Uncovering Adiponectin Replenishing Property of Sujiaonori Algal Biomaterial in Humans
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics of Participants
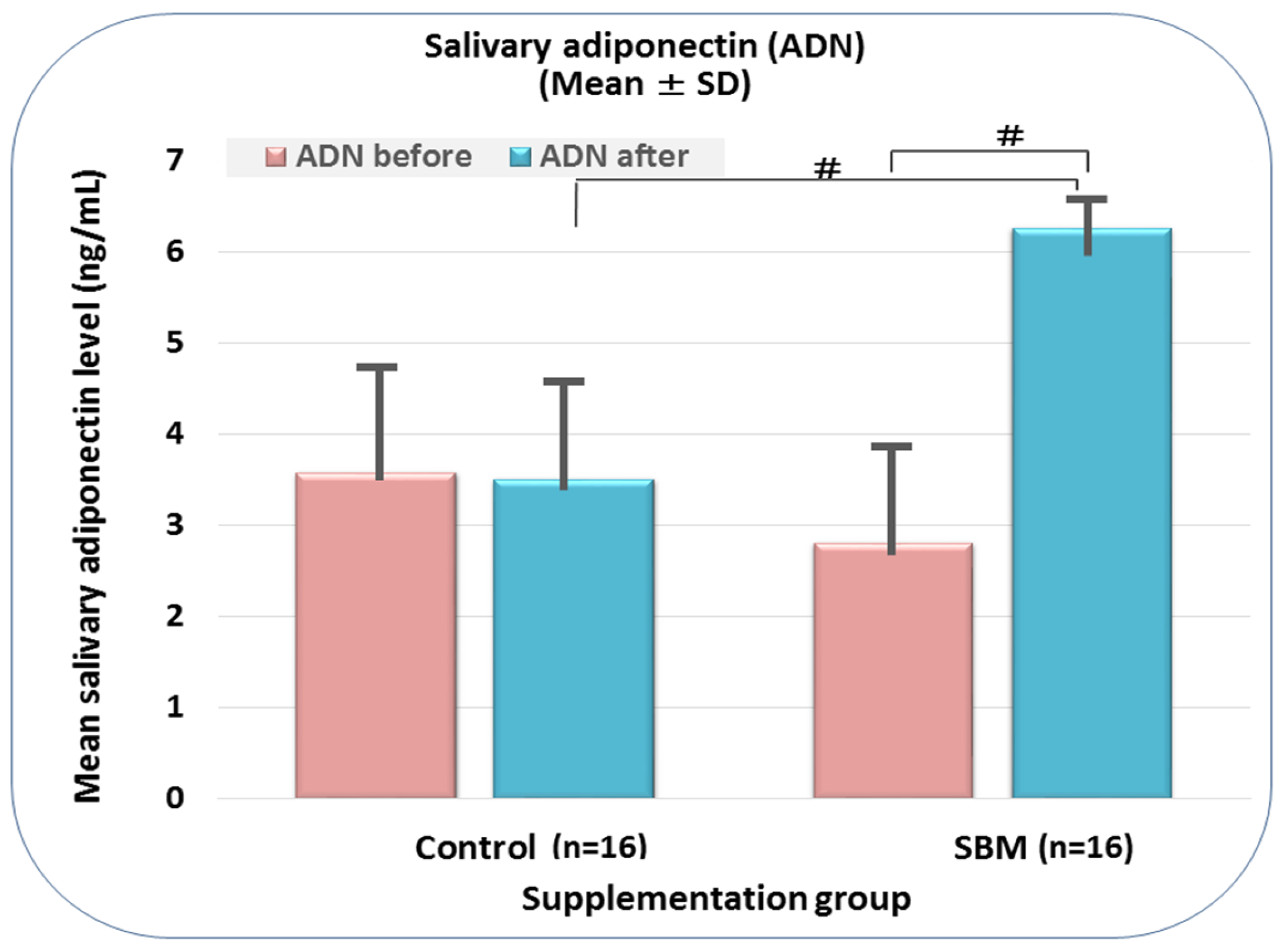
2.2. SBM Supplementation Induces Adiponectin Replenishment
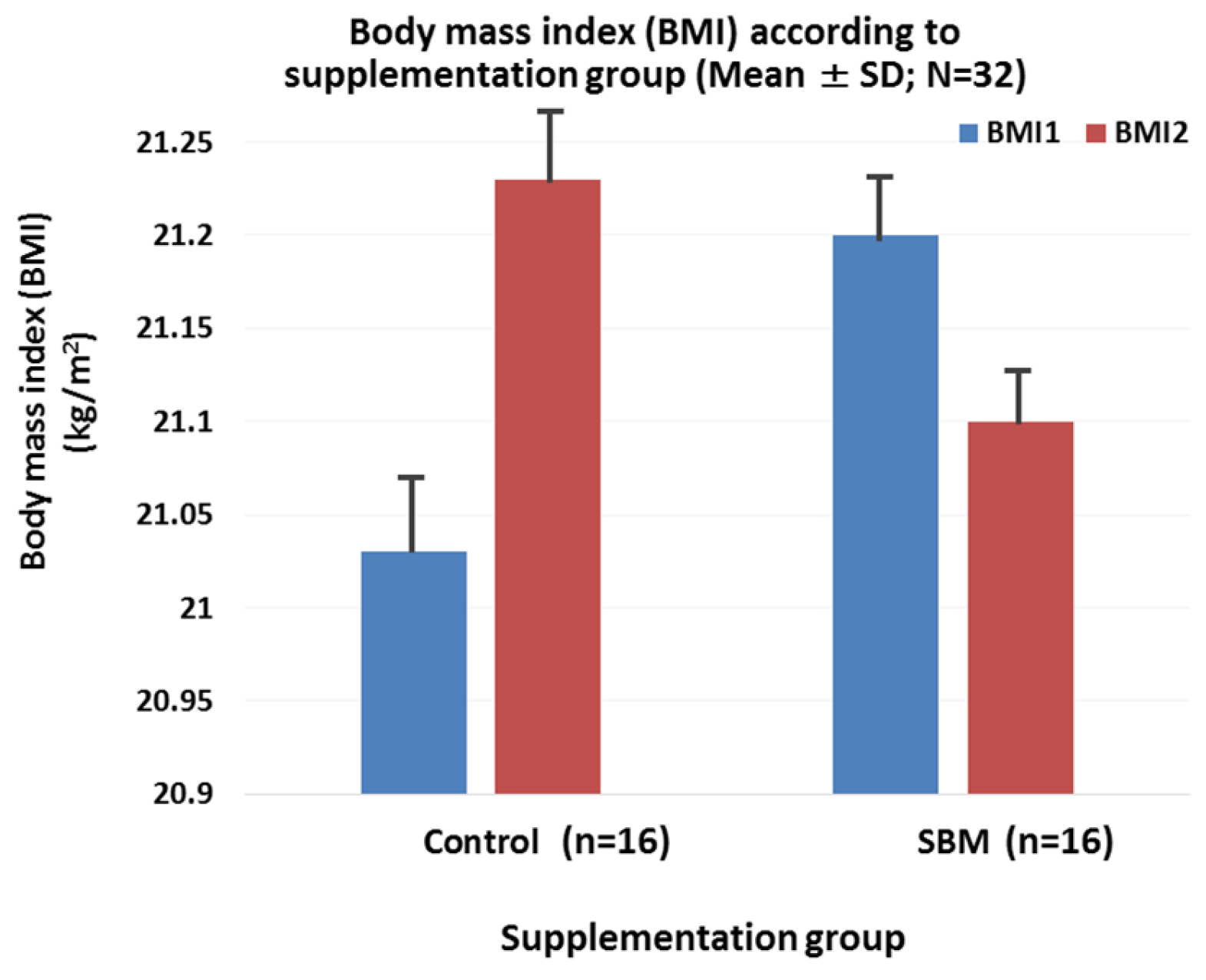
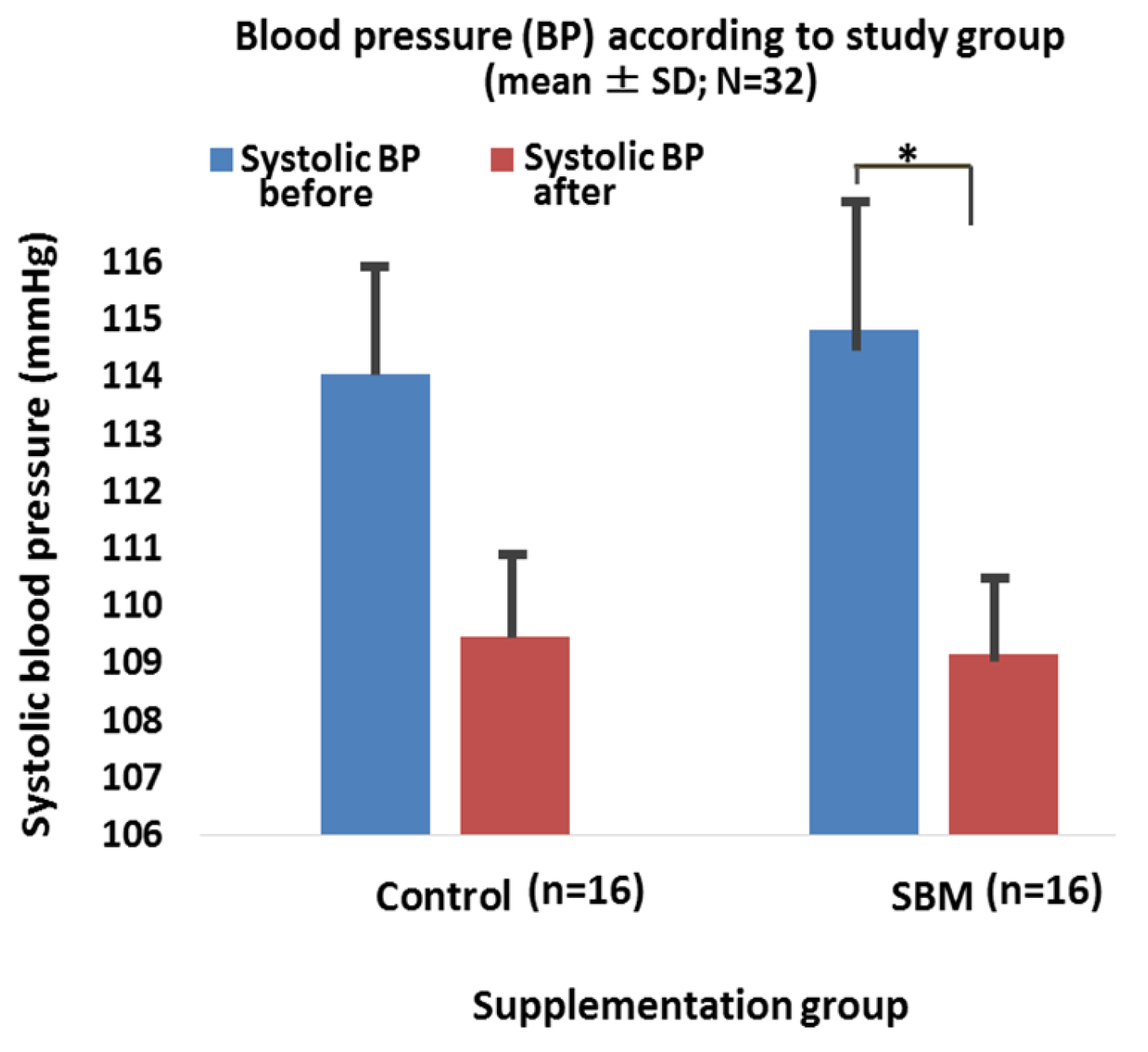
2.3. Effects of SBM Supplementation on BMI, Blood Pressure, and Correlation between Salivary Adiponectin and Outcome Parameters
3. Materials and Methods
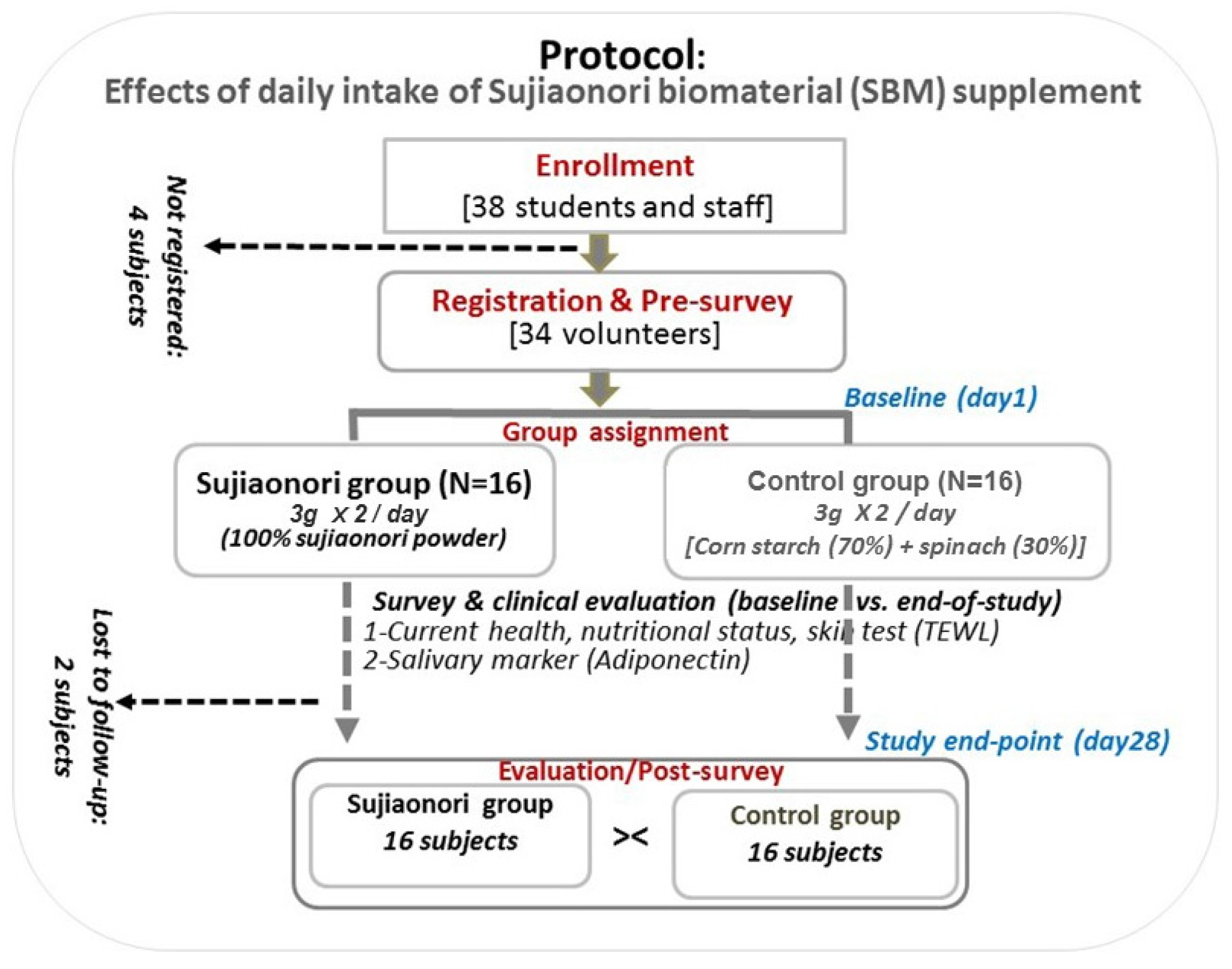
3.1. Study Design, Participants, and Survey Questionnaires
3.2. ELISA Assay and Outcome Variables
3.3. Ethics Approval and Consent to Participate
3.4. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BDHQ | brief self-administered diet history questionnaire |
| BMI | body mass index |
| BP | blood pressure |
| CHD | coronary heart disease |
| DBP | diastolic blood pressure |
| ELISA | enzyme-linked immunosorbent assay |
| SBM | Sujiaonori biomaterial |
| SBP | systolic blood pressure |
References
- Lancaster, G.I.; Febbraio, M.A. Adiponectin sphings into action. Nat. Med. 2011, 17, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Shimomura, I.; Kishida, K.; Nishizawa, H.; Matsuda, M.; Nagaretani, H.; Furuyama, N.; Kondo, H.; Takahashi, M.; Arita, Y.; et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002, 8, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Kihara, S.; Ouchi, N.; Nishida, M.; Arita, Y.; Kumada, M.; Ohashi, K.; Sakai, N.; Shimomura, I.; Kobayashi, H.; et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 2002, 106, 2767–2770. [Google Scholar] [CrossRef] [PubMed]
