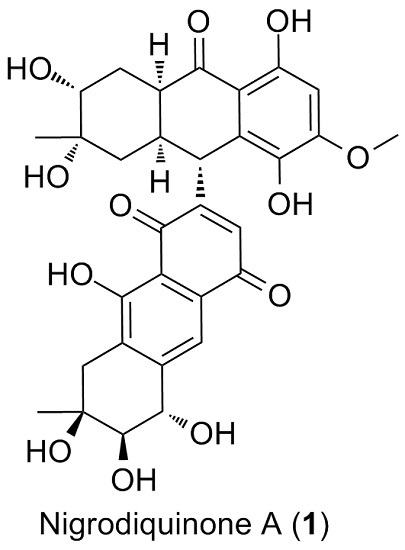
Nigrodiquinone A, a Hydroanthraquinone Dimer Containing a Rare C-9–C-7′ Linkage from a Zoanthid-Derived Nigrospora sp. Fungus
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Extraction and Isolation
3.4. Biological Assays
3.5. Computational Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fsulkner, D.J. Marine natural products. Nat. Prod. Rep. 1987, 4, 539–576. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2008, 25, 35–94. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.M.; Zheng, C.J.; Chen, G.Y.; Song, X.P.; Han, C.R.; Li, G.N.; Fu, Y.H.; Chen, W.H.; Niu, Z.G. Bioactive anthraquinone derivatives from the mangrove-derived fungus Stemphylium sp. 33231. J. Nat. Prod. 2014, 77, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Socha, A.M.; Garcia, D.; Sheffer, R.; Rowley, D.C. Antibiotic bisanthraquinones produced by a Streptomycete isolated from a cyanobacterium associated with Ecteinascidia turbinate. J. Nat. Prod. 2006, 69, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Nash, B.D.; Duffy, S.; Avery, V.M. Albopunctatone, an antiplasmodial anthrone-anthraquinone from the Australian ascidian Didemnum albopunctatum. J. Nat. Prod. 2012, 75, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Shao, C.L.; Guo, Z.Y.; Chen, J.F.; Deng, D.S.; Yang, K.L.; Chen, Y.Y.; Fu, X.M.; She, Z.G.; Lin, Y.C.; et al. Bioactive hydroanthraquinones and anthraquinone dimmers from a soft coral-derived Alternaria sp. fungus. J. Nat. Prod. 2012, 75, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.L.; Wei, M.Y.; Shao, C.L.; Fu, X.M.; Guo, Z.Y.; Xu, R.F.; Zheng, C.J.; She, Z.G.; Lin, Y.C.; Wang, C.Y. Antibacterial anthraquinone derivatives from a sea anemone-derived fungus Nigrospora sp. J. Nat. Prod. 2012, 75, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.J. Organic Stereochemsitry—Experimental and Theoretical Methods; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Zhu, H.J. Current Organic Stereochemistry; Science Presses of China: Beijing, China, 2009. [Google Scholar]
- Zhu, H.J.; Li, W.X.; Hu, D.B.; Wen, M.L. Discussion of absolute configuration for bioactive Griseusins by comparing computed optical rotations and electronic circular dichroism with the experimental results. Tetrahedron 2014, 70, 8236–8243. [Google Scholar] [CrossRef]
- Yu, H.; Li, W.X.; Wang, J.C.; Yang, Q.; Wang, H.J.; Zhang, C.C.; Ding, S.S.; Li, Y.; Zhu, H.J. Pestalotiopsin C, stereochemistry of a new caryophyllene from a fungus of Trichoderma sp. and its tautomerization characteristics in solution. Tetrahedron 2015, 71, 3491–3494. [Google Scholar] [CrossRef]
- He, P.; Wang, X.F.; Guo, X.J.; Zhou, C.Q.; Shen, S.G.; Hu, D.B.; Yang, X.L.; Luo, D.Q.; Dukor, R.; Zhu, H.J. Vibrational circular dichroism study for natural bioactive schizandrin and reassignment of its absolute configuration. Tetrahedron Lett. 2014, 55, 2965–2968. [Google Scholar] [CrossRef]
- He, J.B.; Ji, Y.N.; Hu, D.B.; Zhang, S.; Yan, H.; Liu, X.C.; Luo, H.R.; Zhu, H.J. Structure and absolute configuration of penicilliumine, a new alkaloid from Penicillium commune 366606. Tetrahedron Lett. 2014, 55, 2684–2686. [Google Scholar] [CrossRef]
- Huang, C.H.; Pan, J.H.; Chen, B.; Yu, M.; Huang, H.B.; Zhu, X.; Lu, Y.J.; She, Z.G.; Lin, Y.C. Three bianthraquinone derivatives from the mangrove endophytic fungus Alternaria sp. ZJ9-6B from the South China Sea. Mar. Drugs 2011, 9, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Suemitsu, R.; Horiuchi, K.; Kubota, M.; Okamatse, T. Production of alterporriols, altersolanols and macrosporin by Alternaria porri and A. Solani. Phytochemistry 1990, 29, 1509–1511. [Google Scholar] [CrossRef]
