Propylene Glycol Alginate Sodium Sulfate Alleviates Cerulein‐Induced Acute Pancreatitis by Modulating the MEK/ERK Pathway in Mice
Abstract
:1. Introduction
2. Results
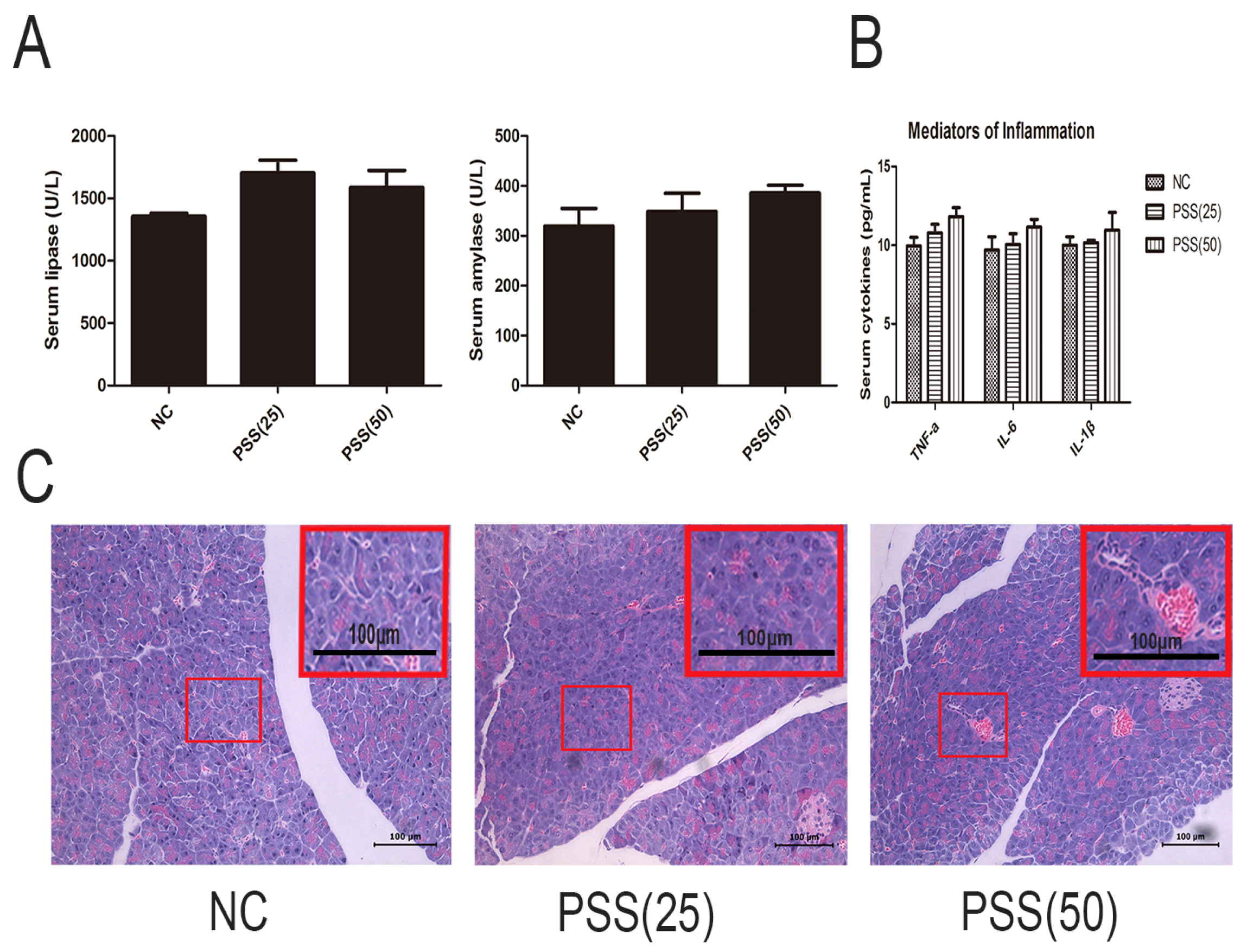