- Satar, N.; Wannamethee, G.; Sarwar, N.; Tchernova, J.; Cherry, L.; Wallace, A.M. Adiponectin and coronary heart disease, a prospective study and meta-analysis. Circulation 2006, 114, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Quon, M.J.; Kim, J.; Koh, K.K. Adiponectin and cardiovascular disease: Response to therapeutic interventions. J. Am. Coll. Cardiol. 2007, 49, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Lam, K.S.L.; Vanhutte, P.M.; Xu, A. Adiponectin and cardiovascular health: An update. Br. J. Pharmacol. 2012, 165, 574–590. [Google Scholar] [CrossRef] [PubMed]
- Katira, A.; Tan, P.H. Adiponectin and its receptors signaling: An anti-cancer therapeutic target and its implications for anti-tumor immunity. Expert. Opin. Ther. Targets 2015, 19, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, I.; Kelesidis, T.; Mantzoros, C.S. Adiponectin and cancer: A systematic review. Br. J. Cancer 2006, 94, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Wang, H.; Hoo, R.L.; Sweeney, G.; Vanhoutte, P.M.; Wang, Y.; Wu, D.; Chu, W.; Qin, G.; Lam, K.S. Selective elevation of adiponectin production by the natural compounds derived from a medicinal herb alleviates insulin resistance and glucose intolerancein obese mice. Endocrinology 2009, 150, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Otvos, L., Jr.; Haspinger, E.; La Russa, F.; Maspero, F.; Graziano, P.; Kovalszky, I.; Lovas, S.; Nama, K.; Hoffmann, R.; Knappe, D.; et al. Design and development of peptide-based adiponectin receptor agonist for cancer treatment. BMC. Biotechnol. 