Secondary Metabolites from Polar Organisms
Abstract
:1. Introduction
2. Microorganisms
2.1. Unicellular Bacteria
2.2. Actinomycetes
2.3. Fungi
3. Lichen
4. Mosses
5. Bryozoans
6. Cnidarians
7. Echinoderms
8. Molluscs
9. Sponges
10. Tunicates
11. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Santiago, I.F.; Soares, M.A.; Rosa, C.A.; Rosa, L.H. Lichensphere: A protected natural microhabitat of the non-lichenised fungal communities living in extreme environments of Antarctica. Extremophiles 2015, 19, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, X.P.; Zhu, T.J.; Yang, L.L.; Li, W.J. Streptomyces fildesensis sp. nov., a novel streptomycete isolated from Antarctic soil. Antonie Van Leeuwenhoek 2011, 100, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Jeong, H.J.; Lee, Y.K.; Moon, E.Y.; Cho, J.C.; Lee, H.K.; Hong, S.G. Actimicrobium antarcticum gen. nov., sp. nov., of the family Oxalobacteraceae, isolated from Antarctic coastal seawater. Curr. Microbiol. 2011, 63, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Snauwaert, I.; Peeters, K.; Willems, A.; Vandamme, P.; Vuyst, L.D.; Hoste, B.; Bruyne, K.D. Carnobacterium iners sp. nov., a psychrophilic, lactic acid-producing bacterium from the littoral zone of an Antarctic pond. Int. J. Syst. Evol. Microbiol. 2013, 63, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ruan, C.; Peng, F.; Deng, Z.; Hong, K. Streptomyces arcticus sp. nov., isolated from the Arctic. Int. J. Syst. Evol. Microbiol. 2016, in press. [Google Scholar] [CrossRef]
- Lebar, M.D.; Heimbegner, J.L.; Baker, B. Cold-water marine natural products. J. Nat. Prod. Rep. 2007, 24, 774–797. [Google Scholar] [CrossRef] [PubMed]
- Wilson, Z.E.; Brimble, M.A. Molecules derived from the extremes of life. Nat. Prod. Rep. 2009, 26, 44–71. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Kelly, M.; Bowling, J.; Sims, J.; Waters, A.; Hamann, M. Advancement into the Arctic region for bioactive sponge secondary metabolites. Mar. Drugs 2011, 9, 2423–2437. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.T.; Lu, X.L.; Liu, X.Y.; Gao, Y.; Hu, B.; Jiao, B.H.; Zheng, H. Bioactive natural products from the antarctic and arctic organisms. Mini Rev. Med. Chem. 2013, 13, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Skropeta, D.; Wei, L. Recent advances in deep-sea natural products. Nat. Prod. Rep. 2013, 31, 999–1025. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pascale, D.; De Santi, C.; Fu, J.; Landfald, B. The microbial diversity of Polar environments is a fertile ground for bioprospecting. Mar. Genom. 2012, 8, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Hua, H.M.; Pei, Y.H.; Yao, X.S. Three new cytotoxic cyclic acylpeptides from marine Bacillus sp. Chem. Pharm. Bull. 2004, 52, 1029–1030. [Google Scholar] [CrossRef] [PubMed]
- Al-Zereini, W.; Schuhmann, I.; Laatsch, H.; Helmke, E.; Anke, H. New aromatic nitro compounds from Salegentibacter sp. T436, an Arctic Sea ice bacterium: Taxonomy, fermentation, isolation and biological activities. J. Antibiot. 2007, 60, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, I.; Yao, C.B.F.; Al-Zereini, W.; Anke, H.; Helmke, E.; Laatsch, H. Nitro derivatives from the Arctic ice bacterium Salegentibacter sp. isolate T436. J. Antibiot. 2009, 62, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Mitova, M.; Tutino, M.L.; Infusini, G.; Marino, G.; De Rosa, S. Exocellular peptides from Antarctic psychrophile Pseudoalteromonas haloplanktis. Mar. Biotechnol. 2005, 7, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Asthana, R.K.; Deepali; Tripathi, M.K.; Srivastava, A.; Singh, A.P.; Singh, S.P.; Nath, G.; Srivastava, R.; Srivastava, B.S. Isolation and identification of a new antibacterial entity from the Antarctic cyanobacterium Nostoc CCC 537. J. Appl. Phycol. 2009, 21, 81–88. [Google Scholar] [CrossRef]
- Mojib, N.; Philpott, R.; Huang, J.P.; Niederweis, M.; Bej, A.K. Antimycobacterial activity in vitro of pigments isolated from Antarctic bacteria. Antonie Van Leeuwenhoek 2010, 98, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, P.; Maida, I.; Esposito, F.P.; Tortorella, E.; Subko, K.; Ezeofor, C.C.; Zhang, Y.; Tabudravu, J.; Jaspars, M.; Fani, R.; et al. Antimicrobial activity of monoramnholipids produced by bacterial strains isolated from the Ross Sea (Antarctica). Mar. Drugs 2016, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Macherla, V.R.; Liu, J.; Bellows, C.; Teisan, S.; Nicholson, B.; Lam, K.S.; Potts, B.C.M. Glaciapyrroles A, B, and C, pyrrolosesquiterpenes from a Streptomyces sp. isolated from an Alaskan marine sediment. J. Nat. Prod. 2005, 68, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Mondol, M.A.M.; Yu, T.K.; Lee, H.S.; Lee, Y.J.; Jung, H.J.; Kim, J.H.; Kwon, H.J. An angiogenesis inhibitor isolated from a marine-derived actinomycete, Nocardiopsis sp. 03N67. Phytochem. Lett. 2010, 3, 194–197. [Google Scholar] [CrossRef]
