Impact of Microalgae-Bacteria Interactions on the Production of Algal Biomass and Associated Compounds
Abstract
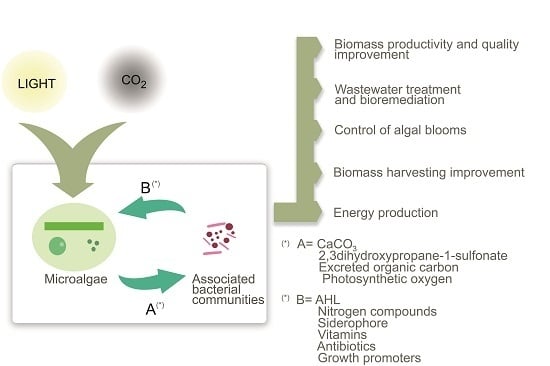
:1. Introduction
2. Algae-Bacteria Interactions: Relevance and Types
2.1. Mutualism
2.2. Commensalism
2.3. Parasitism
3. Algae-Bacteria Interactions: Effects on Biomass Production
4. Algae-Bacteria Interactions: Current and Promising Applications
4.1. Harvesting
4.2. Cell Disruption
4.3. Energy Production
4.4. Nutrient Removal and Wastewater Treatment
4.5. Bioremediation
4.6. Sustainable Aquaculture
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Amin, S.A.; Hmelo, L.R.; Van Tol, H.M.; Durham, B.P.; Carlson, L.T.; Heal, K.R.; Morales, R.L.; Berthiaume, C.T.; Parker, M.S.; Djunaedi, B.; et al. Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature 2015, 522, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.E.; Bashan, Y. Increased growth of the microalga Chlorella vulgaris when co-immobilized and co-cultured in alginate beads with the plant-growth-promoting bacterium Azospirillum brasilense. Appl. Environ. Microbiol. 2000, 66, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Ramanan, R.; Cho, D.H.; Oh, H.M.; Kim, H.S. Role of Rhizobium, a plant growth promoting bacterium, in enhancing algal biomass through mutualistic interaction. Biomass Bioenerg. 2014, 69, 95–105. [Google Scholar] [CrossRef]
- Landa, M.; Blain, S.; Christaki, U.; Monchy, S.; Obernosterer, I. Shifts in bacterial community composition associated with increased carbon cycling in a mosaic of phytoplankton blooms. ISME J. 2015, 10, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, R.; Kang, Z.; Kim, B.H.; Cho, D.H.; Jin, L.; Oh, H.M.; Kim, H.S. Phycosphere bacterial diversity in green algae reveals an apparent similarity across habitats. Algal Res. 2015, 8, 140–144. [Google Scholar] [CrossRef]
- Seyedsayamdost, M.R.; Case, R.J.; Kolter, R.; Clardy, J. The Jekyll-and-Hyde chemistry of Phaeobacter gallaeciensis. Nat. Chem. 2011, 3, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.B.; Smith, A.G. Exploring mutualistic interactions between microalgae and bacteria in the omics age. Curr. Opin. Plant Biol. 2015, 26, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Goecke, F.; Thiel, V.; Wiese, J.; Labes, A.; Imhoff, J.F. Algae as an important environment for bacteria-phylogenetic relationships among new bacterial species isolated from algae. Phycologia 2013, 52, 14–24. [Google Scholar] [CrossRef]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Consortia of cyanobacteria/microalgae and bacteria: Biotechnological potential. Biotechnol. Adv. 2011, 29, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hill, R.T.; Zheng, T.; Hu, X.; Wang, B. Effects of bacterial communities on biofuel producing microalgae: Stimulation, inhibition and harvesting. Crit. Rev. Biotechnol. 2015, 36. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, R.; Kim, B.H.; Cho, D.H.; Oh, H.M.; Kim, H.S. Algae-bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, J.M.; Smith, A.G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Teplitski, M.; Rajamani, S. Signal and nutrient exchange in the interactions between soil algae and bacteria. In Biocommunication in Soil Microorganisms; Witzany, G., Ed.; Springer: Berlin, Germany, 2011; pp. 413–426. [Google Scholar]
- Bolch, C.J.S.; Subramanian, T.A.; Green, D.H. The toxic dinoflagellate Gymnodinium catenatum (Dinophyceae). Requires marine bacteria for growth. J. Phycol. 2011, 47, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Kazamia, E.; Czesnick, H.; Nguyen, T.T.; Croft, M.T.; Sherwood, E.; Sasso, S.; Hodson, S.J.; Warren, M.J.; Smith, A.G. Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria exhibit regulation. Environ. Microbiol. 2012, 14, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, K.E.; Wheeler, G.L.; Leptos, K.C.; Goldstein, R.E.; Smith, A.G. Insights into the evolution of vitamin B12 auxotrophy from sequenced algal genomes. Mol. Boil. Evol. 2011, 28, 2921–2933. [Google Scholar] [CrossRef] [PubMed]