2011, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Otvos, L., Jr.; Knappe, D.; Hoffmann, I.; Kovalszky, I.; Olah, J.; Hewitson, T.D.; Stawikowska, R.; Stawiskowski, M.; Cudic, P.; Lin, F.; et al. Development of second generation peptides modulating cellular adiponectin receptor responses. Front. Chem. 2014, 2, 93. [Google Scholar] [CrossRef] [PubMed]
- Holland, W.L.; Brozinick, J.T.; Wang, L.P.; Hawkins, E.D.; Sargent, K.M.; Liu, Y.; Narra, K.; Hoehn, K.L.; Knotts, T.A.; Siesky, A.; et al. Inhibition of ceramide synthesis ameliorated glucocorticoid, saturated fat, and obesity-induced insulin resistance. Cell Metab. 2007, 5, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Okada-Iwabu, M.; Yamauchi, T.; Iwabu, M.; Honma, T.; Hamagami, K.; Matsuda, K.; Yamaguchi, M.; Tanabe, H.; Kimura-Someya, T.; Shirouzu, M.; et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 2013, 503, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, J.; Li, R.; Lau, W.B.; Yuan, Y.X.; Liang, B.; Li, R.; Gao, E.H.; Koch, W.J.; Ma, X.L.; et al. AdipoRon, the first active adiponectin receptor activator, attenuates postischemic myocardial apoptosis through both AMPK-mediated and AMPK-independent signalings. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E275–E282. [Google Scholar] [CrossRef] [PubMed]
- Ngatu, N.R.; Ikeda, M.; Kanbara, S.; Inoue, M.; Suzuki, M.; Watanabe, H.; Umebara, M.; Nojima, S. Potential health effects of green river algae (Aonori) of the LPP complex, with a reference to Ulva prolifera. Int. J. Gen. Med. 2015, 4, 1–8. [Google Scholar]