- Gao, X.; Lu, Y.; Xing, Y.; Ma, Y.; Lu, J.; Bao, W.; Wang, Y.; Xi, T. A novel anticancer and antifungus phenazine derivative from a marine actinomycete BM-17. Microbiol. Res. 2012, 167, 616–622. [Google Scholar] [CrossRef] [PubMed]
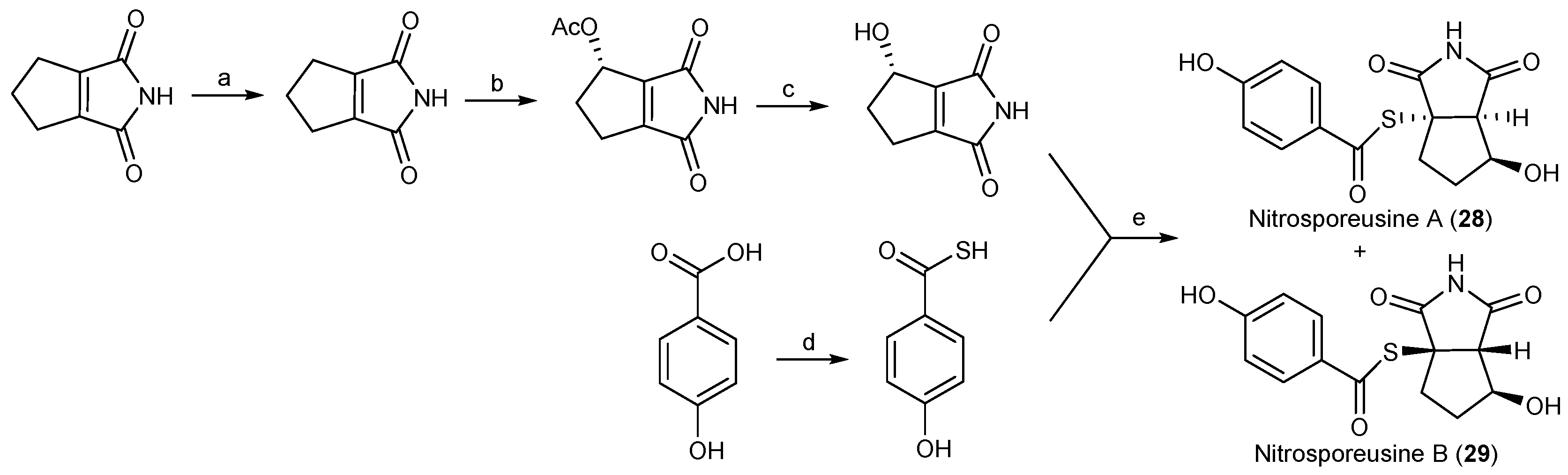
- Yang, A.; Si, L.; Shi, Z.; Tian, L.; Liu, D.; Zhou, D.; Prokch, P.; Lin, W. Nitrosporeusines A and B, unprecedented thioester-bearing alkaloids from the Arctic Streptomyces nitrosporeus. Org. Lett. 2013, 15, 5366–5369. [Google Scholar] [CrossRef] [PubMed]
- Philkhana, S.C.; Jachak, G.R.; Gunjal, V.B.; Dhage, N.M.; Bansode, A.H.; Reddy, D.S. First synthesis of nitrosporeusines, alkaloids with multiple biological activities. Tetrahedron Lett. 2015, 56, 1252–1254. [Google Scholar] [CrossRef]
- Moon, K.; Ahn, C.H.; Shin, Y.; Won, T.H.; Ko, K.; Lee, S.K.; Oh, K.B.; Shin, J.; Nam, S.I.; Oh, D.C. New benzoxazine secondary metabolites from an Arctic Actinomycete. Mar. Drugs 2014, 12, 2526–2538. [Google Scholar] [CrossRef] [PubMed]
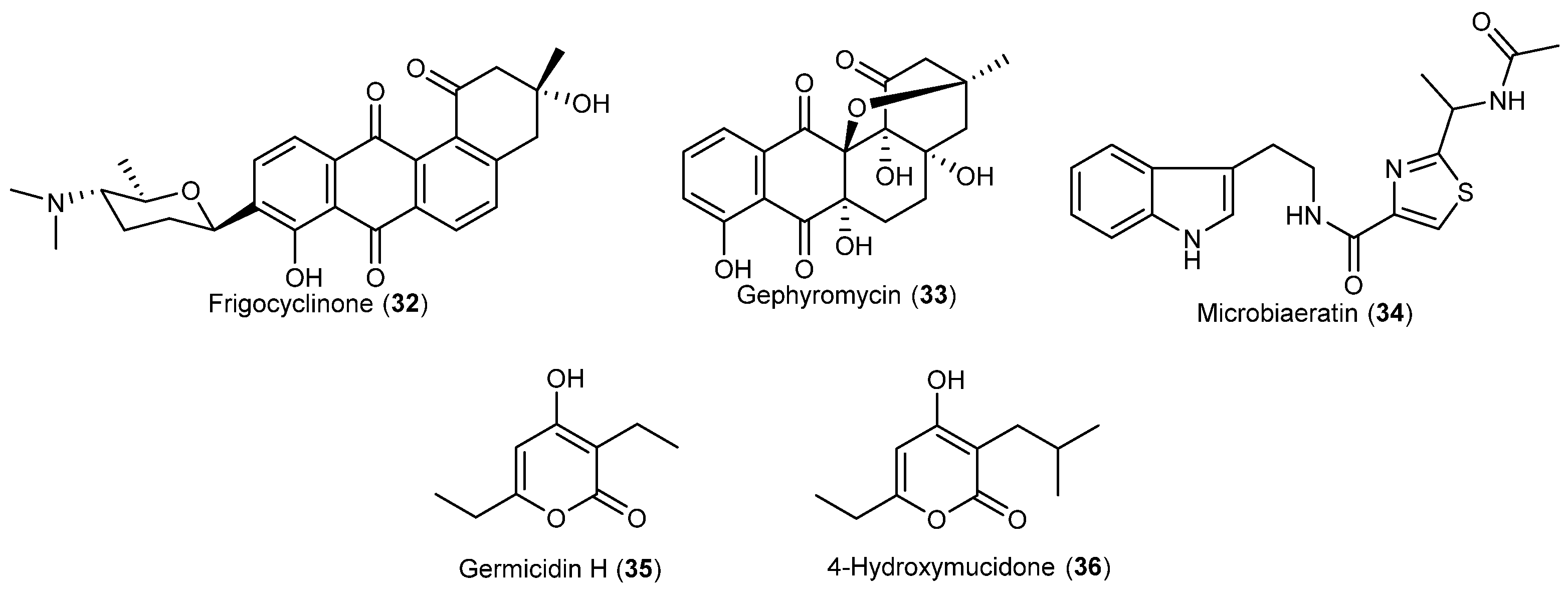
- Bruntner, C.; Binder, T.; Pathom-aree, W.; Goodfellow, M.; Bull, A.T.; Potterat, O.; Puder, C.; Hörer, S.; Schmid, A.; Bolek, W.; et al. Frigocyclinone, a novel angucyclinone antibiotic produced by a Streptomyces griseus strain from Antarctica. J. Antibiot. 2005, 58, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Lang, G.; Maksimenka, K.; Hamm, A.; Gulder, T.A.M.; Dieter, A.; Bull, A.T.; Stach, J.E.M.; Kocher, N.; Müller, W.E.G.; Fiedler, H.P. Gephyromycin, the first bridged angucyclinone, from Streptomyces griseus strain NTK 14. Phytochemistry 2005, 66, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, V.; Kolarova, M.; Aleksieva, K.; Gräfe, U.; Dahse, H.M.; Laatsch, H. Microbiaeratin, a new natural indole alkaloid from a Microbispora aerata strain, isolated from Livingston Island, Antarctica. Prep. Biochem. Biotechnol. 2007, 37, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Saurav, K.; Yu, Z.; Mándi, A.; Kurtán, T.; Li, J.; Tian, X.; Zhang, Q.; Zhang, W.; Zhang, C. α-Pyrones with diverse hydroxy substitutions from three marine-derived Nocardiopsis Strains. J. Nat. Prod. 2016, 79, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, P.W.; Larsen, T.O.; Christophersen, C. Bioactive cyclic peptides from the psychrotolerant fungus Penicillium algidum. J. Antibiot. 2005, 58, 141–144. [Google Scholar] [CrossRef] [PubMed]
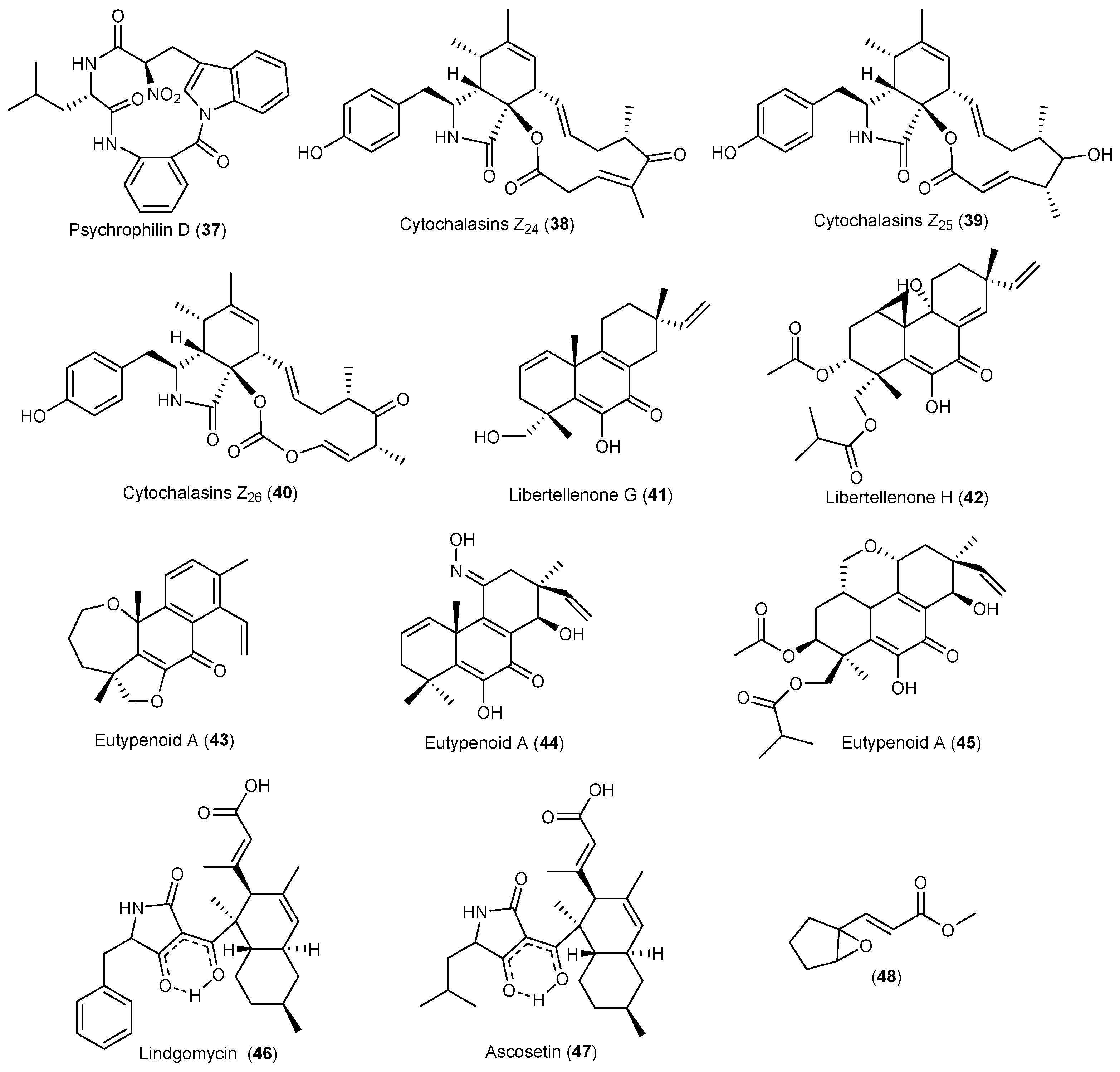
- Liu, J.T.; Hu, B.; Gao, Y.; Zhang, J.P.; Jiao, B.H.; Lu, X.L.; Liu, X.Y. Bioactive tyrosine-derived cytochalasins from fungus Eutypella sp. D-1. Chem. Biodivers. 2014, 11, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.L.; Liu, J.T.; Liu, X.Y.; Gao, Y.; Zhang, J.; Jiao, B.H.; Zheng, H. Pimarane diterpenes from the Arctic fungus Eutypella sp. D-1. J. Antibiot. 2014, 67, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Q.; Chen, X.C.; Chen, Z.Q.; Wang, G.M.; Zhu, S.G.; Yan, Y.F.; Chen, K.X.; Liu, X.Y.; Li, Y.M. Eutypenoids A–C: Novel pimarane diterpenoids from the Arctic fungus Eutypella sp. D-1. Mar. Drugs 2016, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wiese, J.; Labes, A.; Kramer, A.; Schmaljohann, R.; Imhoff, J.F. Lindgomycin, an unusual antibiotic polyketide from a marine fungus of the Lindgomycetaceae. Mar. Drugs 2015, 13, 4617–4632. [Google Scholar] [CrossRef] [PubMed]
- Ondeyka, J.G.; Smith, S.K.; Zink, D.L.; Vicente, F.; Basilio, A.; Bills, G.F.; Polishook, J.D.; Garlisi, C.; Mcguinness, D.; Smith, E.; et al. Isolation, structure elucidation and antibacterial activity of a new tetramic acid, ascosetin. J. Antibiot. 2014, 67, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Kamo, M.; Tojo, M.; Yamazaki, Y.; Itabashi, T.; Takeda, H.; Wakana, D.; Hosoe, T. Isolation of growth inhibitors of the snow rot pathogen Pythium iwayamai from an arctic strain of Trichoderma polysporum. J. Antibiot. 2016, 69, 451–455. [Google Scholar] [CrossRef] [PubMed]
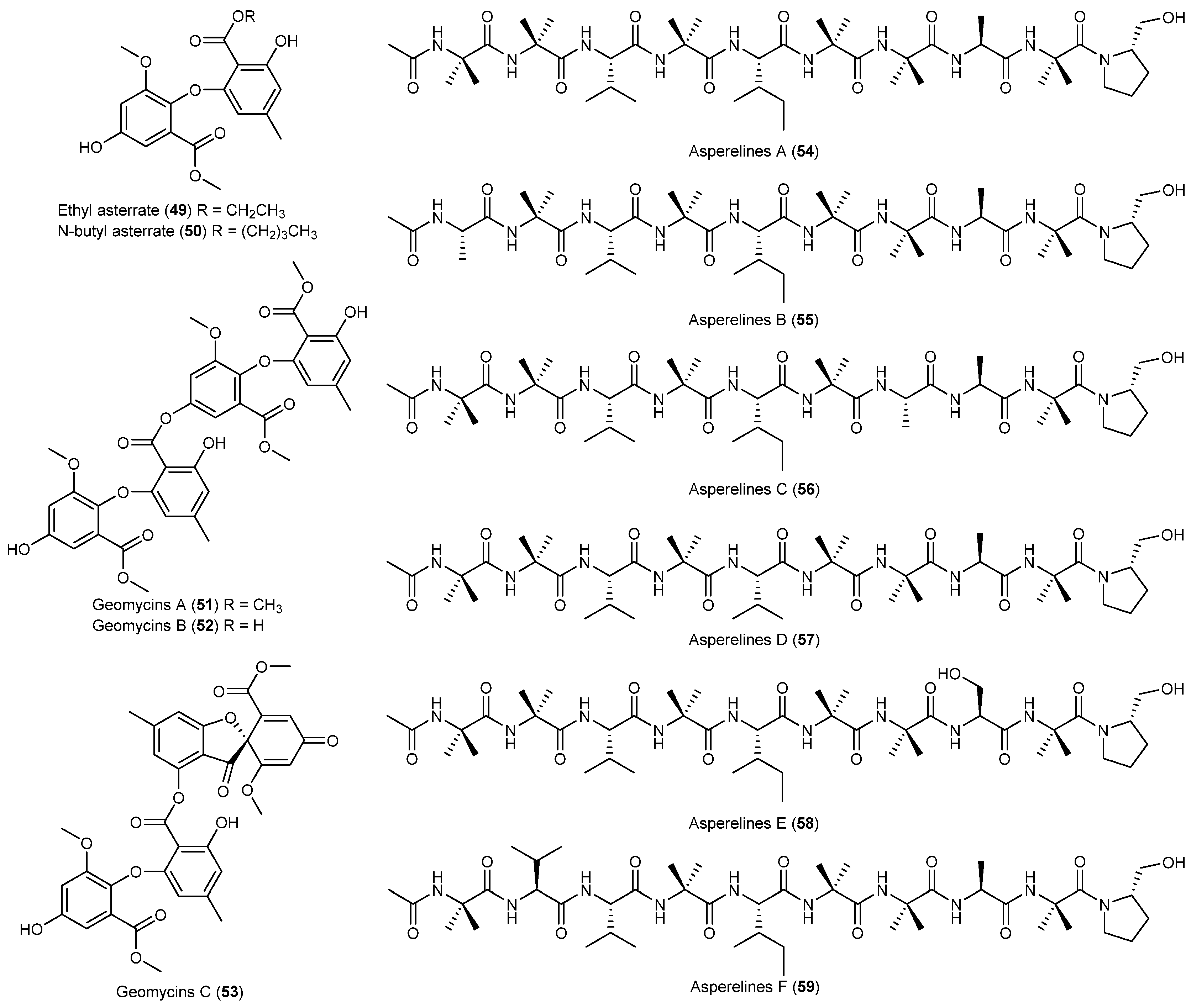
- Li, Y.; Sun, B.; Liu, S.; Jiang, L.; Liu, X.; Zhang, H.; Che, Y. Bioactive asterric acid derivatives from the Antarctic ascomycete fungus Geomyces sp. J. Nat. Prod. 2008, 71, 1643–1646. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Xue, C.; Tian, L.; Xu, M.; Chen, J.; Deng, Z.; Proksch, P.; Lin, W. Asperelines A–F, peptaibols from the marine-derived fungus Trichoderma asperellum. J. Nat. Prod. 2009, 72, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yang, Y.; Liu, D.; Chen, W.; Proksch, P.; Shao, B.; Lin, W. Sequential determination of new peptaibols asperelines G-Z12 produced by marine-derived fungus Trichoderma asperellum using ultrahigh pressure liquid chromatography combined with electrospray-ionization tandem mass spectrometry. J. Chromatogr. A 2013, 1309, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, D.; Luan, Y.; Gu, Q.; Zhu, T. Cytotoxic metabolites from the antarctic psychrophilic fungus Oidiodendron truncatum. J. Nat. Prod. 2012, 75, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ma, H.; Zhu, T.; Li, J.; Gu, Q.; Li, D. Penilactones A and B, two novel polyketides from Antarctic deep-sea derived fungus Penicillium crustosum PRB-2. Tetrahedron 2012, 68, 9745–9749. [Google Scholar] [CrossRef]
