Pseudopterosin A: Protection of Synaptic Function and Potential as a Neuromodulatory Agent
Abstract
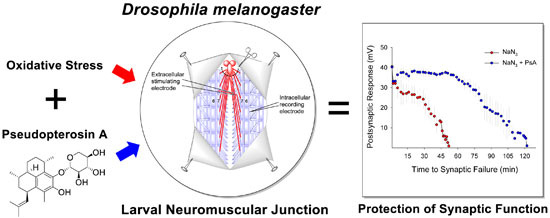
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Animal Material
3.2. Extraction and Isolation
3.3. Fly Stocks
3.4. Electrophysiology
3.5. Pharmacology
3.6. Drosophila NMJ Electrophysiology Statistics
3.7. Preparation of Stocks, Calibration Standards, and Quality Control Samples
3.8. Calibration Curves
3.9. Sample Preparation
3.10. Liquid Chromatography
3.11. Linearity, Precision, and Accuracy of the Bioanalytical Method
3.12. Recovery
3.13. Pharmacokinetic Experiments in Mice
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PsA | pseudopterosin A |
| ROS | reactive oxygen species |
| NMJ | neuromuscular junction |
| NaN3 | sodium azide |
| H2O2 | hydrogen peroxide |
| BBB | blood–brain barrier |
| LLOQ | lower limit of quantification |
| HPLC | high-performance liquid chromatography |
| NBD-Cl | 4-chloro-7-nitrobenzofurazan |
| IV | intravenous |
| AUC | area under the curve |
| RE | relative exposure |
| EJP | excitatory junction potential |
| DMSO | dimethyl sulfoxide |
References
- Inoue, M.; Sato, E.F.; Nishikawa, M.; Park, A.M.; Kira, Y.; Imada, I.; Utsumi, K. Mitochondrial generation of reactive oxygen species and its role in aerobic life. Curr. Med. Chem. 2003, 10, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, J.M.; Segal, M. Peroxide modulation of slow onset potentiation in rat hippocampus. J. Neurosci. 1997, 17, 8695–8701. [Google Scholar] [PubMed]
- Vanden-Hoek, T.L.; Becker, L.B.; Shao, Z.; Li, C.; Schumacker, P.T. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J. Biol. Chem. 1998, 273, 18092–18098. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Shigenaga, M.K.; Park, J.W.; Degan, P.; Ames, B.N. Oxidative damage to DNA during aging: 8-Hydroxy-2′-deoxyguanosine in rat organ DNA and urine. Proc. Natl. Acad. Sci. USA 1990, 87, 4533–4537. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J.; Hurd, H.K.; Hollstein, M.C.; Levin, D.E.; Esterbauer, H.; Ames, B.N. Naturally occurring carbonyl compounds are mutagens in Salmonella tester strain TA104. Mutat. Res. 1985, 148, 25–34. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Starke-Reed, P.E.; Oliver, C.N.; Carney, J.M.; Floyd, R.A. Protein modification in aging. EXS 1992, 62, 64–72. [Google Scholar] [PubMed]
- Wicks, S.; Bain, N.; Duttaroy, A.; Hilliker, A.J.; Phillips, J.P. Hypoxia rescues early mortality conferred by superoxide dismutase deficiency. Free Radic. Biol. Med. 2009, 46, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Réus, G.Z.; Fries, G.R.; Stertz, L.; Badawy, M.; Passos, I.C.; Barichello, T.; Kapczinski, F.; Quevedo, J. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 2015, 300, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Cardaioli, E.; Da Pozzo, P.; Formichi, P.; Gallus, G.N.; Radi, E. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 2012, 322, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Look, S.A.; Fenical, W.; Jacobs, R.S.; Clardy, J. The pseudopterosins: Anti-inflammatory and analgesic natural products from the sea whip Pseudopterogorgia elisabethae. Proc. Natl. Acad. Sci. USA 1986, 83, 6238–6240. [Google Scholar] [CrossRef] [PubMed]