2.1. PSS Does Not Affect Pancreatic Function
2.2. PSS Pretreatment Attenuates Cerulein-Induced Acute Pancreatitis
2.3. PSS Pretreatment Inhibits the Production of TNF-α, IL-6, and IL-1β in Cerulein-Induced Acute Pancreatitis
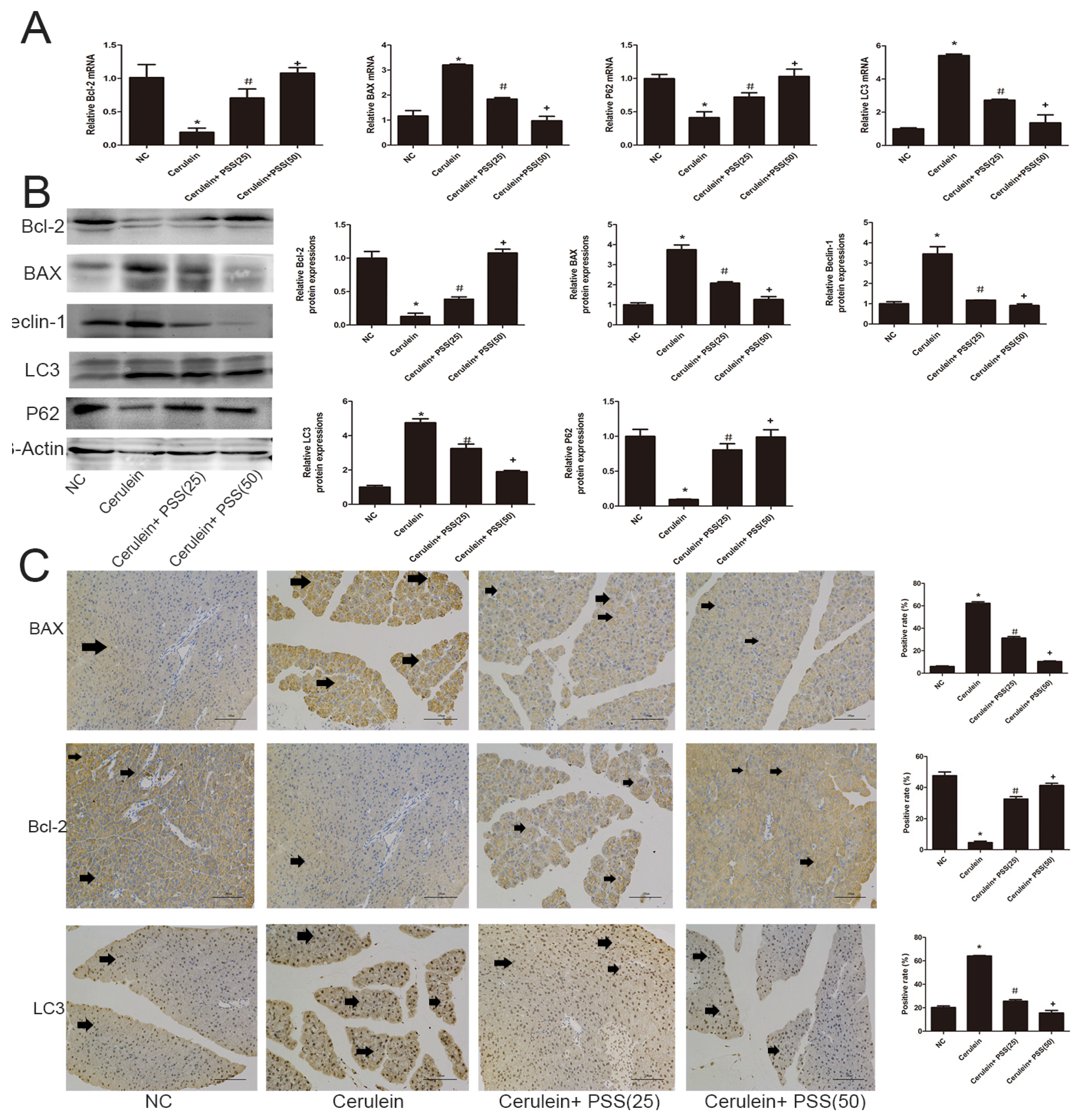
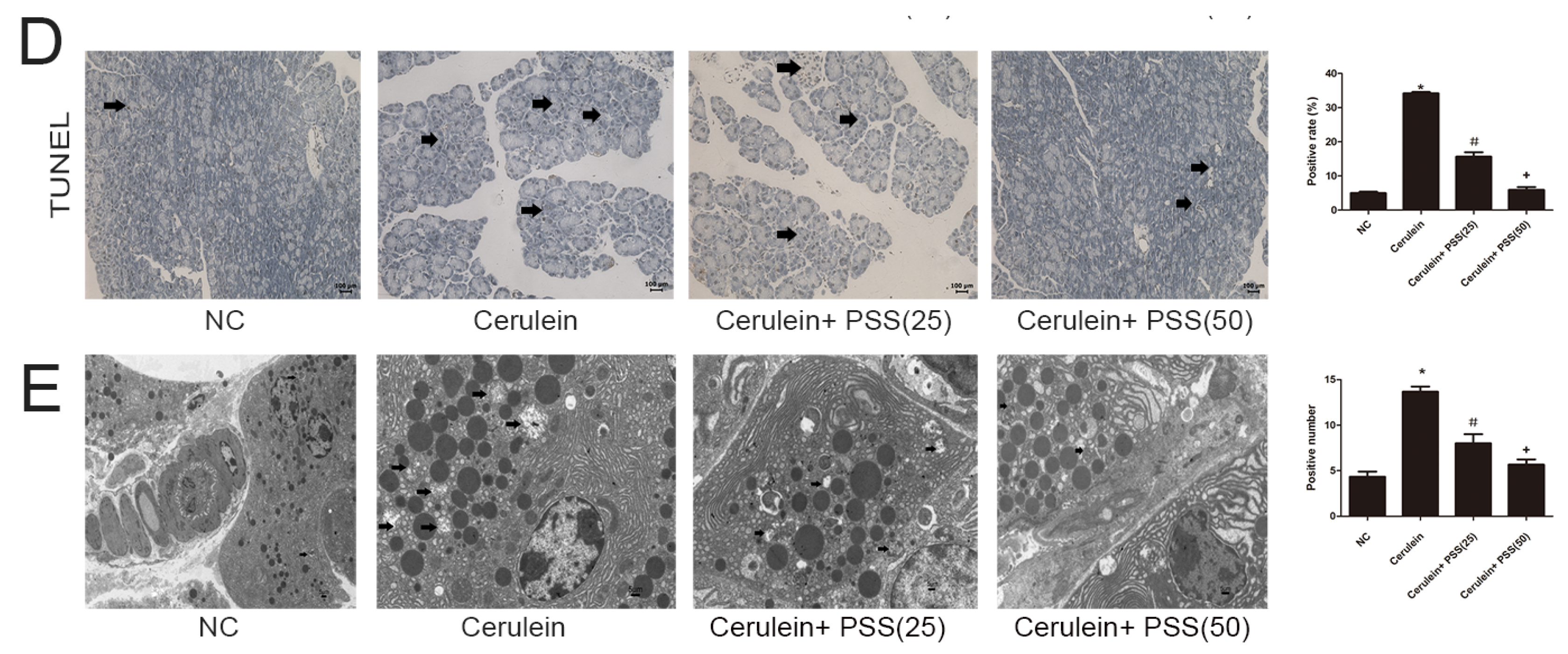
2.4. PSS Pretreatment Decreased Apoptosis and Autophagy in Cerulein-Induced Acute Pancreatitis