- Wang, H.; Lin, A.; Gu, W.; Huan, L.; Gao, S.; Wang, G. The sporulation of the green alga Ulva prolifera is controlled by changes in photosynthetic electron transport chain. Sci. Rep. 2016, 6, 24923. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Therapeutic importance of sulfated polysaccharides from seaweeds: Updating the recent findings. 3 Biotech 2012, 2, 171–185. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, H.; Wang, S.; Wen, S.; Oin, S. Hypolipidemic and antioxidant properties of a polysaccharide fraction from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013, 58, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, S.; Liu, G.; Pei, D.; Liu, Y.; Liu, Y.; Di, D. Polysaccharides from Enteromorpha prolifera enhance the immunity of normal mice. Int. J. Biol. Macromol. 2014, 64, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yang, C.; Liu, B. Biological activities of green macroalgae Enteromorpha prolifera for potential applications. MOJ Food Process. Technol. 2016, 2, 00048. [Google Scholar] [CrossRef]
- Toda, M.; Tsukinoki, R.; Morimoto, K. Measurement of salivary adiponectin levels. Acta Diabetol. 2007, 44, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Huimin, Q.; Sheng, J. The antihyperlipidemic mechanism of high sulfate content ulvan in rats. Mar. Drugs 2015, 13, 3407–3421. [Google Scholar]
- Qi, H.; Sun, Y. Antioxidant activity of high sulfate content derivative of ulvan in hyperlipidemic rats. Int. J. Biol. Macromol. 2015, 76, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Pieczonka, T.D.; Bragiel, A.M. Membrane transporters in salivary exosomes and microvesicles as biomarkers of systemic or oral disease. J. Oral Biosci. 2014, 56, 110–114. [Google Scholar] [CrossRef]