- Spence, J.T.J.; George, J.H. Total synthesis of ent-penilactone A and penilactone B. Org. Lett. 2013, 15, 3891–3893. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Lin, A.; Gu, Q.; Zhu, T.; Li, D. Four new chloro-eremophilane sesquiterpenes from an Antarctic deep-sea derived fungus, Penicillium sp. PR19N-1. Mar. Drugs 2013, 11, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Wu, G.; Gu, Q.; Zhu, T.; Li, D. New eremophilane-type sesquiterpenes from an Antarctic deepsea derived fungus, Penicillium sp. PR19N-1. Arch. Pharm. Res. 2014, 37, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, L.; Wang, W.; Che, Q.; Li, D.; Gu, Q.; Zhu, T. Chrodrimanins I and J from the Antarctic moss-derived fungus Penicillium funiculosum GWT2-24. J. Nat. Prod. 2015, 78, 1442–1445. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, L.; Jiménez, C.; Rodríguez, J.; Areche, C.; Chávez, R.; Henríquez, M.; de la Cruz, M.; Díaz, C.; Segade, Y.; Vaca, I. 3-Nitroasterric acid derivatives from an Antarctic sponge-derived Pseudogymnoascus sp. fungus. J. Nat. Prod. 2015, 78, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, X.; Qin, X.; Tian, X.; Liao, L.; Li, K.; Zhou, X.; Yang, X.; Wang, F.; Zhang, T.; et al. Antiviral merosesquiterpenoids produced by the Antarctic fungus Aspergillus ochraceopetaliformis SCSIO 05702. J. Nat. Prod. 2016, 79, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Ingόlfsdόttir, K. Usnic acid. Phytochemistry 2002, 61, 729–736. [Google Scholar] [CrossRef]
- Kumar, K.C.S.; Müller, K. Lichen metabolites. 1. Inhibitory action against leukotriene B4 biosynthesis by a non-redox mechanism. J. Nat. Prod. 1999, 62, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Paudel, B.; Bhattarai, H.D.; Lee, J.S.; Hong, S.G.; Shin, H.W.; Yim, J.H. Antibacterial potential of Antarctic lichens against human pathogenic Gram-positive bacteria. Phytother. Res. 2008, 22, 1269–1271. [Google Scholar] [CrossRef] [PubMed]
- Paudel, B.; Bhattarai, H.D.; Lee, J.S.; Hong, S.G.; Shin, H.W.; Yim, J.H. Antioxidant activity of polar lichens from King George Island (Antarctica). Polar Biol. 2008, 31, 605–608. [Google Scholar] [CrossRef]
- Ivanova, V.; Kolarova, M.; Aleksieva, K. Diphenylether and macrotriolides occurring in a fungal isolate from the antarctic lichen Neuropogon. Prep. Biochem. Biotechnol. 2007, 37, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.; Yim, J.H.; Lee, H.K.; Park, S.M.; Sohn, J.H.; Oh, H. Stereocalpin A, a bioactive cyclic depsipeptide from the Antarctic lichen Stereocaulon alpinum. Tetrahedron Lett. 2008, 49, 29–31. [Google Scholar] [CrossRef]
- Seo, C.; Sohn, J.H.; Park, S.M.; Yim, J.H.; Lee, H.K.; Oh, H. Usimines A-C, bioactive usnic acid derivatives from the Antarctic lichen Stereocaulon alpinum. J. Nat. Prod. 2008, 71, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Koren, S.; Fantus, I.G. Inhibition of the protein tyrosine phosphatase PTP1B: Potential therapy for obesity, insulin resistance and type-2 diabetes mellitus. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 621–640. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.; Sohn, J.H.; Ahn, J.S.; Yim, J.H.; Lee, H.K.; Oh, H. Protein tyrosine phosphatase 1B inhibitory effects of depsidone and pseudodepsidone metabolites from the Antarctic lichen Stereocaulon alpinum. Bioorg. Med. Chem. Lett. 2009, 19, 2801–2803. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, H.D.; Kim, T.; Oh, H.; Yim, J.H. A new pseudodepsidone from the Antarctic lichen Stereocaulon alpinum and its antioxidant, antibacterial activity. J. Antibiot. 2013, 66, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yim, J.H.; Lee, D.S.; Kim, Y.C.; Oh, H. New diterpene furanoids from the Antarctic lichen Huea sp. Bioorg. Med. Chem. Lett. 2012, 22, 7393–7396. [Google Scholar] [CrossRef] [PubMed]
- Paudel, B.; Bhattarai, H.D.; Koh, H.Y.; Lee, S.G.; Han, S.J.; Lee, H.K.; Oh, H.; Shin, H.W.; Yim, J.H. Ramalin, a novel nontoxic antioxidant compound from the Antarctic lichen Ramalina terebrata. Phytomedicine 2011, 18, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
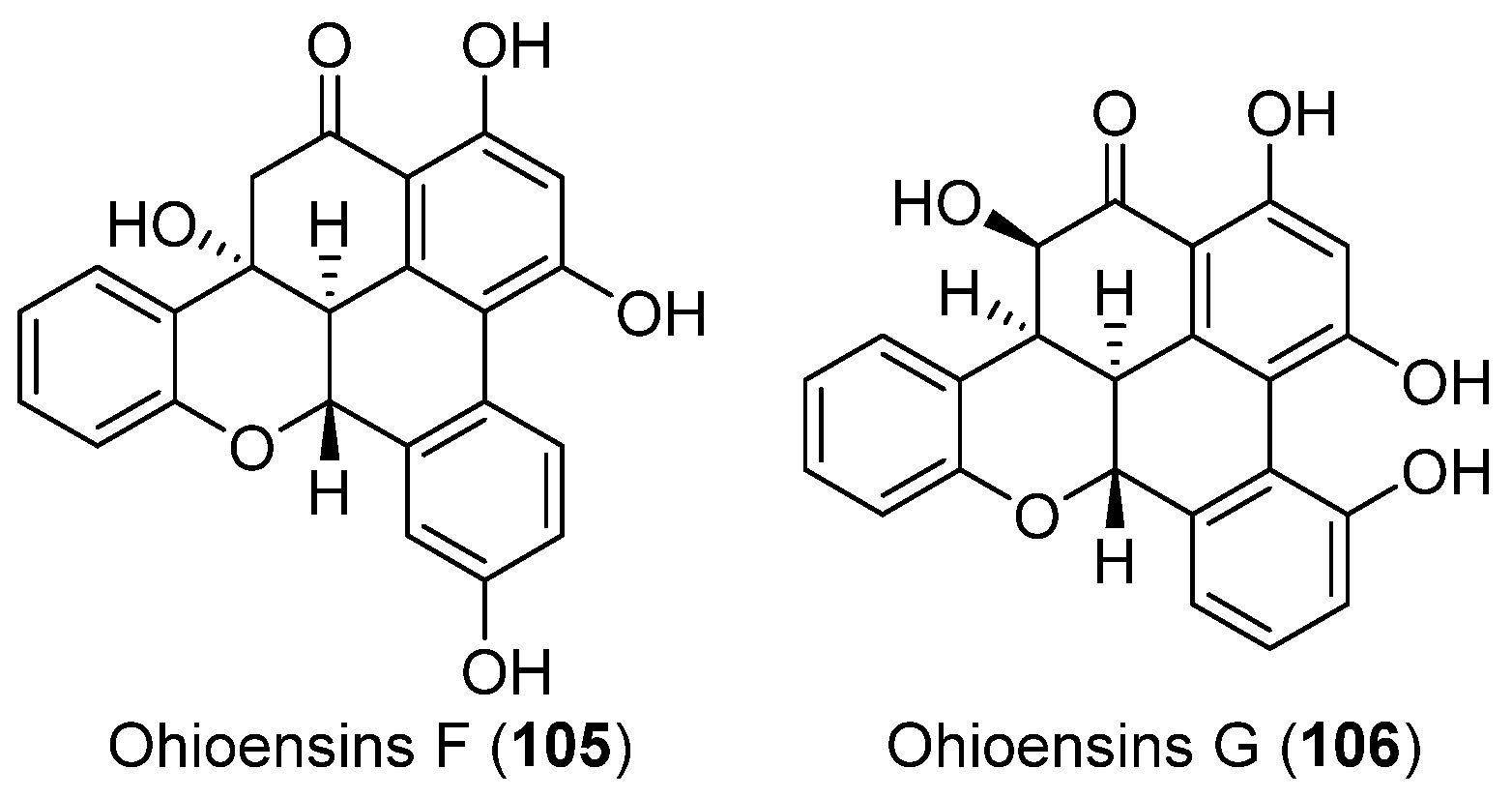
- Seo, C.; Choi, Y.H.; Sohn, J.H.; Ahn, J.S.; Yim, J.H.; Lee, H.K.; Oh, H. Ohioensins F and G: Protein tyrosine phosphatase 1B inhibitory benzonaphthoxanthenones from the Antarctic moss Polytrichastrum alpinum. Bioorg. Med. Chem. Lett. 2008, 18, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.H.; Winson, M.K.; Porter, J.S. Bryozoan metabolites: An ecological perspective. Nat. Prod. Rep. 2007, 24, 659–673. [Google Scholar] [CrossRef] [PubMed]
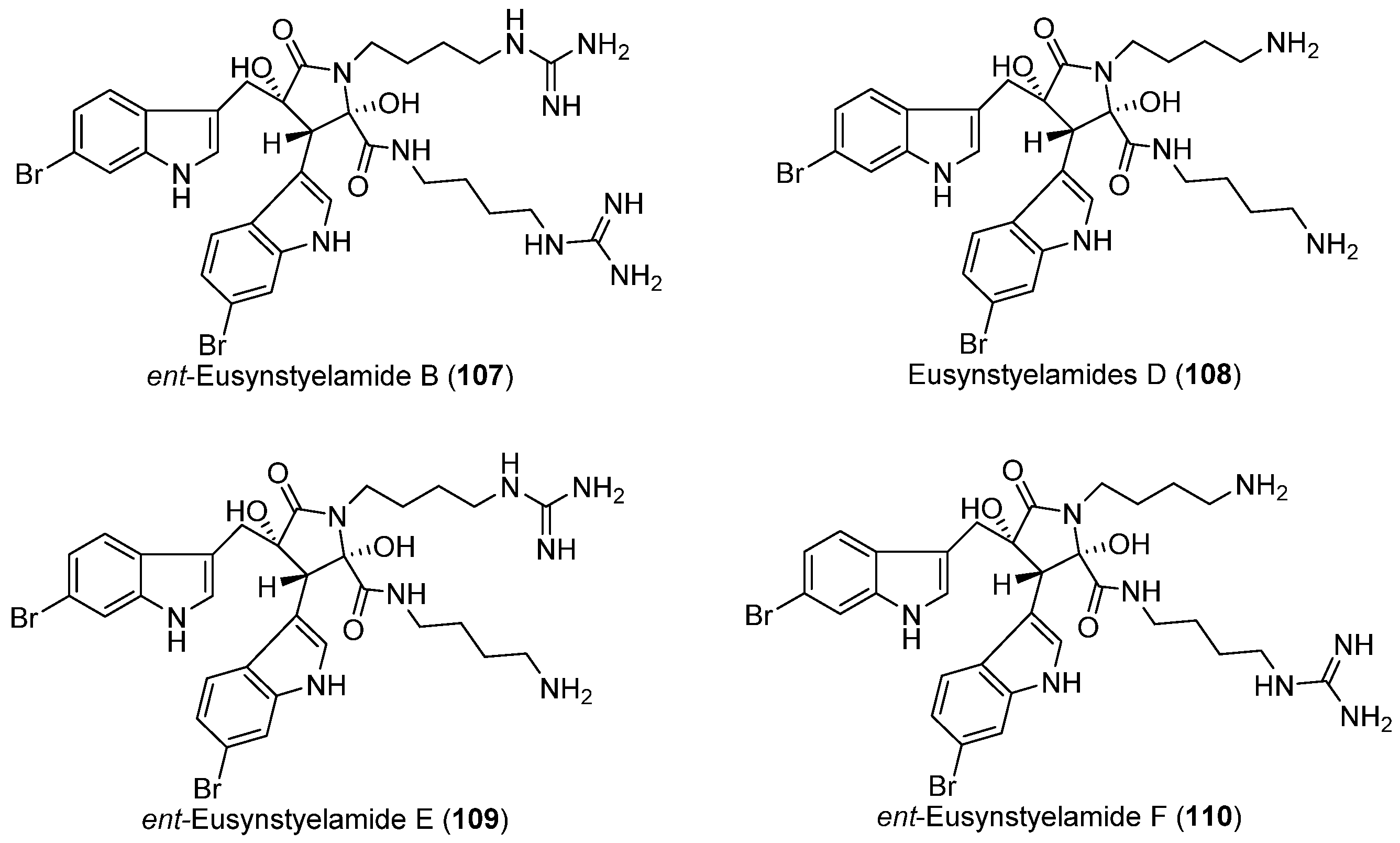