- Look, S.A.; Fenical, W.; Matsumoto, G.K.; Clardy, J. The pseudopterosins: A new class of antiinflammatory and analgesic diterpene pentosides from the marine sea whip Pseudopterogorgia elisabethae (Octocorallia). J. Org. Chem. 1986, 51, 5140–5145. [Google Scholar] [CrossRef]
- Fenical, W. Marine Soft Corals of the Genus Pseudopterogorgia: A Resource for novel anti-inflammatory diterpenoids. J. Nat. Prod. 1987, 50, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Roussis, V.; Wu, Z.; Fenical, W.; Strobel, S.A.; Van Duyne, G.D.; Clardy, J. New anti-inflammatory pseudopterosins from the marine octocoral Pseudopterogorgia elisabethae. J. Org. Chem. 1990, 55, 4916–4922. [Google Scholar] [CrossRef]
- Berrue, F.; McCulloch, M.W.; Kerr, R.G. Marine diterpene glycosides. Bioorg. Med. Chem. 2011, 19, 6702–6719. [Google Scholar] [CrossRef] [PubMed]
- Correa, H.; Aristizabal, F.; Duque, C.; Kerr, R. Cytotoxic and antimicrobial activity of pseudopterosins and seco-pseudopterosins isolated from the octocoral Pseudopterogorgia elisabethae of San Andrés and Providencia Islands (Southwest Caribbean Sea). Mar. Drugs 2011, 9, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.I.; Shi, Y.-P.; Garcia, O.J.; Rodriguez, A.D.; Mayer, A.M.; Sanchez, J.A.; Ortega-Barria, E.; Gonzalez, J. New pseudopterosin and seco-pseudopterosin diterpene glycosides from two Colombian isolates of Pseudopterogorgia elisabethae and their diverse biological activities. J. Nat. Prod. 2004, 67, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Dayan, N.; Grove, G.; Sivalenka, R. Anti-inflammatory activity of pseudopterosins by laser doppler blood flow evaluation. Int. J. Cosmet. Sci. 2009, 31, 480–481. [Google Scholar] [CrossRef]
- Mayer, A.M.; Jacobson, P.B.; Fenical, W.; Jacobs, R.S.; Glaser, K.B. Pharmacological characterization of the pseudopterosins: Novel anti-inflammatory natural products isolated from the Caribbean soft coral, Pseudopterogorgia elisabethae. Life Sci. 1998, 62, PL401–PL407. [Google Scholar] [CrossRef]
- Correa, H.; Valenzuela, A.L.; Ospina, L.F.; Duque, C. Anti-inflammatory effects of the gorgonian Pseudopterogorgia elisabethae collected at the Islands of Providencia and San Andrés (SW Caribbean). J. Inflamm. 2009, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Ata, A.; Kerr, R.G.; Moya, C.E.; Jacobs, R.S. Identification of anti-inflammatory diterpenes from the marine gorgonian Pseudopterogorgia elisabethae. Tetrahedron 2003, 59, 4215–4222. [Google Scholar] [CrossRef]
- Montesinos, M.C.; Gadangi, P.; Longaker, M.; Sung, J.; Levine, J.; Nilsen, D.; Reibman, J.; Li, M.; Jiang, C.-K.; Hirschhorn, R.; et al. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J. Exp. Med. 1997, 186, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Ata, A.; Win, H.Y.; Holt, D.; Holloway, P.; Segstro, E.P.; Jayatilake, G.S. New antibacterial diterpenes from Pseudopterogorgia elisabethae. Helv. Chim. Acta 2004, 87, 1090–1098. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Marine Natural Products and Related Compounds in Clinical and Advanced Preclinical Trials. J. Nat. Prod. 2004, 67, 1216–1238. [Google Scholar] [CrossRef] [PubMed]
- Ettouati, W.S.; Jacobs, R.S. Effect of pseudopterosin A on cell division, cell cycle progression, DNA, and protein synthesis in cultured sea urchin embryos. Mol. Pharmacol. 1987, 31, 500–505. [Google Scholar] [PubMed]
- Mydlarz, L.D.; Jacobs, R.S. Comparison of an inducible oxidative burst in free-living and symbiotic dinoflagellates reveals properties of the pseudopterosins. Phytochemistry 2004, 65, 3231–3241. [Google Scholar] [CrossRef] [PubMed]