- Droop, M.R. Vitamins, phytoplankton and bacteria: Symbiosis or scavenging? J. Plankton Res. 2007, 29, 107–113. [Google Scholar] [CrossRef]
- Hernandez, J.P.; de-Bashan, L.E.; Rodriguez, D.J.; Rodriguez, Y.; Bashan, Y. Growth promotion of the freshwater microalga Chlorella vulgaris by the nitrogen-fixing, plant growth-promoting bacterium Bacillus pumilus from arid zone soils. Eur. J. Soil. Biol. 2009, 45, 88–93. [Google Scholar] [CrossRef]
- Watanabe, K.; Takihana, N.; Aoyagi, H.; Hanada, S.; Watanabe, Y.; Ohmura, N.; Saiki, H.; Tanaka, H. Symbiotic association in Chlorella culture. FEMS Microbiol. Ecol. 2005, 51, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.H.; Ramanan, R.; Heo, J.; Lee, J.; Kim, B.H.; Oh, H.M.; Kim, H.S. Enhancing microalgal biomass productivity by engineering a microalgal-bacterial community. Bioresour. Technol. 2015, 175, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.A.; Green, D.H.; Hart, M.C.; Küpper, F.C.; Sunda, W.G.; Carrano, C.J. Photolysis of iron-siderophore chelates promotes bacterial-algal mutualism. Proc. Natl. Acad. Sci. USA 2009, 106, 17071–17076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ask, J.; Karlsson, J.; Persson, L.; Ask, P.; Byström, P.; Jansson, M. Whole-lake estimates of carbon flux through algae and bacteria in benthic and pelagic habitats of clear-water lakes. Ecology 2009, 90, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.H.; Ramanan, R.; Heo, J.; Kang, Z.; Kim, B.H.; Ahn, C.Y.; Oh, H.M.; Kim, H.S. Organic carbon, influent microbial diversity and temperature strongly influence algal diversity and biomass in raceway ponds treating raw municipal wastewater. Bioresour. Technol. 2015, 191, 481–487. [Google Scholar] [CrossRef] [PubMed]
- González, J.M.; Simó, R.; Massana, R.; Covert, J.S.; Casamayor, E.O.; Pedrós-Alió, C.; Moran, M.A. Bacterial community structure associated with a dimethylsulfoniopropionate producing North Atlantic algal bloom. Appl. Environ. Microbiol. 2000, 66, 4237–4246. [Google Scholar] [CrossRef] [PubMed]
- Grossart, H.P.; Czub, G.; Simon, M. Algae-bacteria interactions and their effects on aggregation and organic matter flux in the sea. Appl. Environ. Microbiol. 2006, 8, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Grover, J.P. Resource competition and community structure in aquatic micro-organisms: Experimental studies of algae and bacteria along a gradient of organic carbon to inorganic phosphorus supply. J. Plankton Res. 2000, 22, 1591–1610. [Google Scholar] [CrossRef]
- Oh, H.M.; Lee, S.J.; Kim, J.H.; Kim, H.S.; Yoon, B.D. Seasonal variation and indirect monitoring of microcystin concentrations in Daechung Reservoir, Korea. Appl. Environ. Microbiol. 2001, 67, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Zapalski, M.K. Is absence of proof a proof of absence? Comments on commensalism. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 302, 484–488. [Google Scholar] [CrossRef]
- Gurung, T.B.; Urabe, J.; Nakanishi, M. Regulation of the relationship between phytoplankton Scenedesmusacutus and heterotrophic bacteria by the balance of light and nutrients. Aquat. Microb. Ecol. 1999, 17, 27–35. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeong, S.Y.; Lee, S.J. Isolation, identification, and algicidal activity of marine bacteria against Cochlodinium polykrikoides. J. Appl. Phycol. 2008, 20, 1069–1078. [Google Scholar] [CrossRef]