- Tadesse, M.; Tabudravu, J.N.; Jaspars, M.; Strøm, M.B.; Hansen, E.; Andersen, J.H.; Kristiansen, P.E.; Haug, T. The antibacterial ent-eusynstyelamide B and eusynstyelamides D, E, and F from the Arctic bryozoan Tegella cf. spitzbergensis. J. Nat. Prod. 2011, 74, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Tapiolas, D.M.; Bowden, B.F.; Abou-Mansour, E.; Willis, R.H.; Doyle, J.R.; Muirhead, A.N.; Liptrot, C.; Llewellyn, L.E.; Wolff, C.W.W.; Wright, A.D.; et al. Eusynstyelamides A, B, and C, nNOS inhibitors, from the ascidian Eusynstyela latericius. J. Nat. Prod. 2009, 72, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
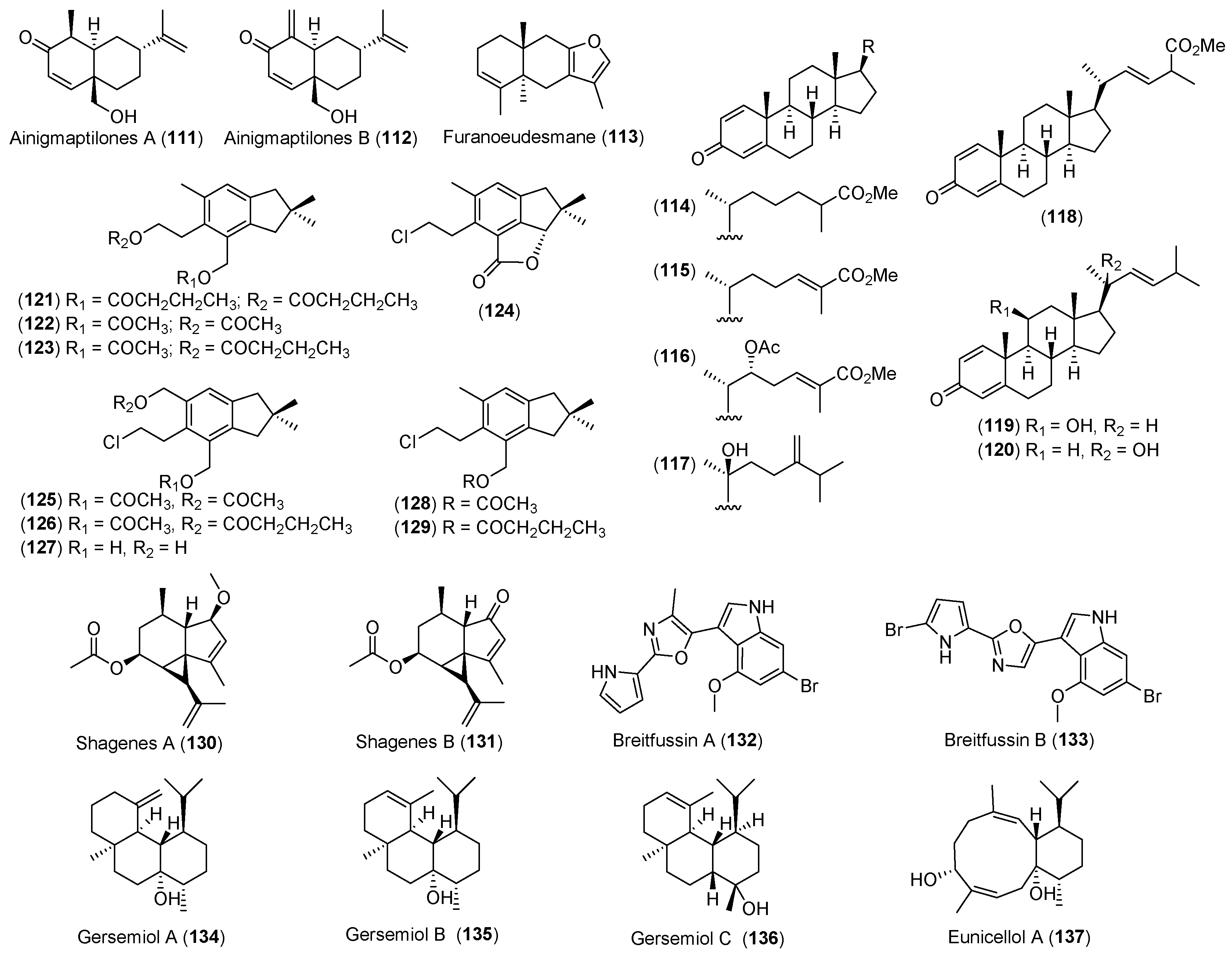
- Iken, K.B.; Baker, B.J. Ainigmaptilones, sesquiterpenes from the Antarctic gorgonian coral Ainigmaptilon antarcticus. J. Nat. Prod. 2003, 66, 888–890. [Google Scholar] [CrossRef] [PubMed]
- Gavagnin, M.; Mollo, E.; Castelluccio, F.; Crispino, A.; Cimino, G. Sesquiterpene metabolites of the antarctic gorgonian Dasystenella acanthina. J. Nat. Prod. 2003, 66, 1517–1519. [Google Scholar] [CrossRef] [PubMed]
- Mellado, G.G.; Zubía, E.; Ortega, M.J.; Lόpez-González, P.J. Steroids from the Antarctic octocoral Anthomastus bathyproctus. J. Nat. Prod. 2005, 68, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Nunez-Pons, L.; Castelluccio, F.; Avila, C.; Gavagnin, M. Illudalane sesquiterpenoids of the alcyopterosin series from the Antarctic marine soft coral Alcyonium grandis. J. Nat. Prod. 2009, 72, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Palermo, J.A.; Rodriguez Brasco, M.F.; Spagnuolo, C.; Seldes, A.M. Illudalane sesquiterpenoids from the soft coral Alcyonium paessleri: The first natural nitrate esters. J. Org. Chem. 2000, 65, 4482–4486. [Google Scholar] [CrossRef] [PubMed]
- Finkielsztein, L.M.; Bruno, A.M.; Renou, S.G.; Moltrasio de Iglesias, G.Y. Design, synthesis, and biological evaluation of alcyopterosin A and illudalane derivatives as anticancer agents. Bioorg. Med. Chem. 2006, 14, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Nakano, Y.; Suzuki, D.; Urabe, H.; Sato, F.J. Selective preparation of benzyltitanium compounds by the metalative Reppe reaction. Its Application to the first synthesis of alcyopterosin A. J. Am. Chem. Soc. 2002, 124, 9682–9683. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Hirata, Y.; Ishihara, S.; Oda, S.; Yukawa, T.; Shirakawa, E.; Hiyama, T. Stannylative cycloaddition of enynes catalyzed by palladium-iminophosphine. J. Am. Chem. Soc. 2004, 126, 15650–15651. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.L.; Snyder, J.K. Intramolecular rhodium-catalyzed [2+2+2] cyclizations of diynes with enones. J. Org. Chem. 2009, 74, 2907–2910. [Google Scholar] [CrossRef] [PubMed]
- Welsch, T.; Tran, H.A.; Witulski, B. Total syntheses of the marine illudalanes alcyopterosin I, L, M, N, and C. Org. Lett. 2010, 12, 5644–5647. [Google Scholar] [CrossRef] [PubMed]
- Von Salm, J.L.; Wilson, N.G.; Vesely, B.A.; Kyle, D.E.; Cuce, J.; Baker, B.J. Shagenes A and B, new tricyclic sesquiterpenes produced by an undescribed Antarctic octocoral. Org. Lett. 2014, 16, 2630–2633. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, K.Ø.; Schuler, B.; Williams, A.J.; Demissie, T.B.; Hansen, E.; Andersen, J.H.; Svenson, J.; Blinov, K.; Repisky, M.; Mohn, F.; et al. A combined atomic force microscopy and computational approach for the structural elucidation of breitfussin A and B: Highly modified halogenated dipeptides from Thuiaria breitfussi. Angew. Chem. Int. Ed. 2012, 51, 12238–12241. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Guttormsen, Y.; Haug, B.E.; Hedberg, C.; Bayer, A. A concise total synthesis of breitfussin A and B. Org. Lett. 2015, 17, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Chen, J.S. Synthesis of breitfussin B by late-stage bromination. Org. Lett. 2015, 17, 3718–3721. [Google Scholar] [CrossRef] [PubMed]
- Angulo-Preckler, C.; Genta-Jouve, G.; Mahajan, N.; de la Cruz, M.; de Pedro, N.; Reyes, F.; Iken, K.; Avila, C.; Thomas, O.P. Gersemiols A-C and eunicellol A, diterpenoids from the Arctic soft coral Gersemia fruticosa. J. Nat. Prod. 2016, 79, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
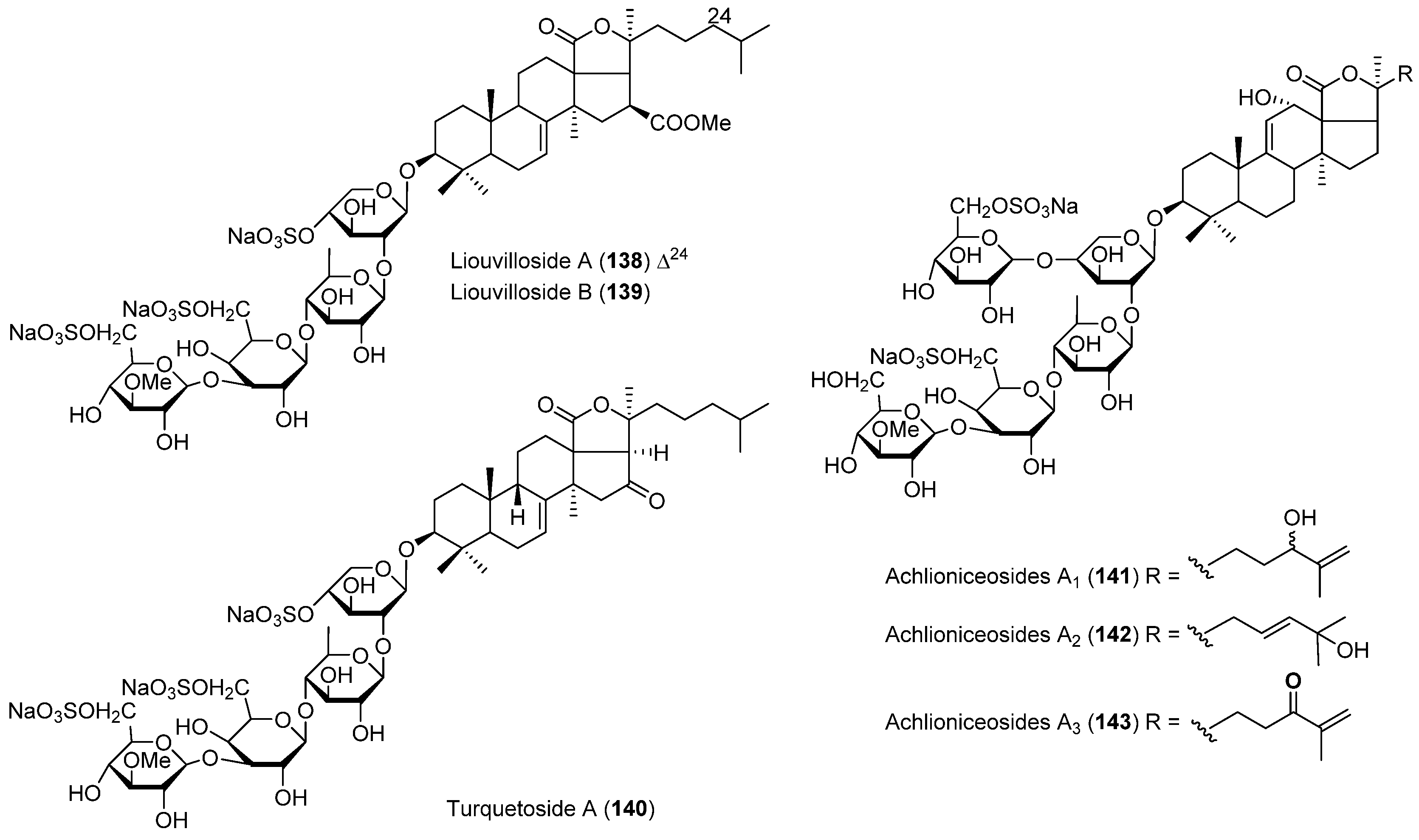
- Maier, M.S.; Roccatagliata, A.J.; Kuriss, A.; Chludil, H.; Seldes, A.M.; Pujol, C.A.; Damonte, E.B. Two new cytotoxic and virucidal trisulfated triterpene glycosides from the Antarctic sea cucumber Staurocucumis liouvillei. J. Nat. Prod. 2001, 64, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjashchenko, P.V.; Dmitrenok, P.S.; Kalinin, V.I.; Taboada, S.; Avila, C. Triterpene glycosides from Antarctic sea cucumbers IV. Turquetoside A, a 3-O-methylquinovose containing disulfated tetraoside from the sea cucumber Staurocucumis turqueti (Vaney, 1906) (=Cucumaria spatha). Biochem. Syst. Ecol. 2013, 51, 45–49. [Google Scholar] [CrossRef]
- Antonov, A.S.; Avilov, S.A.; Kalinovsky, A.I.; Anastyuk, S.D.; Dmitrenok, P.S.; Kalinin, V.I.; Taboada, S.; Bosh, A.; Avila, C.; Stonik, V.A. Triterpene glycosides from Antarctic sea cucumbers. 2. Structure of Achlioniceosides A(1), A(2), and A(3) from the sea cucumber Achlionice violaecuspidata (=Rhipidothuria racowitzai). J. Nat. Prod. 2009, 72, 33–38. [Google Scholar] [CrossRef] [PubMed]
