol. 2005, 25, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Louzao, M.C.; Ares, I.R.; Vieytes, M.R.; Valverde, I.; Vieites, J.M.; Yasumoto, T.; Botana, L.M. The cytoskeleton, a structure that is susceptible to the toxic mechanism activated by palytoxins in human excitable cells. FEBS J. 2007, 274, 1991–2004. [Google Scholar] [CrossRef] [PubMed]
- Valverde, I.; Lago, J.; Reboreda, A.; Vieites, J.M.; Cabado, A.G. Characteristics of palytoxin-induced cytotoxicity in neuroblastoma cells. Toxicol. Vitro 2008, 22, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Hwang, D.F.; Arakawa, O.; Daigo, K.; Sato, S.; Ozaki, H.; Kawai, N.; Ito, M.; Hashimoto, K. Palytoxin as the causative agent in parrotfish poisoning. Toxicon 1987, 26, 34. [Google Scholar]
- Alcala, A.C.; Alcala, L.C.; Garth, J.S.; Yasumura, D.; Yasumoto, T. Human fatality due to ingestion of the crab demania-reynaudii that contained a palytoxin-like toxin. Toxicon 1988, 26, 105–107. [Google Scholar] [CrossRef]
- Onuma, Y.; Satake, M.; Ukena, T.; Roux, J.; Chanteau, S.; Rasolofonirina, N.; Ratsimaloto, M.; Naoki, H.; Yasumoto, T. Identification of putative palytoxin as the cause of clupeotoxism. Toxicon 1999, 37, 55–65. [Google Scholar] [CrossRef]
- Taniyama, S.; Mahmud, Y.; Terada, M.; Takatani, T.; Arakawa, O.; Noguchi, T. Occurrence of a food poisoning incident by palytoxin from a serranid epinephelus sp in japan. J. Nat. Toxins 2002, 11, 277–282. [Google Scholar] [PubMed]
- Wu, M.L.; Yang, C.C.; Deng, J.F.; Wang, K.Y. Hyperkalemia, hyperphosphatemia, acute kidney injury, and fatal dysrhythmias after consumption of palytoxin-contaminated goldspot herring. Ann. Emerg. Med. 2014, 64, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Durando, P.; Ansaldi, F.; Oreste, P.; Moscatelli, P.; Marensi, L.; Grillo, C.; Gasparini, R.; Icardi, G. Ostreopsis ovata and human health: Epidemiological and clinical features of respiratory syndrome outbreaks from a two-year syndromic surveillance, 2005–2006, in north-west italy. Euro Surveill. 2007, 12, E070607.1. [Google Scholar] [PubMed]
- Tichadou, L.; Glaizal, M.; Armengaud, A.; Grossel, H.; Lemee, R.; Kantin, R.; Lasalle, J.L.; Drouet, G.; Rambaud, L.; Malfait, P.; et al. Health impact of unicellular algae of the Ostreopsis genus blooms in the Mediterranean sea: Experience of the french Mediterranean coast surveillance network from 2006 to 2009. Clin. Toxicol. 2010, 48, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Kermarec, F.; Dor, F.; Armengaud, A.; Charlet, F.; Kantin, R.; Sauzade, D.; de Haro, L. Health risks related to Ostreopsis ovata in recreational waters. Environ. Risque Sante 2008, 7, 357–363. [Google Scholar]
- Gallitelli, M.; Ungaro, N.; Addante, L.M.; Silver, N.G.; Sabba, C. Respiratory illness as a reaction to tropical algal blooms occurring in a temperate climate. J. Am. Med. Assoc. 2005, 293, 2599–2600. [Google Scholar]
- Sansoni, G.; Borghini, B.; Camici, G.; Casotti, M.; Righini, P.; Rustighi, C. Algal blooms of Ostreopsis ovata (Gonyaulacales: Dinophyceae): An emerging problem. Biol. Ambient. 2003, 17, 17–23. [Google Scholar]
- Ungaro, N.; Pastorelli, A.M.; Blonda, M.; Assennato, G. Surveillance monitoring of ostreopsis ovata blooms in the apulian seas: Methodological approach and results from the summer season 2007. Biol. Mar. Mediterr. 2008, 15, 62–64. [Google Scholar]