- Lee, Y.K.; Ahn, C.Y.; Kim, H.S.; Oh, H.M. Cyanobactericidal effect of Rhodococcus sp. isolated from eutrophic lake on Microcystis sp. Biotechnol. Lett. 2010, 32, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Su, J.; Tian, Y.; Ning, X.; Hong, H.; Zheng, T. Lysis of a red-tide causing alga, Alexandrium tamarense, caused by bacteria from its phycosphere. Biol. Control. 2010, 52, 123–130. [Google Scholar] [CrossRef]
- Goff, L.J.; Coleman, A.W. Transfer of nuclei from a parasite to its host. Proc. Natl. Acad. Sci. USA 1984, 81, 5420–5424. [Google Scholar] [CrossRef] [PubMed]
- Hancock, L.; Goff, L.; Lane, C. Red algae lose key mitochondrial genes in response to becoming parasitic. Genome Boil. Evol. 2010, 2, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Afi, L.; Metzger, P.; Largeau, C.; Connan, J.; Berkaloff, C.; Rousseau, B. Bacterial degradation of green microalgae: Incubation of Chlorella emersonii and Chlorella vulgaris with Pseudomonas oleovorans and Flavobacteriumaquatile. Org. Geochem. 1996, 25, 117–130. [Google Scholar] [CrossRef]
- Doncaster, C.P.; Jackson, A.; Watson, R.A. Manipulated into giving: When parasitism drives apparent or incidental altruism. Proc. R. Soc. Lond. Biol. 2013, 280. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.K. Cellulases and related enzymes in biotechnology. Biotechnol. Adv. 2000, 18, 355–383. [Google Scholar] [CrossRef]
- Dahiya, N.; Tewari, R.; Hoondal, G.S. Biotechnological aspects of chitinolytic enzymes: A review. Appl. Microbiol. Biotechnol. 2006, 71, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Moran, M.A. Evolutionary ecology of the marine Roseobacter clade. Microbiol. Mol. Biol. Rev. 2014, 78, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Durham, B.P.; Sharma, S.; Luo, H.; Smith, C.B.; Amin, S.A.; Bender, S.J.; Dearth, S.P.; Van Mooy, B.A.; Campagna, S.R.; Kujawinski, E.B.; et al. Cryptic carbon and sulfur cycling between ocean surface plankton. Proc. Natl. Acad. Sci. USA 2015, 112, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Coale, K.H.; Johnson, K.S.; Fitzwater, S.E.; Gordon, R.M.; Tanner, S.; Chavez, F.P.; Ferioli, L.; Sakamoto, C.; Rogers, P.; Millero, F.; et al. A massive phytoplakton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean. Nature 1996, 383, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Dymond, J.; Lyle, M. Flux comparisons between sediments and sediment traps in the eastern tropical Pacific: Implications for atmospheric CO2 variations during the Pleistocene. Limnol. Oceanogr. 1985, 30, 699–712. [Google Scholar] [CrossRef]
- Keller, M.D.; Bellows, W.K.; Guillard, R.R. Dimethyl sulfide production in marine phytoplankton. In Biogenic Sulfur in the Environment; Saltzman, E.S., Cooper, W.J., Eds.; American Chemical Society: Washington, DC, USA, 1989; pp. 167–182. [Google Scholar]
- Cho, B.C.; Azam, F. Major role of bacteria in biogeochemical fluxes in the ocean’s interior. Nature 1988, 332, 441–443. [Google Scholar] [CrossRef]
- Mayali, X.; Franks, P.J.S.; Azam, F. Cultivation and ecosystem role of a marine Roseobacter clade-affiliated cluster bacterium. Appl. Environ. Microbiol. 2008, 74, 2595–2603. [Google Scholar] [CrossRef] [PubMed]
- Villa, J.A.; Ray, E.E.; Barney, B.M. Azotobacter vinelandii siderophore can provide nitrogen to support the culture of the green algae Neochloris oleoabundans and Scenedesmus sp. BA032. FEMS Microbiol. Lett. 2014, 351, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.A.; Reis, A. Microalgal symbiosis in biotechnology. Appl. Microbiol. Biotechnol. 2014, 98, 5839–5846. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.A.; Kuypers, M.M.; Vagner, T.; Paerl, R.W.; Musat, N.; Zehr, J.P. Nitrogen fixation and transfer in open ocean diatom–cyanobacterial symbioses. ISME J. 2011, 5, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Leyva, L.A.; Bashan, Y.; Mendoza, A.; de-Bashan, L.E. Accumulation fatty acids of in Chlorella vulgaris under heterotrophic conditions in relation to activity of acetyl-CoA carboxylase, temperature, and co-immobilization with Azospirillum brasilense. Naturwissenschaften 2014, 101, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Choix, F.J.; de-Bashan, L.E.; Bashan, Y. Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: II. Heterotrophic conditions. Enzym. Microb. Technol. 2012, 51, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Choix, F.J.; de-Bashan, L.E.; Bashan, Y. Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: I. Autotrophic conditions. Enzym. Microb. Technol. 2012, 51, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Seyedsayamdost, M.R.; Carr, G.; Kolter, R.; Clardy, J. Roseobacticides: Small molecule modulators of an algal-bacterial symbiosis. J. Am. Chem. Soc. 2011, 133, 18343–18349. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, G.V.; Steinke, M. Grazing-activated production of dimethyl sulfide (DMS) by two clones of Emiliania huxleyi. Limnol. Oceanogr. 1996, 41, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, G.V.; Steinke, M.; Kirst, G.O. Grazing-activated chemical defence in a unicellular marine alga. Nature 1997, 387, 894–897. [Google Scholar] [CrossRef]
- Rivas, M.O.; Vargas, P.; Riquelme, C.E. Interactions of Botryococcus braunii cultures with bacterial biofilms. Microb. Ecol. 2010, 60, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Silva, A. Effect of the microalga Isochrysisgalbana on the early larval culture of Paralichthys adspersus. Cienc. Mar. 1999, 25, 267–276. [Google Scholar]
- Kazamia, E.; Aldridge, D.C.; Smith, A.G. Synthetic ecology—A way forward for sustainable algal biofuel production? J. Biotech. 2012, 162, 163–169. [Google Scholar] [CrossRef]
- Tulli, F.; Chini Zittelli, G.; Giorgi, G.; Poli, B.M.; Tibaldi, E.; Tredici, M.R. Effect of the inclusion of dried Tetraselmis suecica on growth, feed utilization, and fillet composition of European sea bass juveniles fed organic diets. J. Aquat. Food Prod. Technol. 2012, 188–197. [Google Scholar] [CrossRef]
- Reitan, K.I.; Rainuzzo, J.R.; Øie, G.; Olsen, Y. A review of the nutritional effects of algae in marine fish larvae. Aquaculture 1997, 155, 207–221. [Google Scholar] [CrossRef]
- Forján, E.; Navarro, F.; Cuaresma, M.; Vaquero, I.; Ruíz-Domínguez, M.C.; Gojkovic, Ž.; Vázquez, M.; Márquez, M.; Mogedas, B.; Bermejo, E.; et al. Microalgae: Fast-growth sustainable green factories. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1705–1755. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Q.; Sommerfeld, M.; Puruhito, E.; Chen, Y. Harvesting algal biomass for biofuels using ultrafiltration membranes. Bioresour. Technol. 2010, 101, 5297–5304. [Google Scholar] [CrossRef] [PubMed]
- Wijffels, R.H.; Barbosa, M.J. An Outlook on Microalgal Biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Laughinghouse, H.D.; Anderson, M.A.; Chen, F.; Willliams, E.; Place, A.R.; Zmora, O.; Zohar, Y.; Zheng, T.; Hill, R.T. Novel bacterial isolate from Permian groundwater, capable of aggregating potential biofuel-producing microalga Nannochloropsis oceanica IMET1. Appl. Environ. Microbiol. 2012, 78, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, S.Y.; Praveenkumar, R.; Kim, B.; Seo, J.Y.; Jeon, S.G.; Na, J.G.; Park, J.Y.; Kim, D.M.; Oh, Y.K. Repeated use of stable magnetic flocculant for efficient harvest of oleaginous Chlorella sp. Bioresour. Technol. 2014, 167, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Bitton, G.; Marshall, K.C. Adsorption of microorganisms to Surfaces. Limnol. Oceanogr. 1980, 25, 969–970. [Google Scholar]
- Strumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; John Wiley& Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Powell, R.J.; Hill, R.T. Mechanism of Algal Aggregation by Bacillus sp. Strain RP1137. Appl. Environ. Microbiol. 2014, 80, 4042–4050. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, I.S.; Kim, H.M.; Wi, S.G.; Bae, H.J. Bioethanol production from the nutrient stress induced microalga Chlorella vulgaris by enzymatic hydrolysis and immobilized yeast fermentation. Bioresour. Technol. 2014, 153, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Demuez, M.; González-Fernández, C.; Ballesteros, M. Algicidal microorganisms and secreted algicides: New tools to induce microalgal cell disruption. Biotechnol. Adv. 2015, 33, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Demuez, M.; Mahdy, A.; Tomás-Pejó, E.; González-Fernández, C.; Ballesteros, M. Enzymatic cell disruption of microalgae biomass in biorefinery processes. Biotechnol. Bioeng. 2015, 112, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Carsten, P.; Pohner, G. Interactions of the algicidal bacterium Kordiaalgicida with Diatoms: Regulated protease excretion for specific algal lysis. PLoS ONE 2011, 6, e21032. [Google Scholar] [CrossRef]
- Amin, S.A.; Parker, M.S.; Armbrust, E.V. Interactions between diatoms and bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 667–684. [Google Scholar] [CrossRef] [PubMed]
- Natrah, F.M.I.; Defoirdt, T.; Sorgeloos, P.; Bossier, P. Disruption of bacterial cell-to-cell communication by marine organisms and its relevance to aquaculture. Mar. Biotechnol. 2011, 13, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Natrah, F.M.I.; Bossier, P.; Sorgeloos, P.; Yusoff, F.M.; Defoirdt, T. Significance of microalgal–bacterial interactions for aquaculture. Rev. Aquac. 2014, 6, 48–61. [Google Scholar] [CrossRef]
- Williams, P. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology 2007, 153, 3923–3938. [Google Scholar] [CrossRef] [PubMed]
- Natrah, F.M.I.; Kenmegne, M.M.; Wiyoto, W.; Sorgeloos, P.; Bossier, P.; Defoirdt, T. Effects of microalgae commonly used in aquaculture on acyl-homoserine lactone quorum sensing. Aquaculture 2011, 317, 53–57. [Google Scholar] [CrossRef]
- Teplitski, M.; Chen, H.; Rajamani, S.; Gao, M.; Merighi, M.; Sayre, R.T.; Robinson, J.B.; Rolfe, B.G.; Bauer, W.D. Chlamydomonas reinhardtii secretes compounds that mimic bacterial signals and interfere with quorum sensing regulation in bacteria. Plant Physiol. 2004, 134, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Rajamani, S.; Bauer, W.D.; Robinson, J.B.; Farrow, J.M., III; Pesci, E.C.; Teplitski, M.; Gao, M.; Sayre, R.T.; Phillips, D.A. The vitamin riboflavin and its derivative lumichrome activate the LasR bacterial quorum-sensing receptor. Mol. Plant Microbe Interact. 2008, 21, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Lü, F.; Ji, J.; Shao, L.; He, P. Bacterial bioaugmentation for improving methane and hydrogen production from microalgae. Biotechnol. Biofuels 2013, 6. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, C.; Hidalgo, C.; Zapata, M.; Jeison, D.; Riquelme, C.; Rivas, M. Use of cellulolytic marine bacteria for enzymatic pretreatment in microalgal biogas production. Appl. Environ. Microbiol. 2014, 80, 4199–4206. [Google Scholar] [CrossRef] [PubMed]
- Melis, A.; Happe, T. Hydrogen production. Green algae as a source of energy. Plant Physiol. 2001, 127, 740–748. [Google Scholar] [CrossRef] [PubMed]
- White, H.K.; Reimers, C.E.; Cordes, E.E.; Dilly, G.F.; Girguis, P.R. Quantitative population dynamics of microbial communities in plankton-fed microbial fuel cells. ISME J. 2009, 3, 635–646. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Kan, J.; Mansfeld, F.; Angenent, L.T.; Nealson, K.H. Self-sustained phototrophic microbial fuel cells based on the synergistic cooperation between photosynthetic microorganisms and heterotrophic bacteria. Environ. Sci. Technol. 2009, 43, 1648–1654. [Google Scholar] [PubMed]