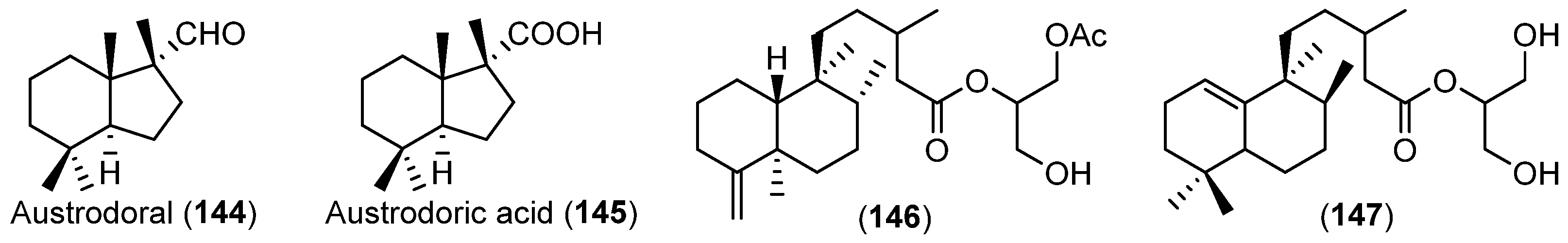
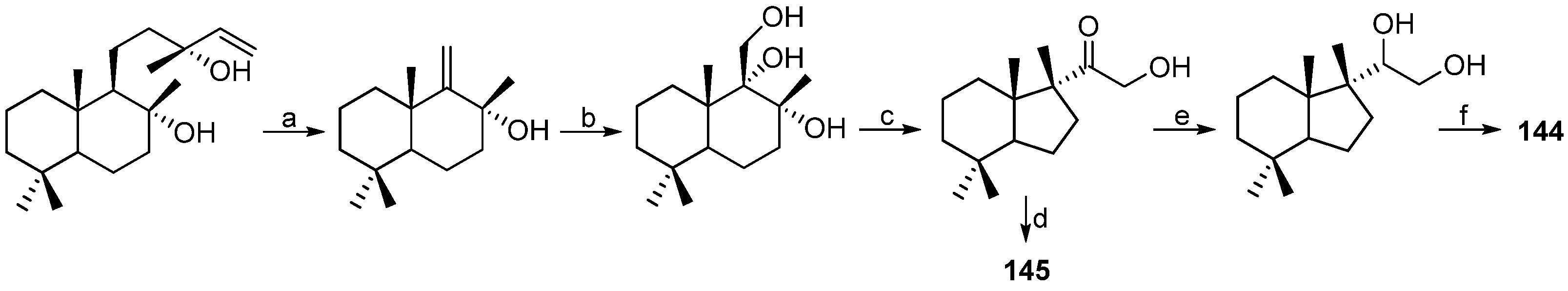
- Gavagnin, M.; Carbone, M.; Mollo, E.; Cimino, G. Austrodoral and austrodoric acid: Nor-sesquiterpenes with a new carbon skeleton from the Antarctic nudibranch Austrodoris kerguelenensis. Tetrahedron Lett. 2003, 44, 1495–1498. [Google Scholar] [CrossRef]
- Alvarez-Manzaneda, E.J.; Chahboun, R.; Barranco, I.; Torres, E.C.; Alvarez, E.; Alvarez-Manzaneda, R. First enantiospecific synthesis of marine nor-sesquiterpene (+)-austrodoral from (–)-sclareol. Tetrahedron Lett. 2005, 46, 5321–5324. [Google Scholar] [CrossRef]
- Gavagnin, M.; Carbone, M.; Mollo, E.; Cimino, G. Further chemical studies on the Antarctic nudibranch Austrodoris kerguelenensis: New terpenoid acylglycerols and revision of the previous stereochemistry. Tetrahedron 2003, 59, 5579–5583. [Google Scholar] [CrossRef]
- Alvarez-Manzaneda, E.J.; Chahboun, R.; Barranco, I.; Torres, E.C.; Alvarez, E.; Alvarez-Manzaneda, R. First enantiospecific synthesis of the antitumor marine sponge metabolite (–)-15-oxopuupehenol from (–)-sclareol. Org. Lett. 2005, 7, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Alvarez-Manzaneda, E.J.; Chahboun, R.; González Díaz, C. New routes toward drimanes and nor-drimanes from (–)-Sclareol. Synlett 2000, 11, 1561–1564. [Google Scholar]
- Diyabalanage, T.; Iken, K.B.; McClintock, J.B.; Amsler, C.D.; Baker, B.J. Palmadorins A–C, diterpene glycerides from the antarctic nudibranch Austrodoris kerguelenensis. J. Nat. Prod. 2010, 73, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Maschek, J.A.; Mevers, E.; Diyabalanage, T.; Chen, L.; Ren, Y.; McClintock, J.B.; Amsler, C.D.; Wu, J.; Baker, B.J. Palmadorin chemodiversity from the Antarctic nudibranch Austrodoris kerguelenensis and inhibition of Jak2/STAT5-dependent HEL leukemia cells. Tetrahedron 2012, 68, 9095–9104. [Google Scholar] [CrossRef]
- Cutignano, A.; Moles, J.; Avila, C.; Fontana, A. Granuloside, a unique linear homosesterterpene from the Antarctic nudibranch Charcotia granulosa. J. Nat. Prod. 2015, 78, 1761–1764. [Google Scholar] [CrossRef] [PubMed]
- Skropeta, D.; Pastro, N.; Zivanovic, A. Kinase inhibitors from marine sponges. Mar. Drugs 2011, 9, 2131–2154. [Google Scholar] [CrossRef] [PubMed]
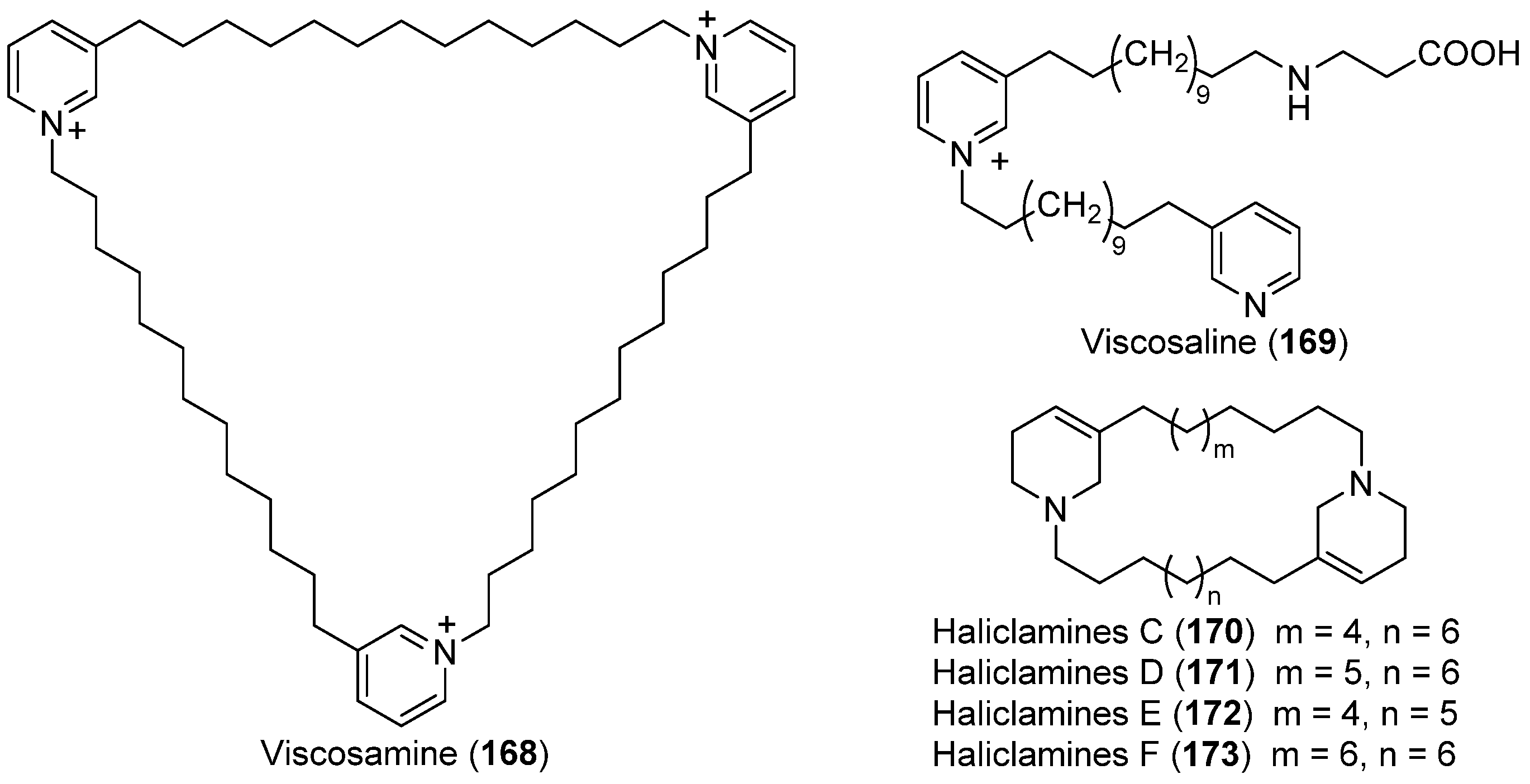
- Volk, C.A.; Köck, M. Viscosamine: The first naturally occurring trimeric 3-alkyl pyridinium alkaloid. Org. Lett. 2003, 5, 3567–3569. [Google Scholar] [CrossRef] [PubMed]
- Volk, C.A.; Köck, M. Viscosaline: New 3-alkyl pyridinium alkaloid from the Arctic sponge Haliclona viscosa. Org. Biomol. Chem. 2004, 2, 1827–1830. [Google Scholar] [CrossRef] [PubMed]
- Volk, C.A.; Lippert, H.; Lichte, E.; Köck, M. Two new haliclamines from the Arctic sponge Haliclona viscosa. Eur. J. Org. Chem. 2004, 3154–3158. [Google Scholar] [CrossRef]
- Schmidt, G.; Timm, C.; Köck, M. New haliclamines E and F from the Arctic sponge Haliclona viscosa. Org. Biomol. Chem. 2009, 7, 3061–3064. [Google Scholar] [CrossRef]
- Timm, C.; Mordhorst, T.; Köck, M. Synthesis of 3-alkyl pyridinium alkaloids from the arctic sponge Haliclona viscosa. Mar. Drugs 2010, 8, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Shorey, B.J.; Lee, V.; Baldwin, J.E. Synthesis of the Arctic sponge alkaloid viscosaline and the marine sponge alkaloid theonelladin C. Tetrahedron 2007, 63, 5587–5592. [Google Scholar] [CrossRef]
- Díaz-Marrero, A.R.; Brito, I.; Dorta, E.; Cueto, M.; San-Martín, A.; Darias, J. Synthesis of the Arctic sponge alkaloid viscosaline and the marine sponge alkaloid theonelladin C. Tetrahedron Lett. 2003, 44, 5939–5942. [Google Scholar]
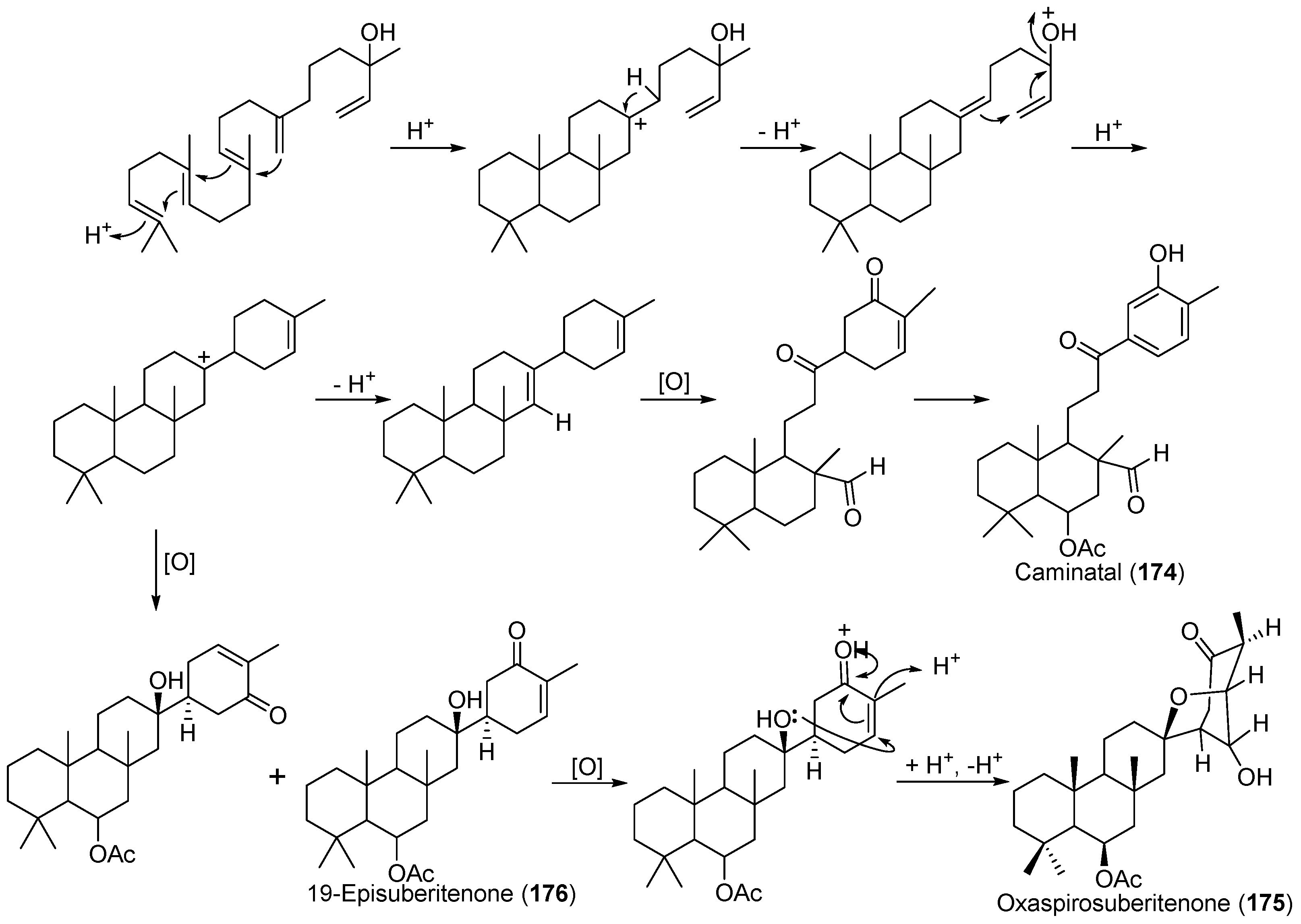
- Díaz-Marrero, A.R.; Brito, I.; Cueto, M.; San-Martín, A.; Darias, J. Suberitane network, a taxonomical marker for Antarctic sponges of the genus Suberites? Novel sesterterpenes from Suberites caminatus. Tetrahedron Lett. 2004, 45, 4707–4710. [Google Scholar] [CrossRef]
- Díaz-Marrero, A.R.; Dorta, E.; Cueto, M.; San-Martín, A.; Darias, J. Conformational analysis and absolute stereochemistry of ‘spongian’-related metabolites. Tetrahedron 2004, 60, 1073–1078. [Google Scholar] [CrossRef]
- Lee, H.S.; Ahn, J.W.; Lee, Y.H.; Rho, J.R.; Shin, J. New sesterterpenes from the Antarctic sponge Suberites sp. J. Nat. Prod. 2004, 67, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.S.; Mutka, T.; Vesley, B.; Amsler, M.O.; McClintock, J.B.; Amsler, C.D.; Perman, J.A.; Singh, M.P.; Maiese, W.M.; Zaworotko, M.J.; et al. Norselic acids A–E, highly oxidized anti-infective steroids that deter mesograzer predation, from the Antarctic sponge Crella sp. J. Nat. Prod. 2009, 72, 1842–1846. [Google Scholar] [CrossRef] [PubMed]