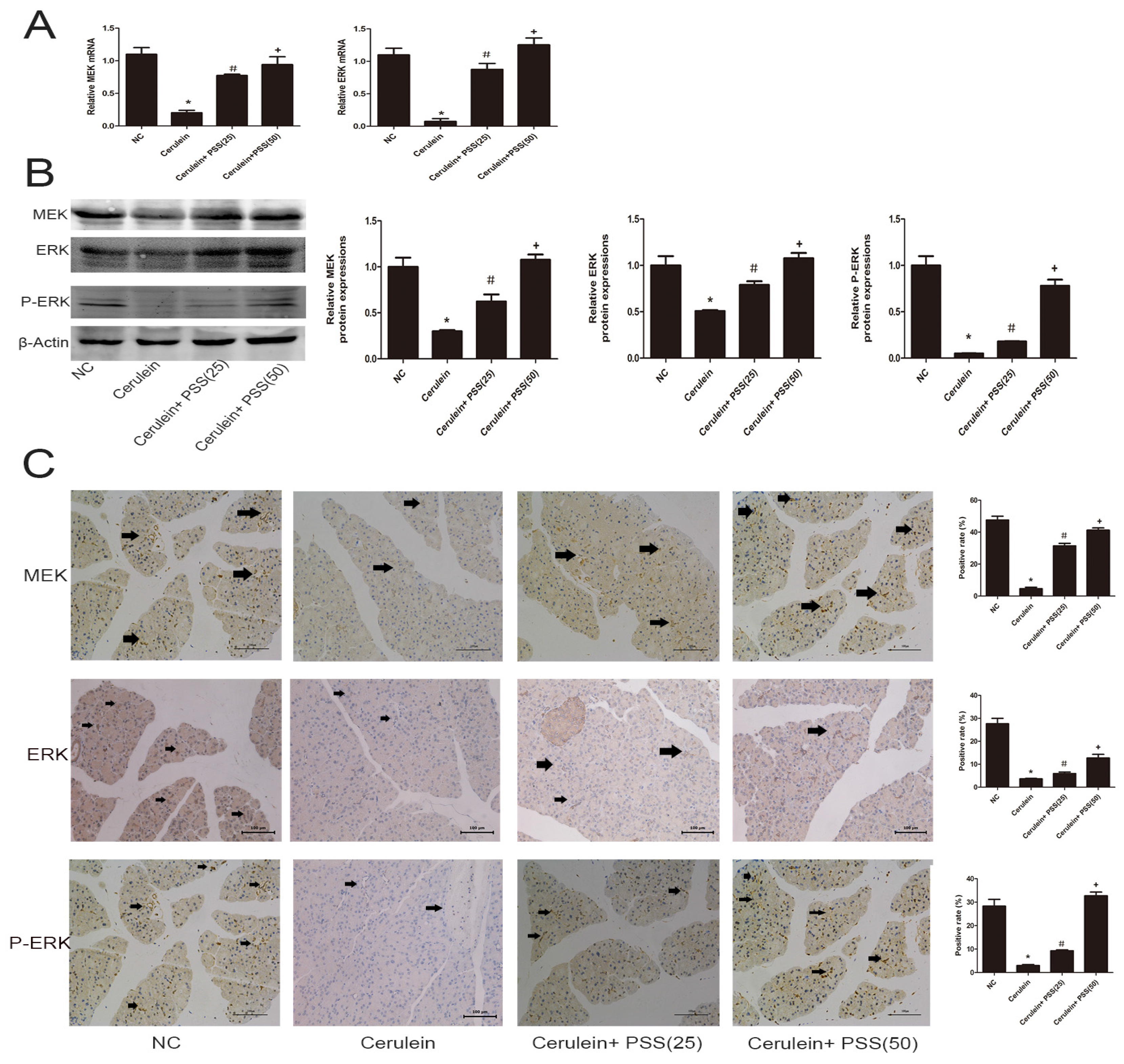
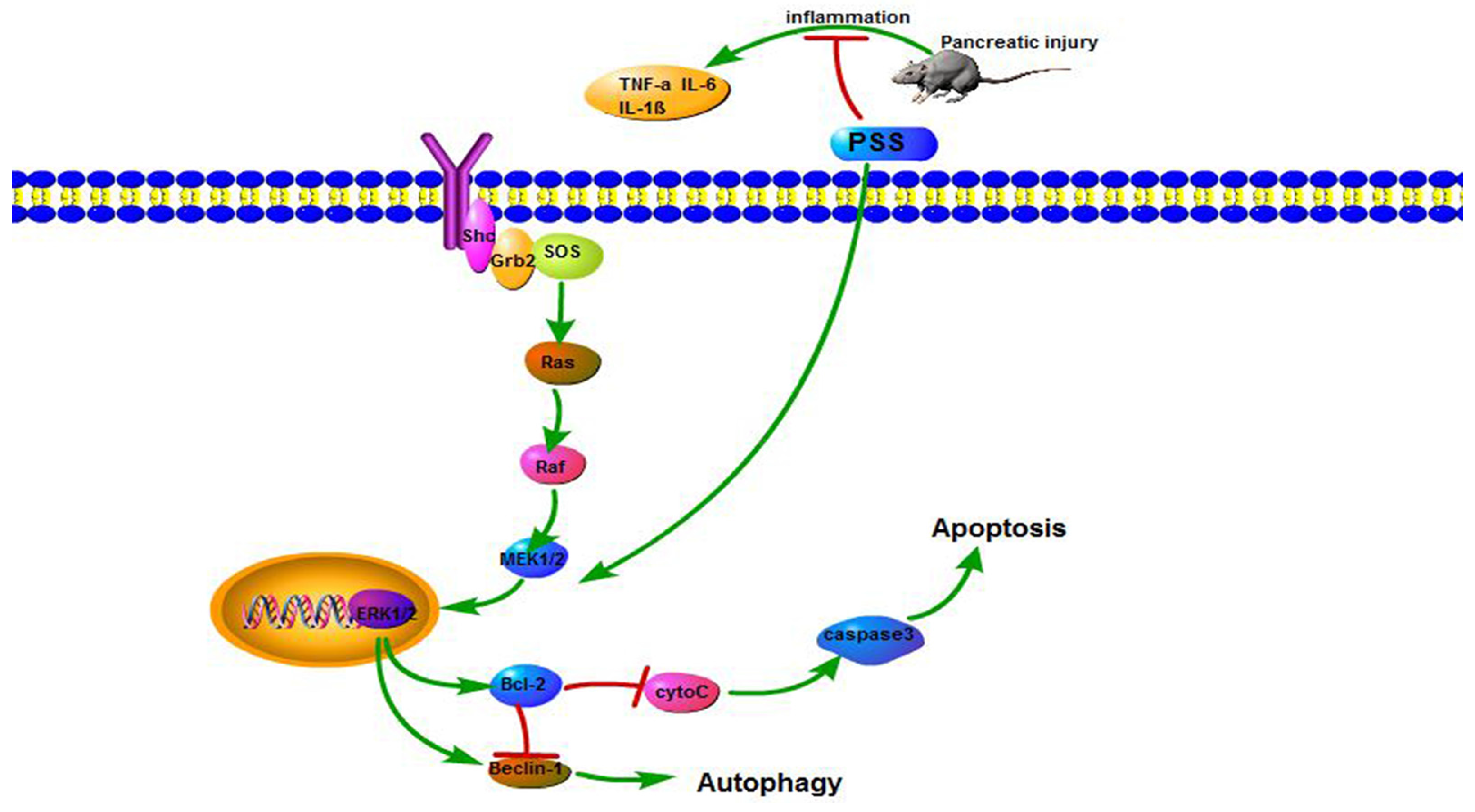
2.5. PSS Pretreatment Regulates the MEK/ERK Signaling Pathway in Cerulein-Induced Acute Pancreatitis
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Experimental Design
- (1)
- normal control group (treated with saline solution)
- (2)
- treated with 25 mg/kg PSS
- (3)
- treated with 50 mg/kg PSS.