- Deeds, J.R.; Schwartz, M.D. Human risk associated with palytoxin exposure. Toxicon 2010, 56, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Deeds, J.R.; Handy, S.M.; White, K.D.; Reimer, J.D. Palytoxin found in Palythoa sp. zoanthids (anthozoa, hexacorallia) sold in the home aquarium trade. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Wieringa, A.; Bertholee, D.; Ter Horst, P.; van den Brand, I.; Haringman, J.; Ciminiello, P. Respiratory impairment in four patients associated with exposure to palytoxin containing coral. Clin. Toxicol. 2014, 52, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Hamade, A.K.; Deglin, S.E.; McLaughlin, J.B.; Deeds, J.R.; Handy, S.M.; Knolhoff, A.M. Suspected palytoxin inhalation exposures associated with zoanthid corals in aquarium shops and homes—Alaska, 2012–2014. Morb. Mortal. Wkly. Rep. 2015, 64, 852–855. [Google Scholar] [CrossRef]
- Hoffmann, K.; Hermanns-Clausen, M.; Buhl, C.; Buchler, M.W.; Schemmer, P.; Mebs, D.; Kauferstein, S. A case of palytoxin poisoning due to contact with zoanthid corals through a skin injury. Toxicon 2008, 51, 1535–1537. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; Khalifa, Y.M.; Espandar, L.; Mifflin, M.D. Aquarium coral keratoconjunctivitis. Arch. Ophthalmol. 2010, 128, 1360–1362. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, Y.; Fuchs, J.; Beuschel, R.; Tschopp, M.; Goldblum, D. Dangerous reef aquaristics: Palytoxin of a brown encrusting anemone causes toxic corneal reactions. Toxicon 2015, 106, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Majlesi, N.; Su, M.K.; Chan, G.M.; Lee, D.C.; Greller, H.A. A case of inhalational exposure to palytoxin. Clin. Toxicol. 2008, 46, 637. [Google Scholar]
- Snoeks, L.; Veenstra, J. Family with fever after cleaning a sea aquarium. Ned. Tijdschr. Geneeskd. 2012, 156, A4200. [Google Scholar] [PubMed]
- Bernasconi, M.; Berger, D.; Tamm, M.; Stolz, D. Aquarism: An innocent leisure activity? Palytoxin-induced acute pneumonitis. Respiration 2012, 84, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Sud, P.; Su, M.K.; Greller, H.A.; Majlesi, N.; Gupta, A. Case series: Inhaled coral vapor-toxicity in a tank. J. Med. Toxicol. 2013, 9, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Rumore, M.; Houst, M. Palytoxin poisoning via inhalation in pediatric siblings. Int. J. Case Rep. Images 2014, 5, 501–504. [Google Scholar] [CrossRef]
- Hall, C.; Levi, D.; Sattler, S. A case of palytoxin poisoning in a home aquarium enthusiast and his family. Case Rep. Emerg. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Nordt, S.P.; Wu, J.; Zahller, S.; Clark, R.F.; Cantrell, F.L. Palytoxin poisoning after dermal contact with zoanthid coral. J. Emerg. Med. 2009, 40, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Oku, N.; Sata, N.U.; Matsunaga, S.; Uchida, H.; Fusetani, N. Identification of palytoxin as a principle which causes morphological changes in rat 3Y1 cells in the zoanthid Palythoa aff. margaritae. Toxicon 2004, 43, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R.J.; Kashiwagi, M.; Moore, R.E.; Norton, T.R. Anticancer activity of zoanthids and the associated toxin, palytoxin, against ehrlich ascites tumor and p-388 lymphocytic leukemia in mice. J. Pharm. Sci. 1974, 63, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Attaway, D.H.; Ciereszko, L.S. Isolation and partial characterization of Caribbean palytoxin. In Proceedings of the 2nd International Coral Reef Symposium, Brisbane, Australia, 22 June–2 July 1973; Cameron, A.M., Cambell, B.M., Cribb, A.B., Endean, R., Jell, J.S., Jones, O.A., Mather, P., Talbot, F.H., Eds.; The Great Barrier Reef Committee: Brisbane, Australia; pp. 497–504.