- Moya, C.E.; Jacobs, R.S. Pseudopterosin A inhibits phagocytosis and alters intracellular calcium turnover in a pertussis toxin sensitive site in Tetrahymena thermophile. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 2006, 143, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Cull-Candy, S.G.; Miledi, R. Glutamate and quisqualate noise in voltage-clamped locust muscle fibres. Nature 1976, 261, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Patlak, J.B.; Gration, K.A.; Usherwood, P.N. Single glutamate-activated channels in locust muscle. Nature 1979, 278, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Jan, L.Y.; Jan, Y.N. Properties of the larval neuromuscular junction in Drosophila melanogaster. J. Physiol. 1976, 262, 189–214. [Google Scholar] [CrossRef] [PubMed]
- Jan, L.Y.; Jan, Y.N. l-Glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J. Physiol. 1976, 262, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Gramates, L.S.; Budnik, V. Assembly and maturation of the Drosophila larval neuromuscular junction. Int. Rev. Neurobiol. 1999, 43, 93–117. [Google Scholar] [CrossRef]
- Collins, C.A.; DiAntonio, A. Synaptic development: Insights from Drosophila. Curr. Opin. Neurobiol. 2007, 17, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.C.; Mlady, G.W.; Kwon, Y.H.; Rose, G.M. Chronic in vivo sodium azide infusion induces selective and stable inhibition of cytochrome c oxidase. J. Neurochem. 1996, 66, 2606–2611. [Google Scholar] [CrossRef] [PubMed]
- Noumi, T.; Maeda, M.; Futai, M. Mode of inhibition of sodium azide on H+-ATPase of Escherichia coli. FEBS Lett. 1987, 213, 381–384. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. Inhibition of superoxide dismutases by azide. Arch. Biochem. Biophys. 1978, 189, 317–322. [Google Scholar] [CrossRef]
- Ciesla, Z.; Mardarowicz, K.; Klopotowski, T. Inhibition of DNA synthesis and cell division in Salmonella typhimurium by azide. Mol. Genet. Genom. 1974, 135, 339–348. [Google Scholar] [CrossRef]
- Halliwell, B. Role of free radicals in the neurodegenerative diseases: Therapeutic implications for antioxidant treatment. Drugs Aging 2001, 18, 685–716. [Google Scholar] [CrossRef] [PubMed]
- Haddad, G.G.; Jiang, C. O2 deprivation in the central nervous system: On mechanisms of neuronal response, differential sensitivity and injury. Prog. Neurobiol. 1993, 40, 277–318. [Google Scholar] [CrossRef]
- Wingrove, J.A.; O’Farrell, P.H. Nitric oxide contributes to behavioral, cellular, and developmental responses to low oxygen in Drosophila. Cell 1999, 98, 105–114. [Google Scholar] [CrossRef]
- Haddad, G.G. Tolerance to low O2: Lessons from invertebrate genetic models. Exp. Physiol. 2006, 91, 277–282. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, P.H. Conserved responses to oxygen deprivation. J. Clin. Investig. 2001, 107, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Caplan, S.L.; Milton, S.L.; Dawson-Scully, K. A cGMP-dependent protein kinase (PKG) controls synaptic transmission tolerance to acute oxidative stress at the Drosophila larval neuromuscular junction. J. Neurophysiol. 