4.4. Drug Treatment
- (1)
- Normal control (n = 12)
- (2)
- Cerulein group (n =12): mice were injected with ten injections of cerulein (100 mg/kg, i.p. at intervals of 1 h) and 5 mg/kg lipopolysaccharide was injected intraperitoneally after the last injection.
- (3)
- Low dose group (n = 12): mice were intraperitoneally injected with 25 mg/kg PSS 1 h before cerulein.
- (4)
- High dose group (n =12): mice were intraperitoneally injected with 50 mg/kg PSS 1 h before cerulein.
4.5. Histological Analysis
4.6. Biochemical Analysis
4.7. Myeloperoxidase Activity
4.8. Real Time-PCR
4.9. Immunohistochemistry
4.10. Western Blot
4.11. TUNEL Staining
4.12. Transmission Electron Microscopy
4.13. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Malleo, G.; Mazzon, E.; Siriwardena, A.K.; Cuzzocrea, S. Role of tumor necrosis factor-alpha in acute pancreatitis: From biological basis to clinical evidence. Shock 2007, 28, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Halangk, W.; Lerch, M.M. Early events in acute pancreatitis. Gastroenterol. Clin. N. Am 2004, 33, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yang, D.; Qiu, P.; Han, Z.; Zeng, P.; He, Y.; Guo, Z.; Xu, L.; Cui, Y.; Zhou, Z.; et al. Efficacy of Heparinoid PSS in Treating Cardiovascular Diseases and Beyond-A Review of 27 Years Clinical Experiences in China. Clin. Appl. Thromb. Hemost. 2016, 22, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Sendler, M.; Maertin, S.; John, D.; Persike, M.; Weiss, F.U.; Kruger, B.; Wartmann, T.; Wagh, P.; Halangk, W.; Schaschke, N.; et al. Cathepsin B Activity Initiates Apoptosis via Digestive Protease Activation in Pancreatic Acinar Cells and Experimental Pancreatitis. J. Biol. Chem. 2016, 291, 14717–14731. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Q.; Li, Y.; Zhou, Z.G.; Wang, C.; Zhan, L.; Zhou, B. Toll-like receptor 4-mediated apoptosis of pancreatic cells in cerulein-induced acute pancreatitis in mice. Hepatobiliary Pancreat Dis. Int. 2010, 9, 645–650. [Google Scholar] [PubMed]
- Hall, J.C.; Crawford, H.C. The conspiracy of autophagy, stress and inflammation in acute pancreatitis. Curr. Opin. Gastroenterol. 2014, 30, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Gukovskaya, A.S.; Gukovsky, I. Autophagy and pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G993–G1003. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Du, W.; Song, L.J.; Zhang, R.; Sun, L.G.; Chen, F.G.; Wei, X.T. Norcantharidin induces growth inhibition and apoptosis of glioma cells by blocking the Raf/MEK/ERK pathway. World J. Surg. Oncol. 2014, 12, 207. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Hu, W.; Song, Z.P.; Chen, Y.G.; Zhang, D.D.; Wang, C.Q. Rapamycin Inhibits Cardiac Hypertrophy by Promoting Autophagy via the MEK/ERK/Beclin-1 Pathway. Front. Physiol. 2016, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Ma, X.; Xiao, Z.; Yao, H.; Liu, Z. Coxsackievirus B3 induces autophagy in HeLa cells via the AMPK/MEK/ERK and Ras/Raf/MEK/ERK signaling pathways. Infect. Genet. Evol. 2015, 36, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Dorn, G.W., 2nd; Diwan, A. The rationale for cardiomyocyte resuscitation in myocardial salvage. J. Mol. Med. 2008, 86, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Gregoric, P.; Sijacki, A.; Stankovic, S.; Radenkovic, D.; Ivancevic, N.; Karamarkovic, A.; Popovic, N.; Karadzic, B.; Stijak, L.; Stefanovic, B.; et al. SIRS score on admission and initial concentration of IL-6 as severe acute pancreatitis outcome predictors. Hepato-Gastroenterology 2010, 57, 349–353. [Google Scholar] [CrossRef] [PubMed]