- Lee, C.H.; Hung, Y.J. Possible new therapeutic approach for obesity-related diseases: role of adiponectin receptor agonists. J. Diabetes Investig. 2015, 6, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Fisman, E.Z.; Tenenbuam, A. Adiponectin: A manifold therapeutic target for meta-bolic syndrome, diabetes, and coronary disease? Cardiovasc. Diabetol. 2014, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Quon, M.J.; Koh, K.K. Modulation of adiponectin as a potential therapeutic strategy. Atherosclerosis 2014, 233, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Lefils-Lacourtablaise, J.; Socorro, M.; Géloën, A.; Daira, P.; Debard, C.; Loizon, E.; Guichardant, M.; Dominguez, Z.; Vidal, H.; Lagarde, M.; et al. The eicosa-pentaenoic acid metabolite 15-Deoxy-δ12,14-Prostaglandin J3 increases adiponectin secretion by adipocytes partly via a PPARγ-dependent mechanism. PLoS ONE 2013, 8, e63997. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.J.; Senanayake, V.K.; Pu, S.; Jenkins, D.J.; Cornnelly, P.W.; Lamarche, B.; Couture, P.; Charest, A.; Baril-Gravel, L.; West, S.G.; et al. DHA-enriched high-oleic acid oil improves lipid profile and lowers predicted cardiovascular disease risk in the canola oil multicenter randomized controlled trial. Am. J. Clin. Nutr. 2014, 100, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.; Fargnoli, J.L.; Hwang, J.J.; van Dam, R.M.; Blackburn, G.L.; Hu, F.B.; Mantzoros, C.S. Coffee consumption is associated