2013, 109, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Hamad, I.; Arda, N.; Pekmez, M.; Karaer, S.; Temizkan, G. Intracellular scavenging activity of Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) in the fission yeast, Schizosaccharomyces pombe. J. Nat. Sci. Biol. Med. 2001, 1, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Jayaraman, A.; Quaglino, A.; Pike, C.J. Androgens selectively protect against apoptosis in hippocampal neurones. J. Neuroendocrinol. 2010, 22, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; West, L.M. Estimating the Lipophilicity of Natural Products using a Polymeric Reversed Phase HPLC Method. J. Liquid Chromatogr. Relat. Technol. 2009, 33, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Kunnecke, B.; Verry, P.; Benardeau, A.; Kienlin, M. Quantitative body composition analysis in awake mice and rats by magnetic resonance relaxometry. Obes. Res. 2004, 12, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.; Delp, M.; Lindstedt, S.; Rhomberg, L.; Beliles, R. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health 1997, 13, 407–484. [Google Scholar] [CrossRef] [PubMed]
- Macleod, G.T.; Hegstrom-Wojtowicz, M.; Charlton, M.P.; Atwood, H.L. Fast calcium signals in Drosophila motor neuron terminals. J. Neurophysiol. 2002, 88, 2659–2663. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.A.; Schuster, C.M.; Goodman, C.S.; Atwood, H.L. Homeostasis of synaptic transmission in Drosophila with genetically altered nerve terminal morphology. J. Neurosci. 1996, 16, 3877–3886. [Google Scholar] [PubMed]
- Zhong, W.; Moya, C.; Jacobs, R.S.; Little, R.D. Synthesis and an Evaluation of the Bioactivity of the C-Glycoside of Pseudopterosin A Methyl Ether. J. Org. Chem. 2008, 73, 7011–7016. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Little, R.D. Exploration and determination of the redox properties of the pseudopterosin class of marine natural products. Tetrahedron 2009, 65, 10784–10790. [Google Scholar] [CrossRef]
- Day, D.R.; Jabaiah, S.; Jacobs, R.S.; Little, R.D. Cyclodextrin Formulation of the Marine Natural Product Pseudopterosin A Uncovers Optimal Pharmacodynamics in Proliferation Studies of Human Umbilical Vein Endothelial Cells. Mar. Drugs 2013, 11, 3258–3271. [Google Scholar] [CrossRef] [PubMed]






| Parameter | Value |
|---|---|
| Distribution Half-life (h) | 0.05 ± 0.005 |
| Elimination Half-life (h) | 3.21 ± 0.30 |
| AUC (h·mg/L) | 14.35 ± 0.95 |
| CL (L/h·kg) | 3.48 ± 0.23 |
| Vss (L/kg) | 10.09 ± 1.35 |
| Cmax (mg/L) | 85.49 ± 7.64 |
| Parameter | Brain | Liver | Kidney |
|---|---|---|---|
| Half-life (h) | 2.91 | 3.16 | 3.98 |
| AUC * (h·mg/kg) | 19.49 | 18.20 | 18.37 |
| Cmax (μg/g) | 10.02 | 10.06 | 3.00 |
| Tmax (h) | 0.5 | 0.17 | 1.5 |
| Relative Exposure ** | 1.36 | 1.27 | 1.28 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caplan, S.L.; Zheng, B.; Dawson-Scully, K.; White, C.A.; West, L.M. Pseudopterosin A: Protection of Synaptic Function and Potential as a Neuromodulatory Agent. Mar. Drugs 2016, 14, 55. https://doi.org/10.3390/md14030055
Caplan SL, Zheng B, Dawson-Scully K, White CA, West LM. Pseudopterosin A: Protection of Synaptic Function and Potential as a Neuromodulatory Agent. Marine Drugs. 2016; 14(3):55. https://doi.org/10.3390/md14030055
Chicago/Turabian StyleCaplan, Stacee Lee, Bo Zheng, Ken Dawson-Scully, Catherine A. White, and Lyndon M. West. 2016. "Pseudopterosin A: Protection of Synaptic Function and Potential as a Neuromodulatory Agent" Marine Drugs 14, no. 3: 55. https://doi.org/10.3390/md14030055