- von Salm, J.L.; Witowski, C.G.; Fleeman, R.M.; McClintock, J.B.; Amsler, C.D.; Shaw, L.N.; Baker, B.J. Darwinolide, a new diterpene scaffold that inhibits methicillin-resistant Staphylococcus aureus biofilm from the Antarctic sponge Dendrilla membranosa. Org. Lett. 2016, 18, 2596–2599. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.K.; Hansen, E.; Moodie, L.W.K.; Isaksson, J.; Sepčić, K.; Cergolj, M.; Svenson, J.; Andersen, J.H. Marine AChE inhibitors isolated from Geodia barretti: Natural compounds and their synthetic analogs. Org. Biomol. Chem. 2016, 14, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.F.; Namikoshi, M. Bioactive nitrogenous metabolites from ascidians. Heterocycles 2007, 74, 53–88. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 2004, 67, 1216–1238. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.S. Ascidians: Producers of amino acid derived metabolites. Chem. Rev. 1993, 93, 1771–1791. [Google Scholar] [CrossRef]
- Reyes, F.; Fernández, R.; Rodríguez, A.; Francesch, A.; Taboada, S.; Ávila, C.; Cuevas, C. Aplicyanins A–F, new cytotoxic bromoindole derivatives from the marine tunicate Aplidium cyaneum. Tetrahedron 2008, 64, 5119–5123. [Google Scholar] [CrossRef]
- Miyata, Y.; Diyabalanage, T.; Amsler, C.D.; McClintock, J.B.; Valeriote, F.A.; Baker, B.J. Ecdysteroids from the Antarctic tunicate Synoicum adareanum. J. Nat. Prod. 2007, 70, 1859–1864. [Google Scholar] [CrossRef] [PubMed]
- Diyabalanage, T.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Palmerolide A, a cytotoxic macrolide from the Antarctic tunicate Synoicum adareanum. J. Am. Chem. Soc. 2006, 128, 5630–5631. [Google Scholar] [CrossRef] [PubMed]
- Noguez, J.H.; Diyabalanage, T.K.K.; Miyata, Y.; Xie, X.S.; Valeriote, F.A.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Palmerolide macrolides from the Antarctic tunicate Synoicum adareanum. Bioorg. Med. Chem. 2011, 19, 6608–6614. [Google Scholar] [CrossRef] [PubMed]
- Mi, Q.; Pezzuto, J.M.; Farnsworth, N.R.; Wani, M.C.; Kinghorn, A.D.; Swanson, S.M. Use of the in vivo hollow fiber assay in natural products anticancer drug discovery. J. Nat. Prod. 2009, 72, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Riesenfeld, C.S.; Murray, A.E.; Baker, B.J. Characterization of the microbial community and polyketide biosynthetic potential in the palmerolide-producing tunicate Synoicum adareanum. J. Nat. Prod. 2008, 71, 1812–1818. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, M.P.; Dudley, G.B. Synthesis of cytotoxic palmerolides. Chem. Eur. J. 2013, 19, 16146–16168. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, B.; Lebreton, S.; De Brabander, J.K. Total synthesis and structure revision of the marine metabolite palmerolide A. J. Am. Chem. Soc. 2007, 129, 6386–6387. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Guduru, R.; Sun, Y.P.; Banerji, B.; Chen, D.Y.K. Total synthesis of the originally proposed and revised structures of palmerolide A. Angew. Chem. Int. Ed. 2007, 46, 5896–5900. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Sun, Y.P.; Guduru, R.; Banerji, B.; Chen, D.Y.K. Total synthesis of the originally proposed and revised structures of palmerolide A and isomers thereof. J. Am. Chem. Soc. 2008, 130, 3633–3644. [Google Scholar] [CrossRef] [PubMed]
- Ravu, V.R.; Leung, G.Y.C.; Lim, C.S.; Ng, S.Y.; Sum, R.J.; Chen, D.Y.K. Chemical synthesis and biological evaluation of second-generation palmerolide A analogues. Eur. J. Org. Chem. 2011, 2011, 463–468. [Google Scholar] [CrossRef]
- Penner, M.; Rauniyar, V.; Kaspar, L.T.; Hall, D.G. Catalytic asymmetric synthesis of palmerolide A via organoboron methodology. J. Am. Chem. Soc. 2009, 131, 14216–14217. [Google Scholar] [CrossRef] [PubMed]
- Jägel, J.; Maier, M.E. Formal total synthesis of palmerolide A. Synthesis 2009, 17, 2881–2892. [Google Scholar]
- Gowrisankar, P.; Pujari, S.A.; Kaliappan, K.P. A formal total synthesis of palmerolide A. Chem. Eur. J. 2010, 16, 5858–5862. [Google Scholar] [CrossRef] [PubMed]
- Pujari, S.A.; Gowrisankar, P.; Kaliappan, K.P. A Shimizu non-aldol approach to the formal total synthesis of palmerolide A. Chem. Asian J. 2011, 6, 3137–3151. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.R.; Pawar, A.B. Enantioselective formal synthesis of palmerolide A. Org. Lett. 2011, 13, 4252–4255. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.B.; Prasad, K.R. Formal total synthesis of palmerolide A. Chem. Eur. J. 2012, 18, 15202–15206. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, M.P.; Jones, D.M.; Dudley, G.B. Formal synthesis of palmerolide A, featuring alkynogenic fragmentation and syn-selective vinylogous aldol chemistry. Org. Lett. 2013, 15, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Kaliappan, K.P.; Gowrisankar, P. Synthetic studies on a marine natural product, palmerolide A: Synthesis of C1-C9 and C15-C21 fragments. Synlett 2007, 1537–1540. [Google Scholar] [CrossRef]
- Jägel, J.; Schmauder, A.; Binanzer, M.; Maier, M.E. A concise route to the C3–C23 fragment of the macrolide palmerolide A. Tetrahedron 2007, 63, 13006–13017. [Google Scholar] [CrossRef]