- Debbab, A.; Aly, A.H.; Edrada-Ebel, R.; Wray, V.; Müller, W.E.G.; Totzke, F.; Zirrgiebel, U.; Schächtele, C.; Kubbutat, M.H.G.; Lin, W.H.; et al. Bioactive metabolites from the endophytic fungus Stemphylium globuliferum isolated from Mentha pulegium. J. Nat. Prod. 2009, 72, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Rideout, J.A.; Sutherland, M.D. Pigments of marine animals. XV. Bianthrones and related polyketides from Lamprometra palmate gyges and other species of crinoids. Aust. J. Chem. 1985, 38, 793–808. [Google Scholar] [CrossRef]
- Qin, X.Y.; Yang, K.L.; Li, J.; Wang, C.Y.; Shao, C. L. Phylogenetic diversity and antibacterial activity of culturable fungi derived from the zoanthid Palythoa haddoni in the South China Sea. Mar. Biotechnol. 2015, 17, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Grassauer, A.; Weinmuellner, R.; Meier, C.; Pretsch, A.; Prieschl-Grassauer, E.; Unger, H. Iota-carrageenan is a potent inhibitor of rhinovirus infection. Virol. J. 2008, 5. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]



| Position | δH (J in Hz) | δC, Mult. | H–H COSY | HMBC |
|---|---|---|---|---|
| 1 | 1.48, t, (13.5) 1.88, ddd, (13.5, 3.6, 2.3) | 41.5, CH2 | H-1a | C-3 |
| 1a | 2.86, m | 34.5, CH | H-1, 4a, 9 | |
| 2 | 70.6, C | |||
| 3 | 3.31, m | 71.0, CH | H-4 | |
| 4 | 1.65, ddd, (12.6, 12.0, 4.8) 2.52, ddd, (12.7, 4.5, 2.7) | 36.8, CH2 | H-3, 4a | C-2, 10 |
| 4a | 3.07, m | 41.5, CH | H-1a, 4 | |
| 5 | 159.1, C | |||
| 6 | 6.51, s | 98.7, CH | C-10a | |
| 7 | 155.7, C | |||
| 8 | 136.0, C | |||
| 9 | 4.68, br s | 36.8, CH | C-1, 1a, 9a, 10a, 6′, 8′ | |
| 9a | 124.9, C | |||
| 10 | 203.5, C | |||
| 10a | 109.6, C | |||
| 11 | 1.17, s | 26.8, CH3 | C-1, 2, 3 | |
| 12 | 3.93, s | 55.8, CH3 | C-7 | |
| 1′ | 2.68, d (19.0), 3.07, d (19.0) | 36.8, CH2 | C-3′, 4a′, 9′ | |
| 1a′ | 130.2, C | |||
| 2′ | 70.9, C | |||
| 3′ | 3.54, d (8.7) | 77.4, CH | H-4′ | |
| 4′ | 4.74, br d (8.7) | 71.5, CH | H-3′ | |
| 4a′ | 148.1, C | |||
| 5′ | 183.7, C | |||
| 6′ | 6.13, d (1.1) | 135.3, CH | C-8′, 9 | |
| 7′ | 129.7, C | |||
| 8′ | 189.9, C | |||
| 9′ | 159.6, C | |||
| 9a′ | 113.0, C | |||
| 10′ | 7.76, br s | 117.3, CH | C-1a′, 4′, 5′, 9a′ | |
| 10a′ | 100.0, C | |||
| 11′ | 1.43, s | 26.3, CH3 | C-1′, 2′, 3′ | |
| 2-OH | 3.17, br s | |||
| 3-OH | 3.71, d (4.9) | |||
| 5-OH | 12.90, s | C-6, 10a | ||
| 8-OH | 7.61, s | |||
| 2′-OH | 3.67, br s | |||
| 3′-OH | 4.27, br s | |||
| 4′-OH | 4.87, br s | |||
| 9′-OH | 12.54, s | C-1a′, 9′, 9a′ |
| Virus | IC50 (μM) | |||||
| 1 | 2 | 3 | 4 | 5 | Ribavirin b | |
| RSV | - | - | - | - | 74.0 | 78.0 |
| Cox-B3 | - | - | - | 93.7 | - | 39.0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.-F.; Hou, X.-M.; Yang, K.-L.; Cao, F.; Yang, R.-Y.; Wang, C.-Y.; Shao, C.-L. Nigrodiquinone A, a Hydroanthraquinone Dimer Containing a Rare C-9–C-7′ Linkage from a Zoanthid-Derived Nigrospora sp. Fungus. Mar. Drugs 2016, 14, 51. https://doi.org/10.3390/md14030051
Xu W-F, Hou X-M, Yang K-L, Cao F, Yang R-Y, Wang C-Y, Shao C-L. Nigrodiquinone A, a Hydroanthraquinone Dimer Containing a Rare C-9–C-7′ Linkage from a Zoanthid-Derived Nigrospora sp. Fungus. Marine Drugs. 2016; 14(3):51. https://doi.org/10.3390/md14030051
Chicago/Turabian StyleXu, Wei-Feng, Xue-Mei Hou, Kai-Lin Yang, Fei Cao, Rui-Yun Yang, Chang-Yun Wang, and Chang-Lun Shao. 2016. "Nigrodiquinone A, a Hydroanthraquinone Dimer Containing a Rare C-9–C-7′ Linkage from a Zoanthid-Derived Nigrospora sp. Fungus" Marine Drugs 14, no. 3: 51. https://doi.org/10.3390/md14030051