- De Waele, J.J.; Blot, S. The value of IL-6 in predicting the severity of acute pancreatitis. J. Clin. Gastroenterol. 2007, 41, 534. [Google Scholar] [CrossRef] [PubMed]
- Pooran, N.; Indaram, A.; Singh, P.; Bank, S. Cytokines (IL-6, IL-8, TNF): Early and reliable predictors of severe acute pancreatitis. J. Clin. Gastroenterol. 2003, 37, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Burlacu, A. Regulation of apoptosis by Bcl-2 family proteins. J. Cell. Mol. Med. 2003, 7, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells Devot. Mol. Cell. Mech. 1998, 3, 697–707. [Google Scholar] [CrossRef]
- Hawkins, C.J.; Vaux, D.L. The role of the Bcl-2 family of apoptosis regulatory proteins in the immune system. Semin. Immunol. 1997, 9, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Shakor, A.B.A.; Atia, M.; Alshehri, A.S.; Sobota, A.; Kwiatkowska, K. Ceramide generation during curcumin-induced apoptosis is controlled by crosstalk among Bcl-2, Bcl-xL, caspases and glutathione. Cell. Signal. 2015, 27, 2220–2230. [Google Scholar] [CrossRef] [PubMed]
- Vitagliano, O.; Addeo, R.; D’Angelo, V.; Indolfi, C.; Indolfi, P.; Casale, F. The Bcl-2/Bax and Ras/Raf/MEK/ERK signaling pathways: Implications in pediatric leukemia pathogenesis and new prospects for therapeutic approaches. Expert Rev. Hematol. 2013, 6, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Steelman, L.S.; Franklin, R.A.; Abrams, S.L.; Chappell, W.H.; Wong, E.W.; Lehmann, B.D.; Terrian, D.M.; Basecke, J.; Stivala, F.; et al. Targeting the RAF/MEK/ERK, PI3K/AKT and p53 pathways in hematopoietic drug resistance. Adv. Enzyme Regul. 2007, 47, 64–103. [Google Scholar] [CrossRef] [PubMed]
- Sebolt-Leopold, J.S. Development of anticancer drugs targeting the MAP kinase pathway. Oncogene 2000, 19, 6594–6599. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Tafuri, A.; et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta 2007, 1773, 1263–1284. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Panda, P.K.; Sinha, N.; Das, D.N.; Bhutia, S.K. Autophagy and apoptosis: Where do they meet? Apoptosis 2014, 19, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Gordy, C.; He, Y.W. The crosstalk between autophagy and apoptosis: Where does this lead? Protein Cell 2012, 3, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; Le Toumelin, G.; Criollo, A.; Rain, J.C.; Gautier, F.; Juin, P.; Tasdemir, E.; Pierron, G.; Troulinaki, K.; Tavernarakis, N.; et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007, 26, 2527–2539. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Whiteman, M.W.; Lian, H.; Wang, G.; Singh, A.; Huang, D.; Denmark, T. A non-canonical MEK/ERK signaling pathway regulates autophagy via regulating Beclin 1. J. Biol. Chem. 2009, 284, 21412–21424. [Google Scholar] [CrossRef] [PubMed]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Kleeman, L.K.; Jiang, H.H.; Gordon, G.; Goldman, J.E.; Berry, G.; Herman, B.; Levine, B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J. Virol. 1998, 72, 8586–8596. [Google Scholar] [PubMed]