- Cantagrel, G.; Meyer, C.; Cossy, J. Synthetic studies towards the marine natural product palmerolide A: Synthesis of the C3-C15 and C16-C23 fragments. Synlett 2007, 19, 2983–2986. [Google Scholar]
- Lebar, M.D.; Baker, B.J. Synthesis of the C3–14 fragment of palmerolide A using a chiral pool based strategy. Tetrahedron 2010, 66, 1557–1562. [Google Scholar] [CrossRef]
- Prasad, K.R.; Pawar, A.B. Stereoselective synthesis of C1–C18 region of palmerolide A from tartaric acid. Synlett 2010, 7, 1093–1095. [Google Scholar] [CrossRef]
- Wen, Z.K.; Xu, Y.H.; Loh, T.P. Palladium-catalyzed cross-coupling of unactivated alkenes with acrylates: Application to the synthesis of the C13–C21 fragment of palmerolide A. Chem. Eur. J. 2012, 18, 13284–13287. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.M.; Dudley, G.B. Synthesis of the C1–C15 region of palmerolide A using refined Claisen-type addition-bond cleavage methodology. Synlett 2010, 2, 223–226. [Google Scholar]
- Lisboa, M.P.; Jeong-Im, J.H.; Jones, D.M.; Dudley, G.B. Toward a new palmerolide assembly strategy: Synthesis of C16–C24. Synlett 2012, 23, 1493–1496. [Google Scholar]
- Jena, B.K.; Mohapatra, D.K. Synthesis of the C1–C15 fragment of palmerolide A via protecting group dependent RCM reaction. Tetrahedron Lett. 2013, 54, 3415–3418. [Google Scholar] [CrossRef]
- Florence, G.J.; Wlochal, J. Synthesis of the originally proposed structure of palmerolide C. Chem. Eur. J. 2012, 18, 14250–14254. [Google Scholar] [CrossRef] [PubMed]
- Appleton, D.R.; Chuen, C.S.; Berridge, M.V.; Webb, V.L.; Copp, B.R. Rossinones A and B, biologically active meroterpenoids from the Antarctic ascidian, Aplidium species. J. Org. Chem. 2009, 74, 9195–9198. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Pons, L.; Carbone, M.; Vázquez, J.; Rodríguez, J.; Nieto, R.M.; Varela, M.M.; Gavagnin, M.; Avila, C. Natural products from Antarctic colonial Ascidians of the genera Aplidium and Synoicum: Variability and defensive role. Mar. Drugs 2012, 10, 1741–1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Chen, J.; Yang, Z.; Tang, Y. Rapid biomimetic total synthesis of (±)-rossinone B. Org. Lett. 2010, 12, 5554–5557. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Núñez-Pons, L.; Paone, M.; Castelluccio, F.; Avila, C.; Gavagnin, M. Rossinone-related meroterpenes from the Antarctic ascidian Aplidium fuegiense. Tetrahedron 2012, 68, 3541–3544. [Google Scholar] [CrossRef]
- Tadesse, M.; Strøm, M.B.; Svenson, J.; Jaspars, M.; Milne, B.F.; Tørfoss, V.; Andersen, J.H.; Hansen, E.; Stensvåg, K.; Haug, T. Synoxazolidinones A and B: Novel bioactive alkaloids from the ascidian Synoicum pulmonaria. Org. Lett. 2010, 21, 4752–4755. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, M.; Svenson, J.; Jaspars, M.; Strøm, M.B.; Abdelrahman, M.H.; Andersen, J.H.; Hansen, E.; Kristiansen, P.E.; Stensvåg, K.; Haug, T. Synoxazolidinone C; a bicyclic member of the synoxazolidinone family with antibacterial and anticancer activities. Tetrahedron Lett. 2011, 52, 1804–1806. [Google Scholar] [CrossRef]
- Hopmann, K.H.; Šebestík, J.; Novotná, J.; Stensen, W.; Urbanová́, M.; Svenson, J.; Svendsen, J.S.; Bouř, P.; Ruud, K. Determining the absolute configuration of two marine compounds using vibrational chiroptical spectroscopy. J. Org. Chem. 2012, 77, 858–886. [Google Scholar] [CrossRef] [PubMed]
- Trepos, R.; Cervin, G.; Hellio, C.; Pavia, H.; Stensen, W.; Stensvåg, K.; Svendsen, J.S.; Haug, T.; Svenson, J. Antifouling compounds from the sub-Arctic ascidian Synoicum pulmonaria: Synoxazolidinones A and C, pulmonarins A and B, and synthetic analogues. J. Nat. Prod. 2014, 77, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, M.; Svenson, J.; Sepčić, K.; Trembleau, L.; Engqvist, M.; Andersen, J.H.; Jaspars, M.; Stensvåg, K.; Haug, T. Isolation and synthesis of pulmonarins A and B, acetylcholinesterase inhibitors from the colonial ascidian Synoicum pulmonaria. J. Nat. Prod. 2014, 77, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouslimani, A.; Sanchez, L.M.; Garg, N.; Dorrestein, P.C. Mass spectrometry of natural products: Current, emerging and future technologies. Nat. Prod. Rep. 2014, 31, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Weissman, K.J. The structural biology of biosynthetic megaenzymes. Nat. Chem. Biol. 2015, 11, 660–670. [Google Scholar] [CrossRef] [PubMed]


| PHYLUM/Class | Species | Compounds | Bioactivity | Region | References |
|---|---|---|---|---|---|
| MICROORGANISMS | |||||
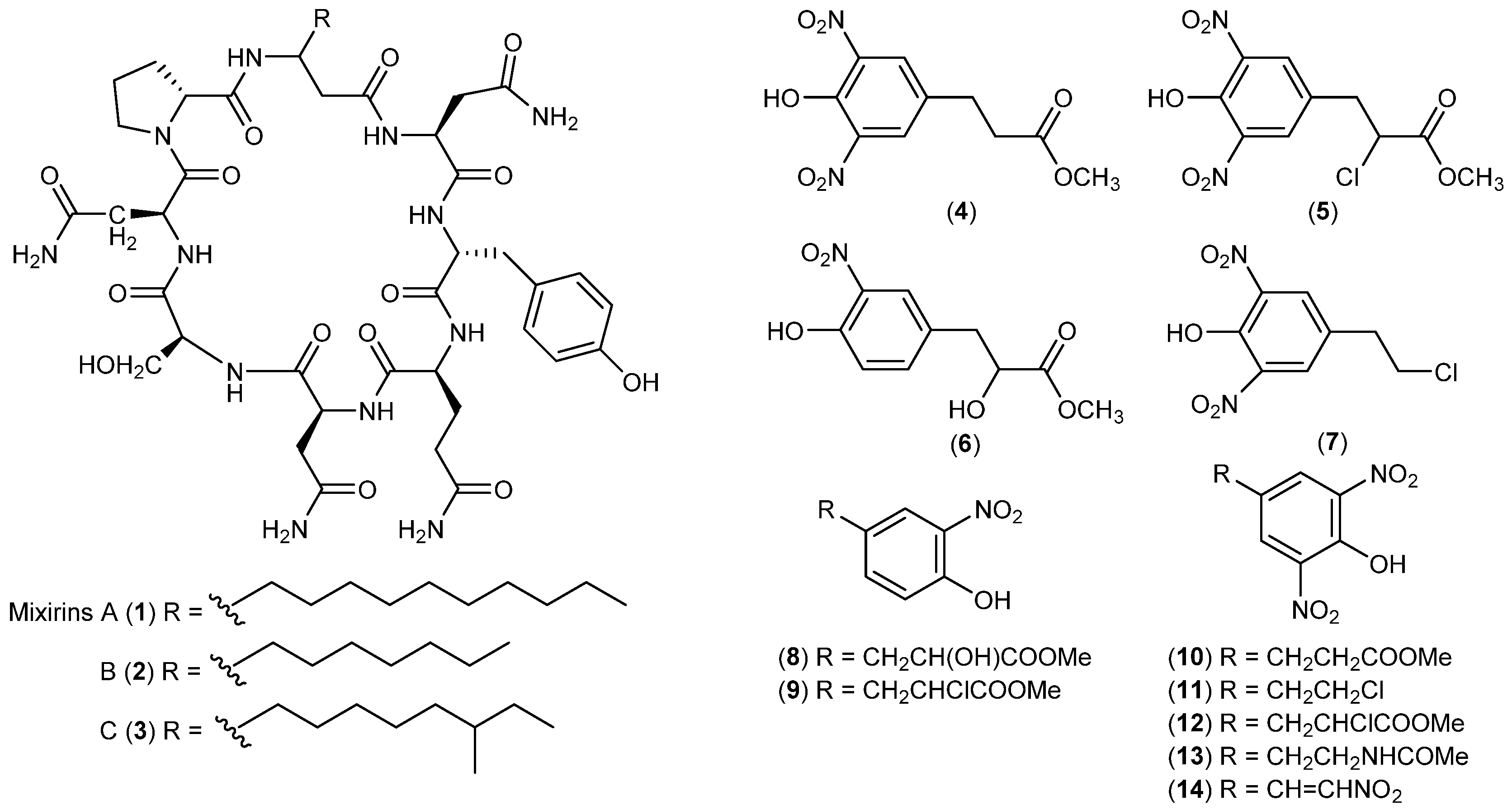
| Bacteria | Bacillus sp. | 1–3 | Cytotoxic | Arctic | [15] |
| Salegentibacter sp. | 4–14 | Antimicrobial and cytotoxic | Arctic | [16,17] | |
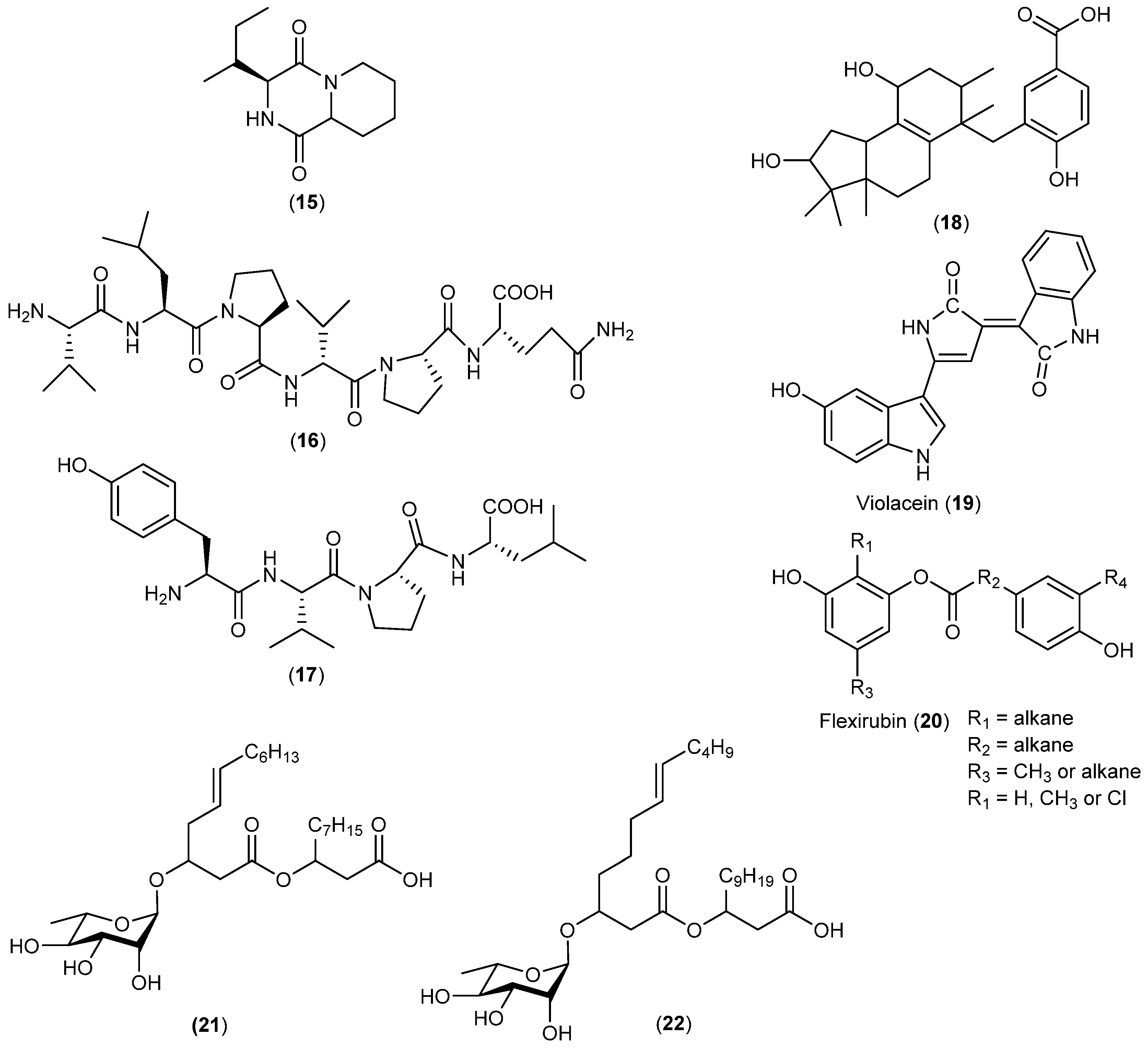
| Pseudoalteromonas haloplanktis | 15 16, 17 | Rradical scavenging | Antarctic | [18] | |
| Nostoc sp. | 18 | Antibacterial | Antarctic | [19] | |
| Janthinobacterium sp. | 19, 20 | Antimycobacterial | Antarctic | [20] | |
| Pseudomonas sp. | 21, 22 | Antibacterial | Antarctic | [21] | |
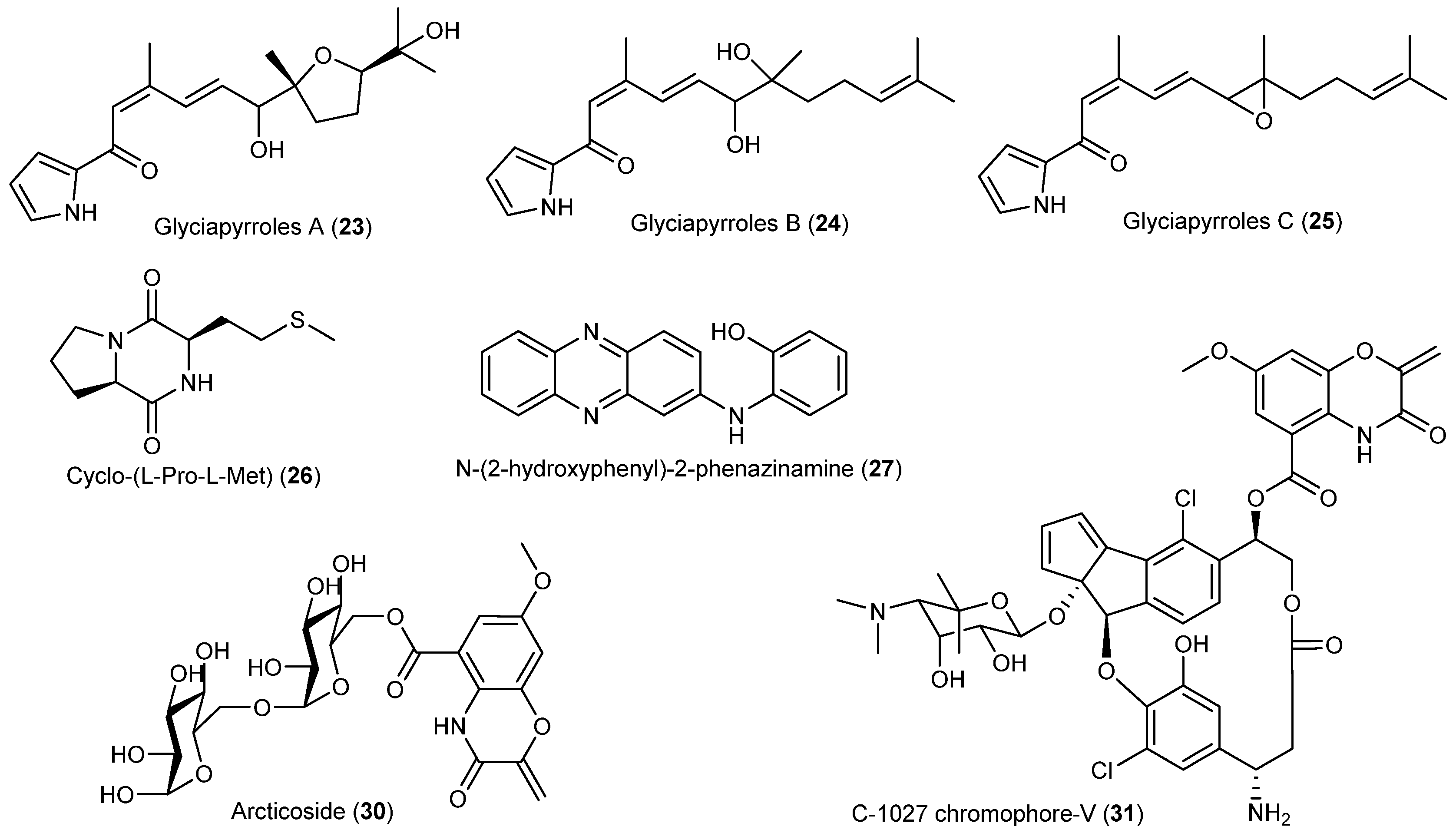