with higher plasma adiponectin concentrations in women with or without type 2 diabetes: A prospective cohort study. Diabetes Care 2008, 31, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Kotani, K.; Fujiwara, S.; Hamada, T.; Tsuzaki, K.; Sakane, N. Coffee consumption is associated with higher plasma adiponectin concentrations in women with or without type-2 diabetes. Diabetes Care 2008, 31, e46. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.S.; Guo, F.R.; Chen, S.C.; Lue, B.H.; Chiu, T.Y.; Chen, C.Y.; Hung, S.H.; Chuang, L.M.; Chen, C.Y. Smokers show reduced circulating adiponectin levels and adiponectin mRNA expression in peripheral blood mononuclear cells. Atherosclerosis 2011, 218, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Kotani, K.; Hazama, A.; Hagimoto, A.; Saika, K.; Shigeta, M.; Katanoda, K.; Nakamura, M. Adiponectin and smoking status: A systematic review. J. Atheroscler. Thromb. 2012, 19, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, N.; Ishizaka, Y.; Toda, E.I.; Hashimoto, H.; Nagai, R.; Yamakado, M. Hypertension is the most common component of metabolic syndrome and the greatest contributor to carotid arteriosclerosis in apparently healthy Japanese individuals. Hypertens. Res. 2005, 28, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, T.; Fujita, T.; Kawamori, R.; Miyazaki, S.; Teramukai, S.; Igarashi, M. OMEGA study: Design, baseline data, metabolic syndrome prevalence in a large-scale observational study of hypertensive patients: The Olmesartan Mega Study to determine the relationship between cardiovacular endpoints and blood pressure goal achievement study. Hypertens. Res. 2008, 31, 2011–2017. [Google Scholar] [PubMed]