- Beress, L.; Zwick, J.; Kolkenbrock, H.J.; Kaul, P.N.; Wassermann, O. A method for the isolation of the caribbean palytoxin (C-PTX) from the coelenterate (zooanthid) Palythoa caribaeorum. Toxicon 1983, 21, 285–290. [Google Scholar] [CrossRef]
- Gleibs, S.; Mebs, D. Distribution and sequestration of palytoxin in coral reef animals. Toxicon 1999, 37, 1521–1527. [Google Scholar] [CrossRef]
- Uemura, D.; Hirata, Y.; Iwashita, T.; Naoki, H. Studies on palytoxins. Tetrahedron 1985, 41, 1007–1017. [Google Scholar] [CrossRef]
- Kimura, S.; Hashimoto, Y. Purification of toxin in a zoanthid Palythoa tuberculosa. Publ. Seto Mar. Biol. Lab. 1973, 20, 713–718. [Google Scholar]
- Hirata, Y.; Uemura, D.; Ueda, K.; Takano, S. Several compounds from Palythoa tuberculosa (Coelenterata). Pure Appl. Chem. 1979, 51, 1875–1883. [Google Scholar] [CrossRef]
- Ellis, S. Farming Soft Corals for the Marine Aquarium Trade; Center for Tropical and Subtropical Aquaculture: Waimanalo, HI, USA, 1999. [Google Scholar]
- Ellis, S.; Sharron, L. The Culture of Soft Corals (Order: Alcyonacea) for the Marine Aquarium Trade; Center for Tropical and Subtropical Aquaculture: Waimanalo, HI, USA, 1999. [Google Scholar]
- Ottuso, P. Aquatic dermatology: Encounters with the denizens of the deep (and not so deep)—A review. Part II: The vertebrates, single-celled organisms, and aquatic biotoxins. Int. J. Dermatol. 2013, 52, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Tubaro, A.; del Favero, G.; Beltramo, D.; Ardizzone, M.; Forino, M.; de Bortoli, M.; Pelin, M.; Poli, M.; Bignami, G.; Ciminiello, P.; et al. Acute oral toxicity in mice of a new palytoxin analog: 42-hydroxy-palytoxin. Toxicon 2011, 57, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Sosa, S.; del Favero, G.; de Bortoli, M.; Vita, F.; Soranzo, M.R.; Beltramo, D.; Ardizzone, M.; Tubaro, A. Palytoxin toxicity after acute oral administration in mice. Toxicol. Lett. 2009, 191, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Del Favero, G.; Sosa, S.; Poli, M.; Tubaro, A.; Sbaizero, O.; Lorenzon, P. In vivo and in vitro effects of 42-hydroxy-palytoxin on mouse skeletal muscle: Structural and functional impairment. Toxicol. Lett. 2014, 225, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Prandi, S.; Sala, G.L.; Bellocci, M.; Alessandrini, A.; Facci, P.; Bigiani, A.; Rossini, G.P. Palytoxin induces cell lysis by priming a two-step process in MCF-7 cells. Chem. Res. Toxicol. 2011, 24, 1283–1296. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Benedettini, G.; Onorari, M.; Serena, F.; Battocchi, C.; et al. First finding of Ostreopsis cf. ovata toxins in marine aerosols. Environ. Sci. Technol. 2014, 48, 3532–3540. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Suganuma, M.; Nakayasu, M.; Hakii, H.; Horiuchi, T.; Takayama, S.; Sugimura, T. Palytoxin is a non-12-O-tetradecanoylphorbol-13-acetate type tumor promoter in two-stage mouse skin carcinogenesis. Carcinogenesis 1986, 7, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Wattenberg, E.V. Palytoxin: Exploiting a novel skin tumor promoter to explore signal transduction and carcinogenesis. Am. J. Physiol. Cell Physiol. 2007, 292, 24–32. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelin, M.; Brovedani, V.; Sosa, S.; Tubaro, A. Palytoxin-Containing Aquarium Soft Corals as an Emerging Sanitary Problem. Mar. Drugs 2016, 14, 33. https://doi.org/10.3390/md14020033
Pelin M, Brovedani V, Sosa S, Tubaro A. Palytoxin-Containing Aquarium Soft Corals as an Emerging Sanitary Problem. Marine Drugs. 2016; 14(2):33. https://doi.org/10.3390/md14020033
Chicago/Turabian StylePelin, Marco, Valentina Brovedani, Silvio Sosa, and Aurelia Tubaro. 2016. "Palytoxin-Containing Aquarium Soft Corals as an Emerging Sanitary Problem" Marine Drugs 14, no. 2: 33. https://doi.org/10.3390/md14020033
APA StylePelin, M., Brovedani, V., Sosa, S., & Tubaro, A. (2016). Palytoxin-Containing Aquarium Soft Corals as an Emerging Sanitary Problem. Marine Drugs, 14(2), 33. https://doi.org/10.3390/md14020033