- Gomez, I.; Weykam, G.; Klöser, H.; Wiencke, C. Photosynthetic light requirements, metabolic carbon balance and zonation of sublittoral macroalgae from King George Island (Antarctica). Mar. Ecol. Prog. Ser. 1997, 148, 281–293. [Google Scholar] [CrossRef]
- Tender, L.M.; Gray, S.A.; Groveman, E.; Lowy, D.A.; Kauffman, P.; Melhado, J.; Tyce, R.C.; Flynn, D.; Petrecca, R.; Dobarro, J. The first demonstration of a microbial fuel cell as a viable power supply: Powering a meteorological buoy. J. Power Sources 2008, 179, 571–575. [Google Scholar] [CrossRef]
- Donovan, C.; Dewan, A.; Heo, D.; Beyenal, H. Batteryless, wireless sensor powered by a sediment microbial fuel cell. Environ. Sci. Technol. 2008, 42, 8591–8596. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, E.M.; de Godos, I.; Rodríguez, E.M.; García-Encina, P.A.; Muñoz, R.; Bécares, E. Molecular characterization of bacterial communities in algal–bacterial photobioreactors treating piggery wastewater. Ecol. Eng. 2012, 40, 121–130. [Google Scholar] [CrossRef]
- Hernández, D.; Riaño, B.; Coca, M.; García-González, M.C. Treatment of agro-industrial wastewater using microalgae–bacteria consortium combined with anaerobic digestion of the produced biomass. Bioresour. Technol. 2013, 135, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Van der Ha, D.; Nachtergaele, L.; Kerckhof, F.M.; Rameiyanti, D.; Bossier, P.; Verstraete, W.; Boon, N. Conversion of biogas to bioproducts by algae and methane oxidizing bacteria. Environ. Sci. Technol. 2012, 46, 13425–13431. [Google Scholar] [CrossRef] [PubMed]
- Bahr, M.; Stams, A.J.; De la Rosa, F.; García-Encina, P.A.; Muñoz, R. Assessing the influence of the carbon oxidation-reduction state on organic pollutant biodegradation in algal–bacterial photobioreactors. Appl. Microbiol. Biotechnol. 2011, 90, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Kang, Z.; Ramanan, R.; Choi, J.E.; Cho, D.H.; Oh, H.M.; Kim, H.S. Nutrient removal and biofuel production in high rate algal pond using real municipal wastewater. J. Microbiol. Biotechnol. 2014, 24, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Christenson, L.B.; Sims, R.C. Rotating algal biofilm reactor and spool harvester for wastewater treatment with biofuels by-products. Biotechnol. Bioeng. 2012, 109, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.P. Wastewater treatment with suspended and non-suspended algae. J. Phycol. 1998, 34, 757–763. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, H.M.; Jo, B.H.; Lee, S.A.; Shin, S.Y.; Kim, H.S.; Lee, S.H.; Ahn, C.Y. Higher biomass productivity of microalgae in an attached growth system, using wastewater. J. Microbiol. Biotechnol. 2014, 24, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- McLean, B.M.; Baskaran, K.; Connor, M.A. The use of algal-bacterial biofilms to enhance nitrification rates in lagoons: Experience under laboratory and pilot-scale conditions. Water Sci. Technol. 2000, 42, 187–194. [Google Scholar]
- Rusten, B.; Eikebrokk, B.; Ulgenes, Y.; Lygren, E. Design and operations of the Kaldnes moving bed biofilm reactors. Aquac. Eng. 2006, 34, 322–331. [Google Scholar] [CrossRef]
- Boivin, M.E.Y.; Greve, G.D.; Garcia-Meza, J.V.; Massieux, B.; Sprenger, W.; Kraak, M.H.S.; Breure, A.M.; Rutgers, M.; Admiraal, W. Algal-bacterial interactions in metal contaminated floodplain sediments. Environ. Pollut. 2007, 145, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; Guieysse, B. Algal-bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef] [PubMed]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Mixotrophic cyanobacteria and microalgae as distinctive biological agents for organic pollutant degradation. Environ. Int. 2013, 51, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Quigg, A. Micronutrients. In The Physiology of Microalgae; Borowitzka, M.A., Beardall, J., Raven, J.A., Eds.; Springer: Cham, Switzerland, 2016; pp. 211–231. [Google Scholar]