| Actinomyces | Streptomyces sp. | 23–25 | Arctic | [22] | |
| Nocardiopsis sp. | 26 | Anti-angiogenesis | Arctic | [23] | |
| Nocardia dassonvillei | 27 | Antifungal and cytotoxic | Arctic | [24] | |
| Streptomyces nitrosporeus | 28, 29 | Antiviral | Arctic | [25] | |
| Streptomyces sp. | 30 | Enzyme inhibitory | Arctic | [27] | |
| 31 | Enzyme inhibitory and cytotoxic | ||||
| Streptomyces griseus | 32 | Antibacterial | Antarctic | [28] | |
| Streptomyces griseus | 33 | Neuroprotective | Antarctic | [29] | |
| Microbispora aerata | 34 | Antiproliferative and cytotoxic | Antarctic | [30] | |
| Nocardiopsis sp. | 35, 36 | Antarctic | [31] | ||
| Fungi | Penicillium algidum | 37 | Anticancer | Arctic | [32] |
| Eutypella sp. | 38–40 | Cytotoxicity | Arctic | [33,34,35] | |
| 41 | Antibacterial | ||||
| 42 | Cytotoxicity | ||||
| 43–45 | Immunosuppression | ||||
| Lindgomycetaceae | 46, 47 | Antibacterial | Arctic | [36,37] | |
| Trichoderma polysporum | 48 | Antifungal | Arctic | [38] | |
| Geomyces sp. | 49, 50 | Antarctic | [39] | ||
| 51–53 | Antifungal and antibacterial | ||||
| Trichoderma asperellum | 54–59 | Antifungal | Antarctic | [40] | |
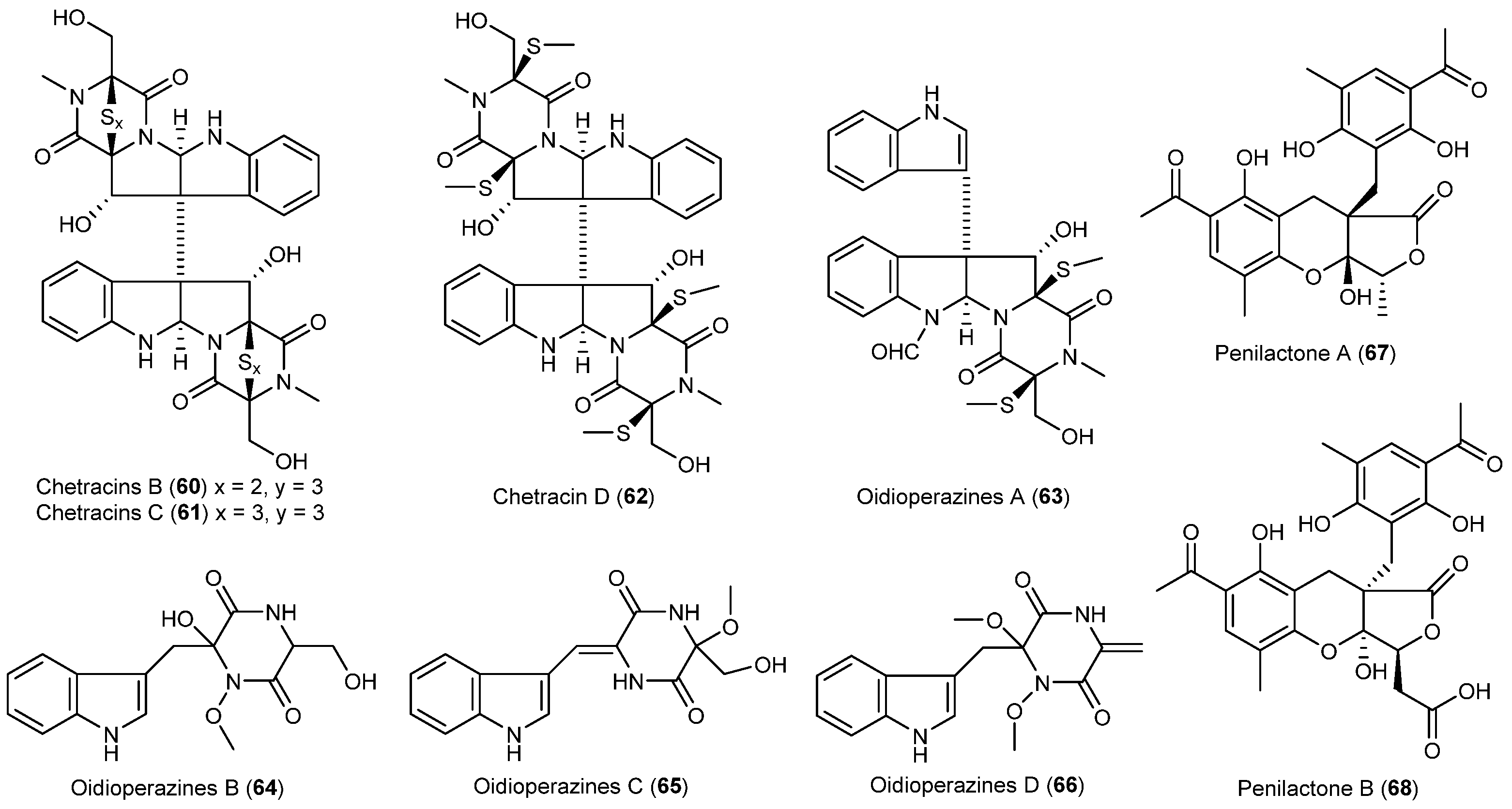
| Oidiodendron truncatum | 60–62 | Cytotoxic | Antarctic | [42] | |
| 63–66 | |||||
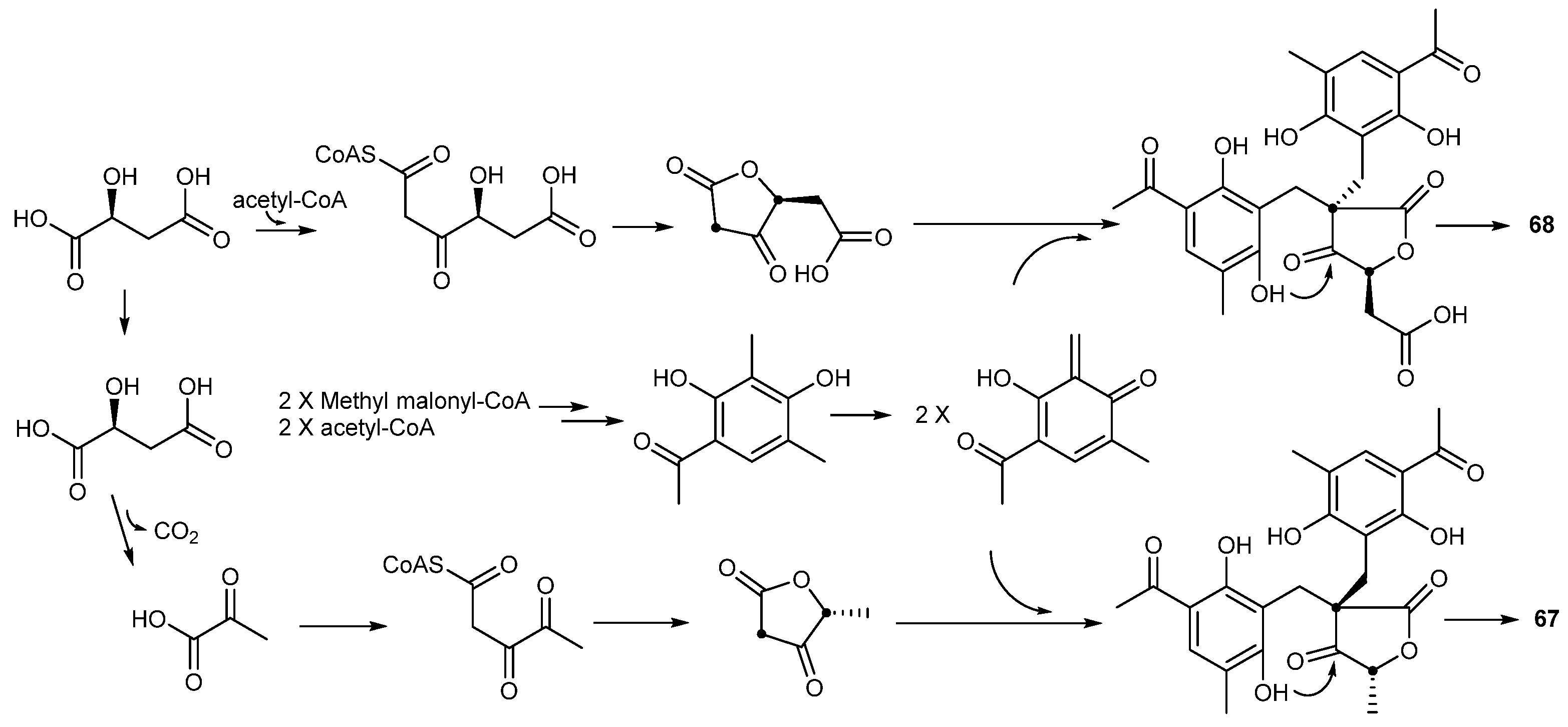
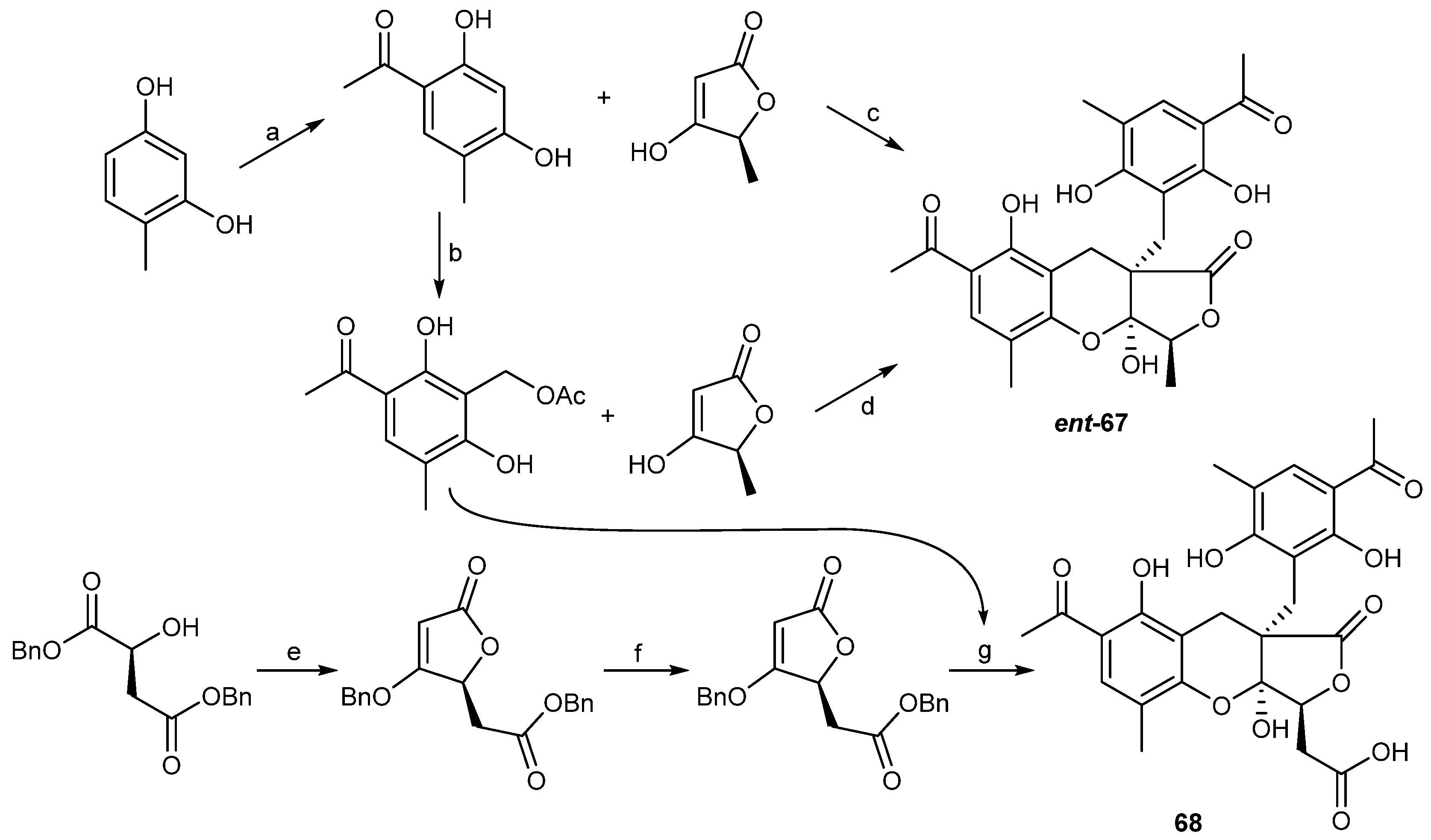
| Penicillium crustosu m | 67, 68 | NF-κB inhibitory | Antarctic | [43] | |
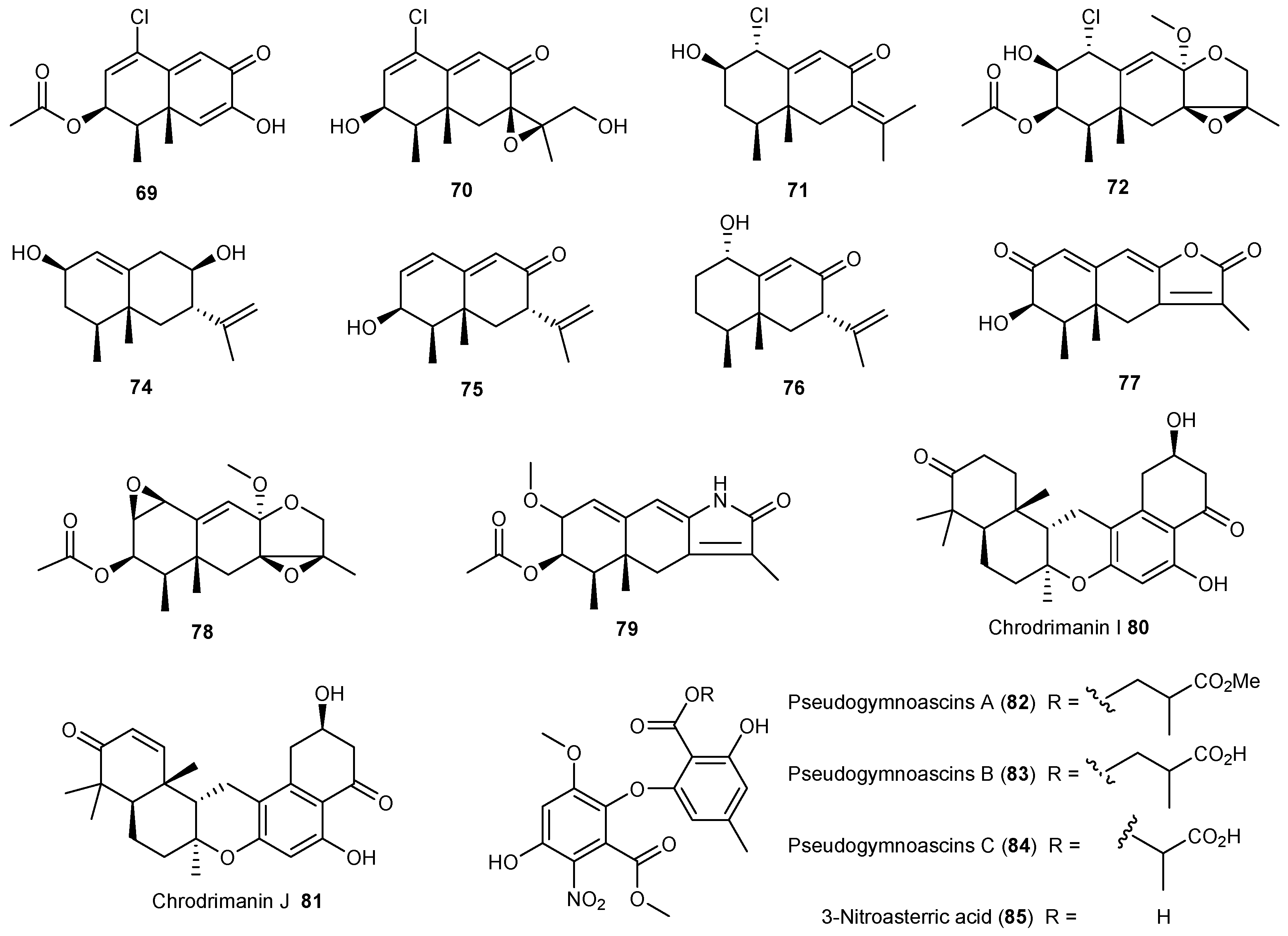
| Penicillium sp. | 69 | Moderate cytotoxic | Antarctic | [45,46] | |
| 70–78 | |||||
| 79 | Cytotoxic | ||||
| Penicillium funiculosum | 80, 81 | Antarctic | [47] | ||
| Pseudogymnoascus sp. | 82–85 | Antarctic | [48] | ||
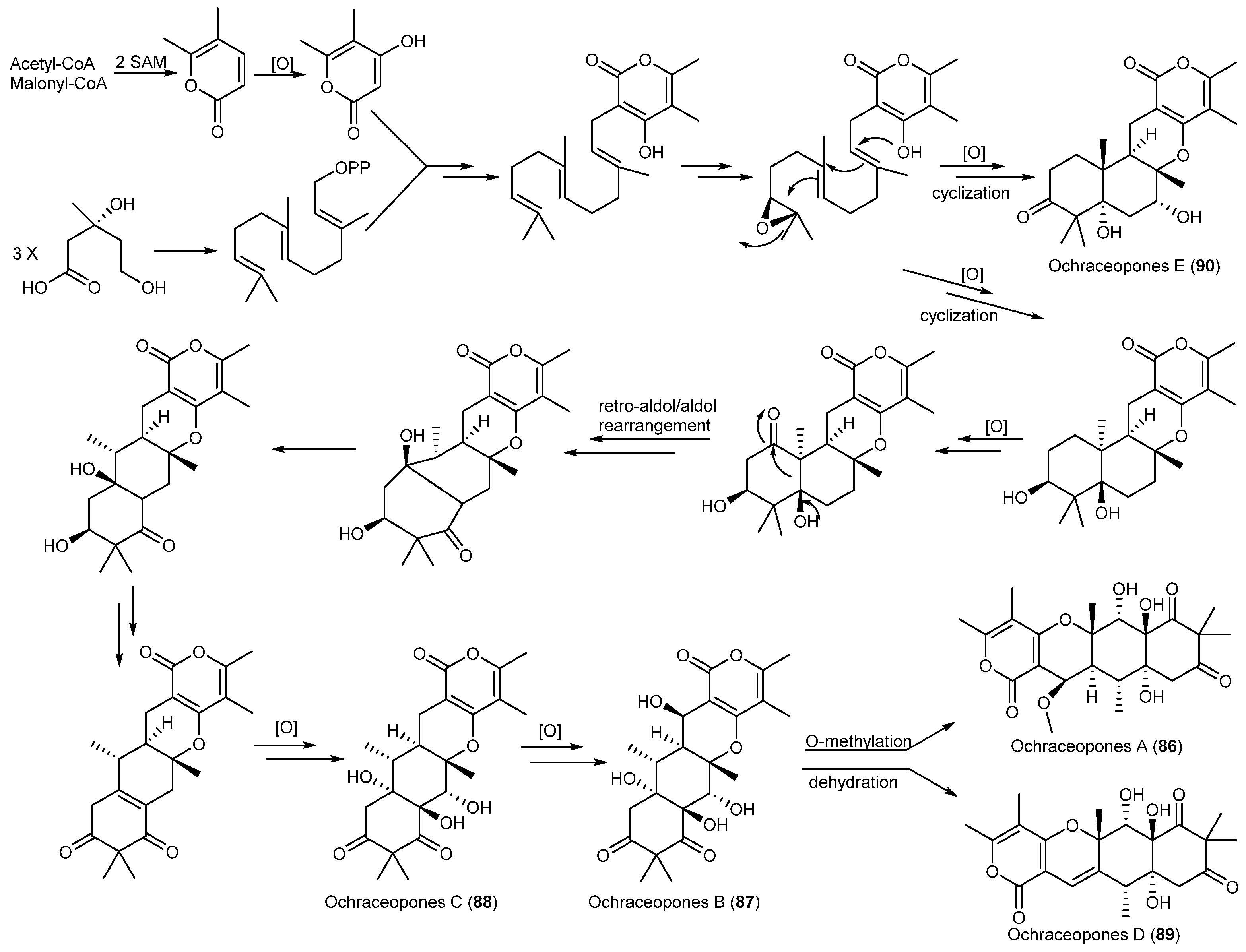
| Aspergillus ochraceopetaliformis | 86–90 | Antiviral | Antarctic | [49] | |
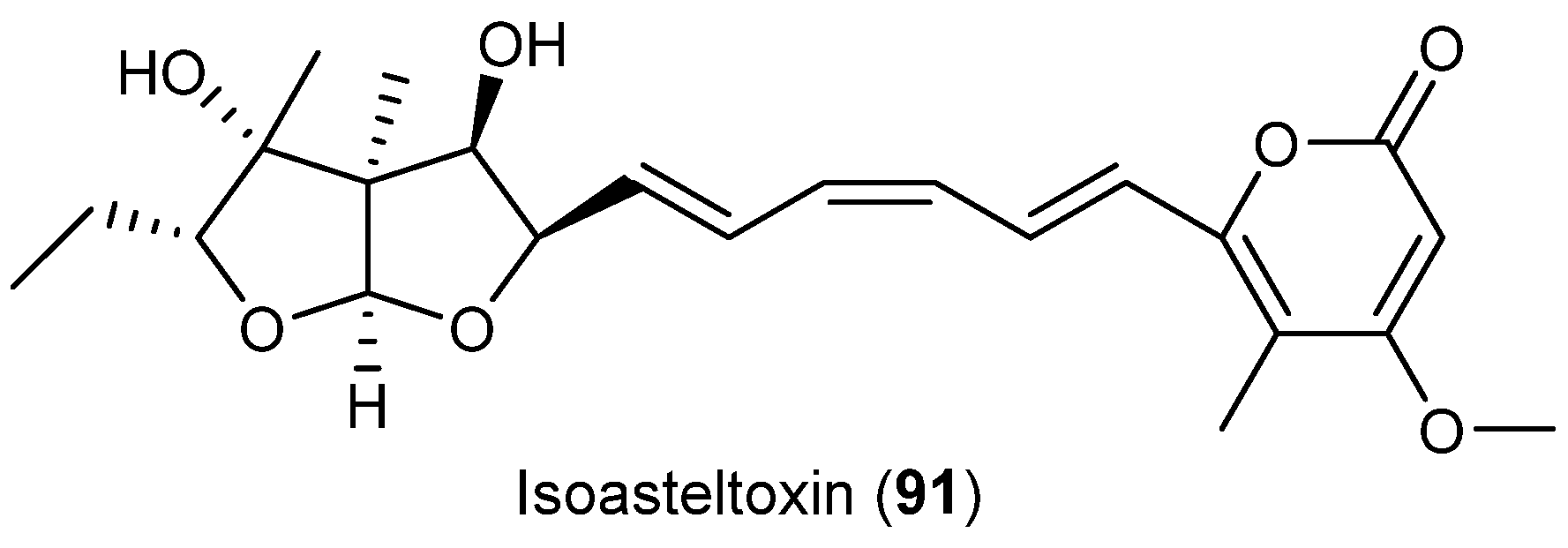
| 91 | |||||
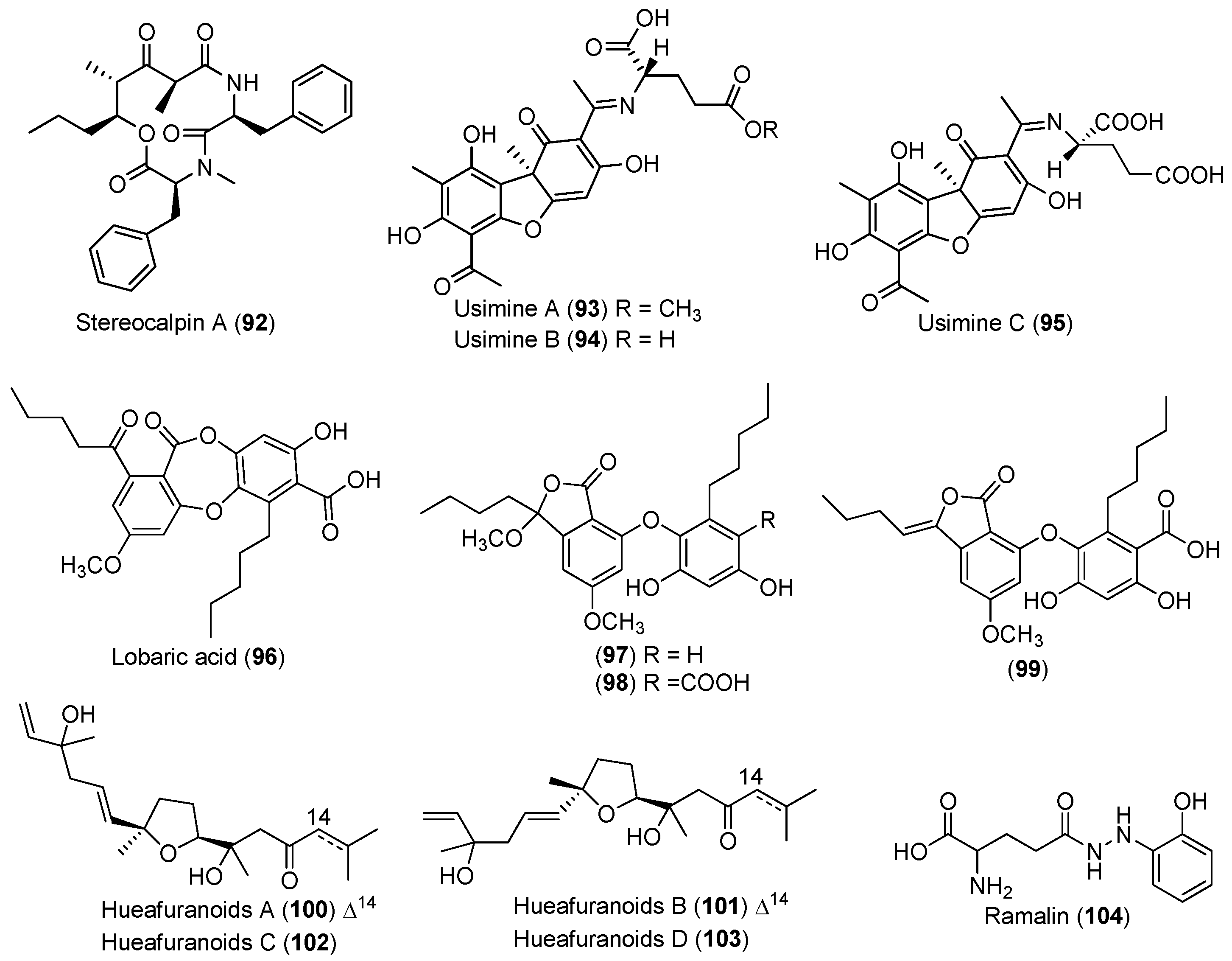
| LICHEN | Stereocaulon alpinum | 92 | Cytotoxic and enzyme inhibitory | Antarctic | [55,56,57,58,59] |
| 93–98 | Enzyme inhibitory | ||||
| 99 | Antioxidant and antibacterial | ||||
| Huea sp. | 100–103 | Enzyme inhibitory | Antarctic | [60] | |
| Ramalina terebrata | 104 | Antioxidant | Antarctic | [61] | |
| MOSS | Polytrichastrum alpinum | 105, 106 | Enzyme inhibitory | Antarctic | [62] |
| BRYOZOANS | Tegella cf. spitzbergensis | 107–110 | Antibacterial | Arctic | [64] |
| CNIDARIANS | Ainigmaptilon antarcticus | 111, 112 | Predation inhibitory | Antarctic | [66] |
| Dasystenella acanthina | 113 | Ichthyotoxic | Antarctic | [67] | |
| Anthomastus bathyproctus | 114–120 | Weak cytotoxic | Antarctic | [68] | |
| Alcyonium grandis | 121–129 | Antarctic | [69] | ||
| Undescribed octocoral | 130, 131 | Antiparasitic | Antarctic | [76] | |
| Thuiria breitfussi | 132, 133 | Arctic | [77] | ||
| Gersemia fruticosa | 134–136 | Arctic | [80] | ||
| 137 | Antibacterial | ||||
| ECHINODERMS | Staurocucumis liouvillei | 138, 139 | Antiviral | Antarctic | [81] |
| Staurocucumis turqueti | 140 | Antarctic | [82] | ||
| Achlionice violaecuspidata | 141–143 | Antarctic | [83] | ||
| MOLLUSCS | Austrodoris kerguelenensis | 144–147 | Antarctic | [84,85] | |
| Austrodoris kerguelenensis | 148–166 | Erythroleukemia inhibitory | Antarctic | [89] | |
| Charcotia granulosa | 167 | Antarctic | [91] | ||
| SPONGES | Haliclona viscosa | 168–173 | Arctic | [93,94,95,96] | |
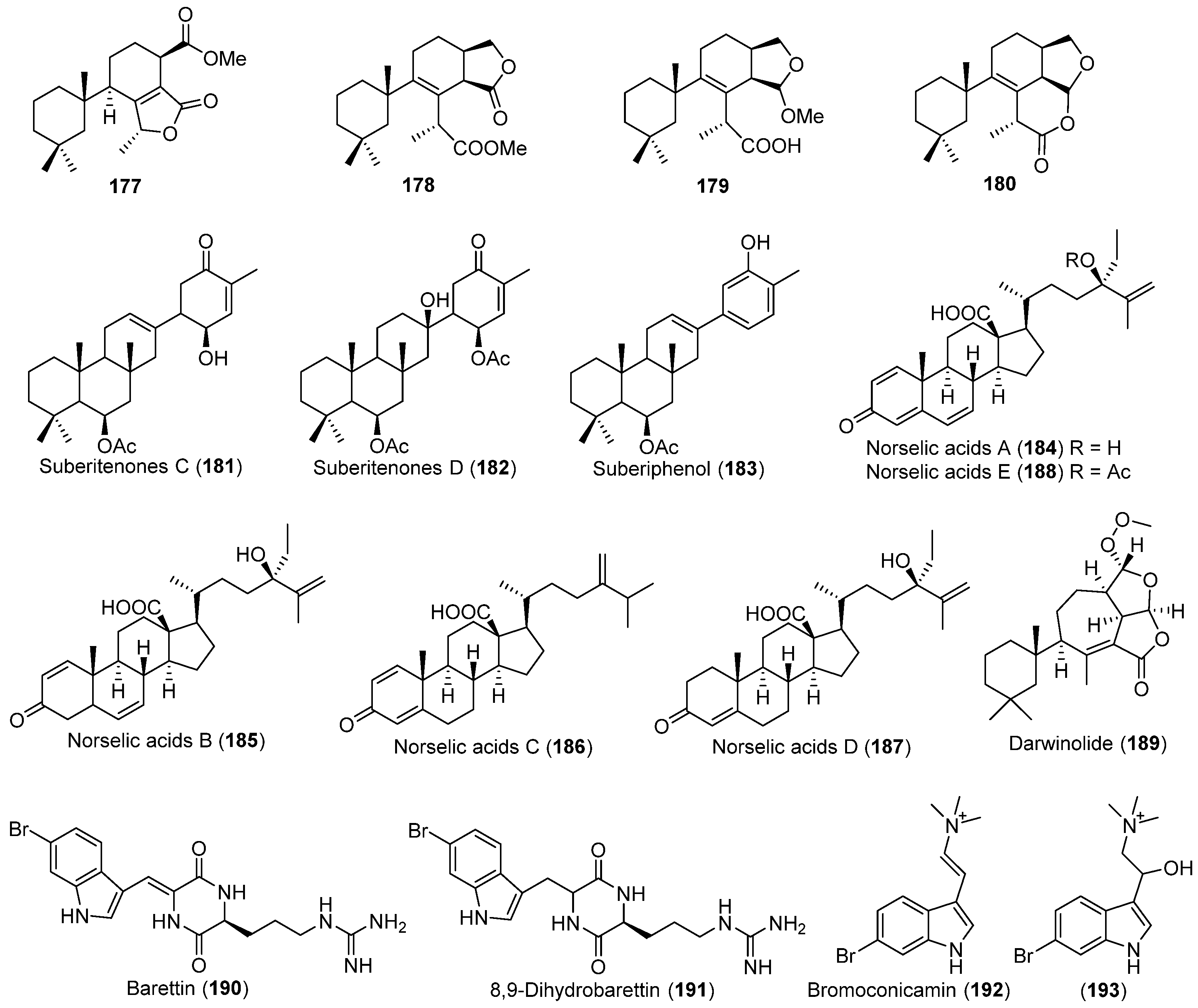
| Suberites caminatus | 174–176 | Antarctic | [99,100] | ||
| Dendrilla membranosa | 177–180 | Antarctic | [101] | ||
| Suberites sp. | 181–183 | Antarctic | [102] | ||
| Crella sp. | 184–188 | Antibacterial and antifungal | Antarctic | [103] | |
| Dendrilla membranosa | 189 | Antibacterial | Antarctic | 104] | |
| Geodia barretti | 190 | Enzyme inhibitory | Arctic | [105] | |
| 191–193 | |||||
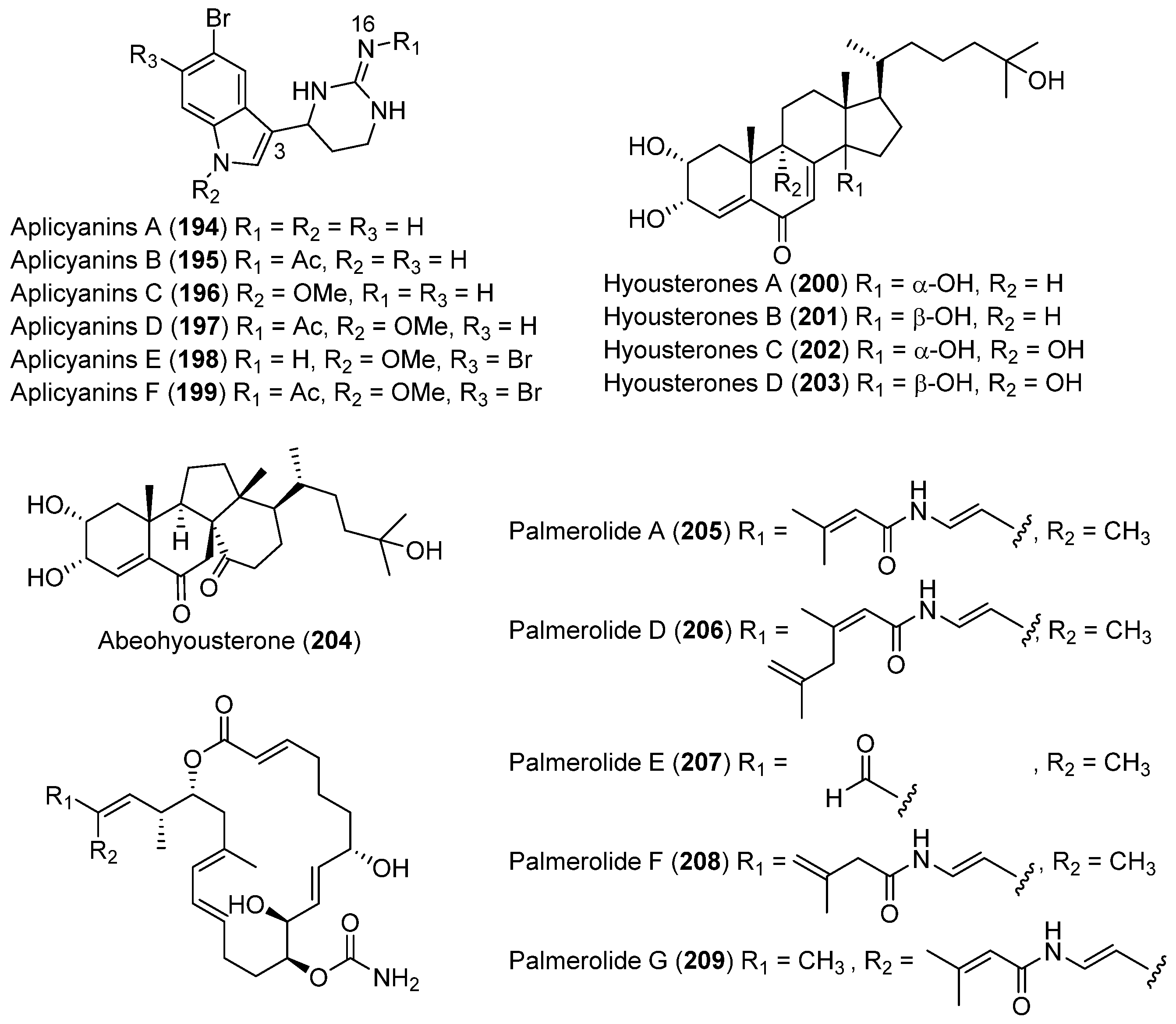
| TUNICATES | Aplidium cyaneum | 194–199 | Antimitotic | Antarctic | [109] |
| Synoicum adareanum | 200–203 | Moderate cytotoxic | Antarctic | [110,111,112] | |
| 204 | |||||
| 205–209 | Cytotoxic and enzyme inhibitory | ||||
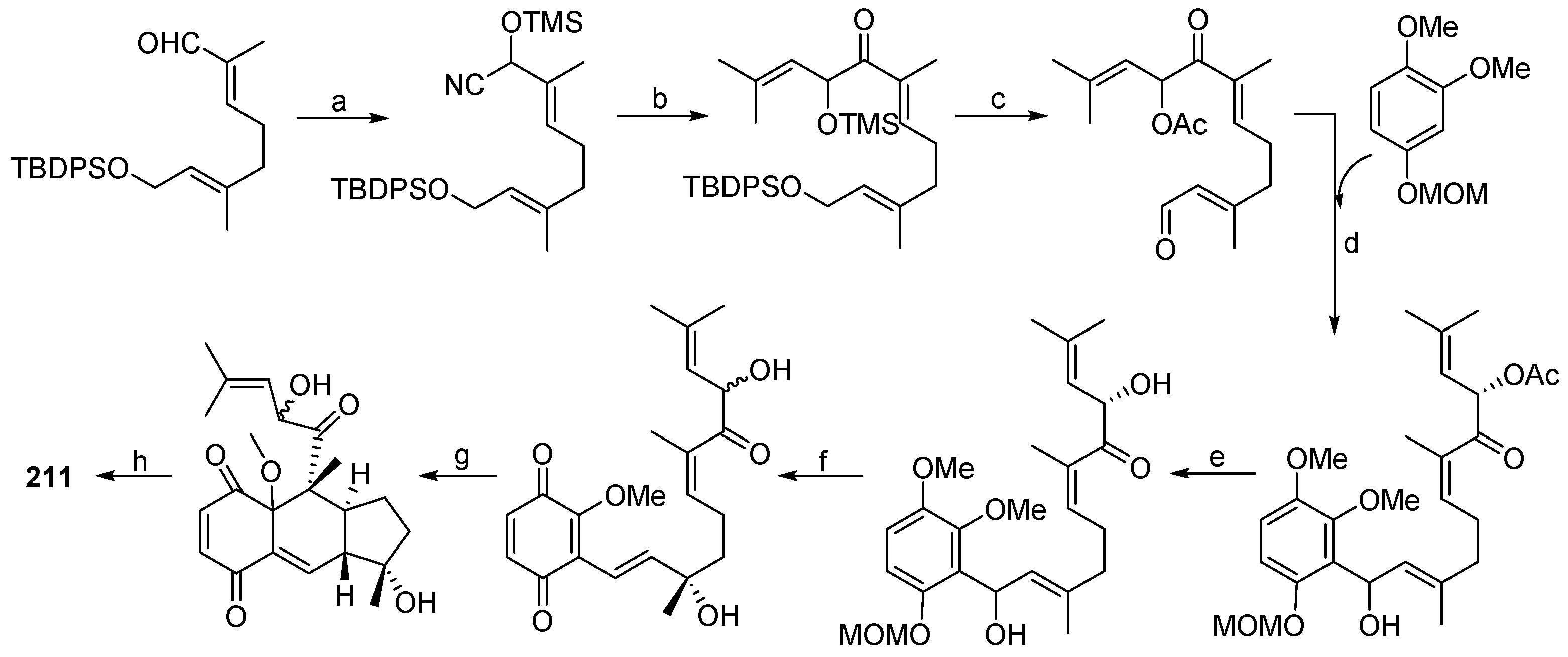
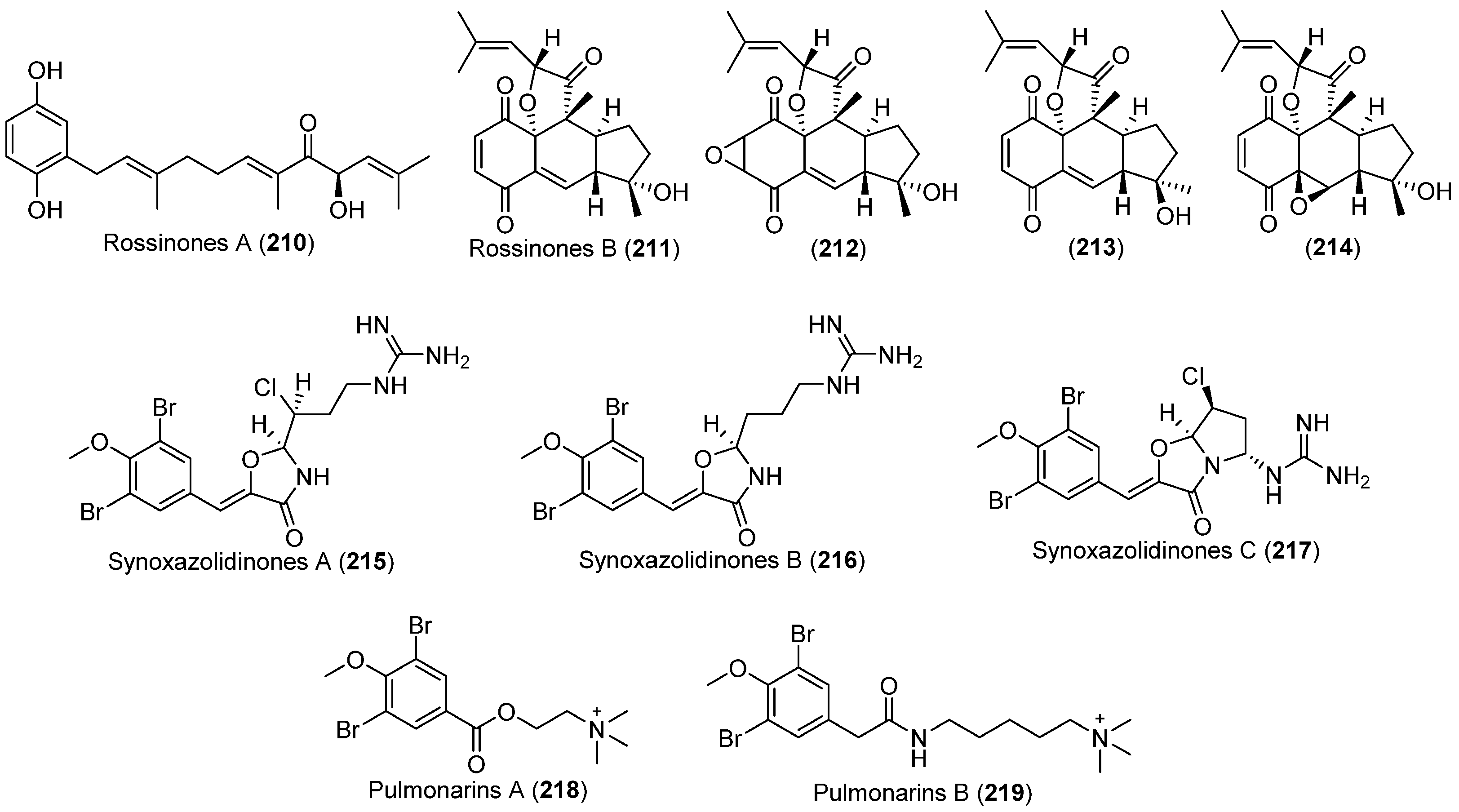
| Aplidium sp. | 210, 211 | Antiinflammatory, antiviral and antiproliferative | Antarctic | [137] | |
| Aplidium fuegiense | 212–214 | Antarctic | [140] | ||
| Synoicum pulmonaria | 215–217 | Antibacterial and antifungal | Arctic | [141,142,143,144,145] | |
| 218, 219 | Enzyme inhibitory and antibacterial |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Li, Y.-L.; Zhao, F.-C. Secondary Metabolites from Polar Organisms. Mar. Drugs 2017, 15, 28. https://doi.org/10.3390/md15030028
Tian Y, Li Y-L, Zhao F-C. Secondary Metabolites from Polar Organisms. Marine Drugs. 2017; 15(3):28. https://doi.org/10.3390/md15030028
Chicago/Turabian StyleTian, Yuan, Yan-Ling Li, and Feng-Chun Zhao. 2017. "Secondary Metabolites from Polar Organisms" Marine Drugs 15, no. 3: 28. https://doi.org/10.3390/md15030028