- Ohashi, K.; Kihara, S.; Ouchi, N.; Kumada, M.; Fujita, K.; Hiuge, A.; Hibuse, T.; Ryo, M.; Nishizawa, H.; Maeda, N.; et al. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension 2006, 47, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Sakata, S.; Tsuchihashi, T.; Oniki, H.; Tominaga, M.; Arakawa, K.; Sakaki, M.; Kitazono, T. Relationship between salt intake as estimated by a brief self-administered diet-history questionnaire (BDHQ) and 24-h urinary salt excretion in hypertensive patients. Hypertens. Res. 2015, 38, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Smith, L. New AHA recommendations for blood pressure measurement. Am. Fam. Physician 2005, 72, 1391–1398. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngatu, N.R.; Ikeda, M.; Watanabe, H.; Tanaka, M.; Inoue, M.; Kanbara, S.; Nojima, S. Uncovering Adiponectin Replenishing Property of Sujiaonori Algal Biomaterial in Humans. Mar. Drugs 2017, 15, 32. https://doi.org/10.3390/md15020032
Ngatu NR, Ikeda M, Watanabe H, Tanaka M, Inoue M, Kanbara S, Nojima S. Uncovering Adiponectin Replenishing Property of Sujiaonori Algal Biomaterial in Humans. Marine Drugs. 2017; 15(2):32. https://doi.org/10.3390/md15020032
Chicago/Turabian StyleNgatu, Nlandu Roger, Mitsunori Ikeda, Hiroyuki Watanabe, Mamoru Tanaka, Masataka Inoue, Sakiko Kanbara, and Sayumi Nojima. 2017. "Uncovering Adiponectin Replenishing Property of Sujiaonori Algal Biomaterial in Humans" Marine Drugs 15, no. 2: 32. https://doi.org/10.3390/md15020032