- Mahdavi, H.; Prasad, V.; Liu, Y.; Ulrich, A.C. In situ biodegradation of naphthenic acids in oil sands tailings pond water using indigenous algae–bacteria consortium. Bioresour. Technol. 2015, 187, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.G.; Kim, W.; Nam, K.; Kim, S.; Lee, B.; Park, M.S.; Yang, J.W. A comprehensive study on algal-bacterial communities shift during thiocyanate degradation in a microalga-mediated process. Bioresour. Technol. 2015, 191, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toi, H.T.; Boeckx, P.; Sorgeloos, P.; Bossier, P.; Van Stappen, G. Co-feeding of microalgae and bacteria may result in increased N assimilation in Artemia as compared to mono-diets, as demonstrated by a 15N isotope uptake laboratory study. Aquaculture 2014, 422, 109–114. [Google Scholar] [CrossRef]
- Van Den Hende, S.; Claessens, L.; De Muylder, E.; Boon, N.; Vervaeren, H. Microalgal bacterial flocs originating from aquaculture wastewater treatment as diet ingredient for Litopenaeus vannamei (Boone). Aquac. Res. 2014. [Google Scholar] [CrossRef]

| Microalga | Bacterium | Mediators from Microalgae | Mediators from Bacteria | Reference |
|---|---|---|---|---|
| Algal growth improvement/production cost decrease | ||||
| E. huxleyi | P. gallaeciensis | Dimethylsulphonio-propionate | Promoters and antibiotics | Seyedsayamdost et al. (2011) [53] |
| B. braunii | Rhizobium sp. | AHL | Rivas et al. (2010) [56] | |
| L. rostrate | M. loti | Vitamin B12 | Kazamia et al. (2012) [16] | |
| T. pseudonana CCMP1335 | R. pomeroyi DSS-3 | 2,3-dihydroxy-propane-1-sulfonate | Vitamin B12 | Durham et al. (2015) [41] |
| S. trochoidea | Marinobacter | Organic molecules | Vibrioferrin | Amin et al. (2009) [22] |
| S. trochoidea | Roseobacter | Organic molecules | Vibrioferrin | Amin et al. (2009) [22] |
| N. oleoabundans | A. vinelandii | Siderophore | Santos et al. (2014) [48] | |
| Scenedesmus sp. | A. vinelandii | Siderophore | Santos et al. (2014) [48] | |
| Accumulation of fatty acids and lipids | ||||
| C. vulgaris | A. brasilense | Siderophore mediated nitrogen fixation | Leyva et al. (2014) [50] | |
| Heterotrophic accumulation of starch and carbohydrates | ||||
| C. vulgaris | A. brasilense | Siderophore mediated nitrogen fixation | Choix et al. (2012) [51] | |
| C. sorokiniana | A. brasilense | Siderophore mediated nitrogen fixation | Choix et al (2012) [51] | |
| Photoautotrophic accumulation of starch and carbohydrates | ||||
| C. vulgaris | A. brasilense | Siderophore mediated nitrogen fixation | Choix et al (2012) [52] | |
| C. sorokiniana | A. brasilense | Siderophore mediated nitrogen fixation | Choix et al (2012) [52] | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes, J.L.; Garbayo, I.; Cuaresma, M.; Montero, Z.; González-del-Valle, M.; Vílchez, C. Impact of Microalgae-Bacteria Interactions on the Production of Algal Biomass and Associated Compounds. Mar. Drugs 2016, 14, 100. https://doi.org/10.3390/md14050100
Fuentes JL, Garbayo I, Cuaresma M, Montero Z, González-del-Valle M, Vílchez C. Impact of Microalgae-Bacteria Interactions on the Production of Algal Biomass and Associated Compounds. Marine Drugs. 2016; 14(5):100. https://doi.org/10.3390/md14050100
Chicago/Turabian StyleFuentes, Juan Luis, Inés Garbayo, María Cuaresma, Zaida Montero, Manuel González-del-Valle, and Carlos Vílchez. 2016. "Impact of Microalgae-Bacteria Interactions on the Production of Algal Biomass and Associated Compounds" Marine Drugs 14, no. 5: 100. https://doi.org/10.3390/md14050100
APA StyleFuentes, J. L., Garbayo, I., Cuaresma, M., Montero, Z., González-del-Valle, M., & Vílchez, C. (2016). Impact of Microalgae-Bacteria Interactions on the Production of Algal Biomass and Associated Compounds. Marine Drugs, 14(5), 100. https://doi.org/10.3390/md14050100