| Pathologic Changes | Acinar Edema | Vacuolization | Inflammation | Acinar Cell Necrosis |
|---|---|---|---|---|
| Normal control | 0.3 ± 0.1 | 0 ± 0 | 0.1 ± 0 | 0.1 ± 0.1 |
| Cerulein | 3.2 ± 0.5 * | 0.4 ± 0.2 * | 0.7 ± 0.2 * | 0.8 ± 0.3 * |
| Cerulein+ astaxanthin (20) | 1.6 ± 0.3 *,** | 0.2 ± 0.1 *,** | 0.3 ± 0.1 *,** | 0.4 ± 0.2 *,** |
| Cerulein+ astaxanthin (40) | 1.0 ± 0.3 *,** | 0.1 ± 0.1 *,** | 0.2 ± 0.1 *,** | 0.2 ± 0.1 *,** |
| Pathological Factors | Histological Score | Pathologic Change |
|---|---|---|
| 0 | none | |
| cell necrosis | 1 | <10% necrosis |
| 2 | <40% necrosis | |
| 3 | >40%necrosis | |
| 0 | none | |
| vacuolization | 1 | <20% acini with vacuoles |
| 2 | <50% acini | |
| 3 | >50% acini | |
| 0 | none | |
| inflammation | 1 | inflammatory cells present at interlobular areas |
| 2 | present at intralobular areas | |
| 3 | present at interacini | |
| 0 | none | |
| acinar edema | 1 | interlobular edema |
| 2 | intralobular edema | |
| 3 | interacinar edema |
| Gene | Primer Sequence (5′-3′) |
|---|---|
| IL-1β | Forward: CGATCGCGCAGGGGCTGGGCGG |
| Reverse: AGGAACTGACGGTACTGATGGA | |
| IL-6 | Forward: CTGCAAGAGACTTCCATCCAG |
| Reverse: AGTGGTATAGACAGGTCTGTTGG | |
| TNF-α | Forward: CAGGCGGTGCCTATGTCTC |
| Reverse: CGATCACCCCGAAGTTCAGTAG | |
| Bcl-2 | Forward: GCTACCGTCGTCGTGACTTCGC |
| Reverse: CCCCACCGAACTCAAAGAAGG | |
| Bax | Forward: AGACAGGGGCCTTTTTGCTAC |
| Reverse: AATTCGCCGGAGACACTCG | |
| LC3 | Forward: GACCGCTGTAAGGAGGTGC |
| Reverse: AGAAGCCGAAGGTTTCTTGGG | |
| P62 | Forward: GAGGCACCCCGAAACATGG |
| Reverse: ACTTATAGCGAGTTCCCACCA | |
| MEK | Forward: TCCTCACCAGGTTTAGAATTGC |
| Reverse: GCGAGTTTCTCACGTCGGA | |
| ERK | Forward: ACTGCTGGGCATAACGCTTTT |
| Reverse: GAGGAGGATCTTGAGAGCCTT | |
| β-actin | Forward: GGCTGTATTCCCCTCCATCG |
| Reverse: CCAGTTGGTAACAATGCCATGT |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, Y.; Li, L.; Liu, H.; Hu, L.; Dai, Y.; Chen, J.; Xu, S.; Chen, W.; Xu, X.; et al. Propylene Glycol Alginate Sodium Sulfate Alleviates Cerulein‐Induced Acute Pancreatitis by Modulating the MEK/ERK Pathway in Mice. Mar. Drugs 2017, 15, 45. https://doi.org/10.3390/md15020045
Zhang H, Li Y, Li L, Liu H, Hu L, Dai Y, Chen J, Xu S, Chen W, Xu X, et al. Propylene Glycol Alginate Sodium Sulfate Alleviates Cerulein‐Induced Acute Pancreatitis by Modulating the MEK/ERK Pathway in Mice. Marine Drugs. 2017; 15(2):45. https://doi.org/10.3390/md15020045
Chicago/Turabian StyleZhang, Hui, Yueyue Li, Linqiang Li, Hua Liu, Liangkai Hu, Ying Dai, Jianqing Chen, Shuqi Xu, Weimin Chen, Xiaorong Xu, and et al. 2017. "Propylene Glycol Alginate Sodium Sulfate Alleviates Cerulein‐Induced Acute Pancreatitis by Modulating the MEK/ERK Pathway in Mice" Marine Drugs 15, no. 2: 45. https://doi.org/10.3390/md15020045




