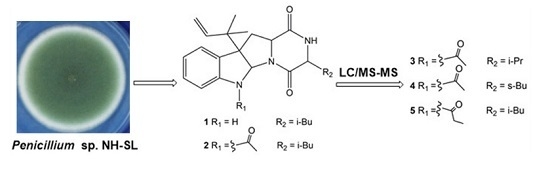
Mass Spectrometric Characteristics of Prenylated Indole Derivatives from Marine-Derived Penicillium sp. NH-SL
Abstract
:1. Introduction
2. Results and Discussion
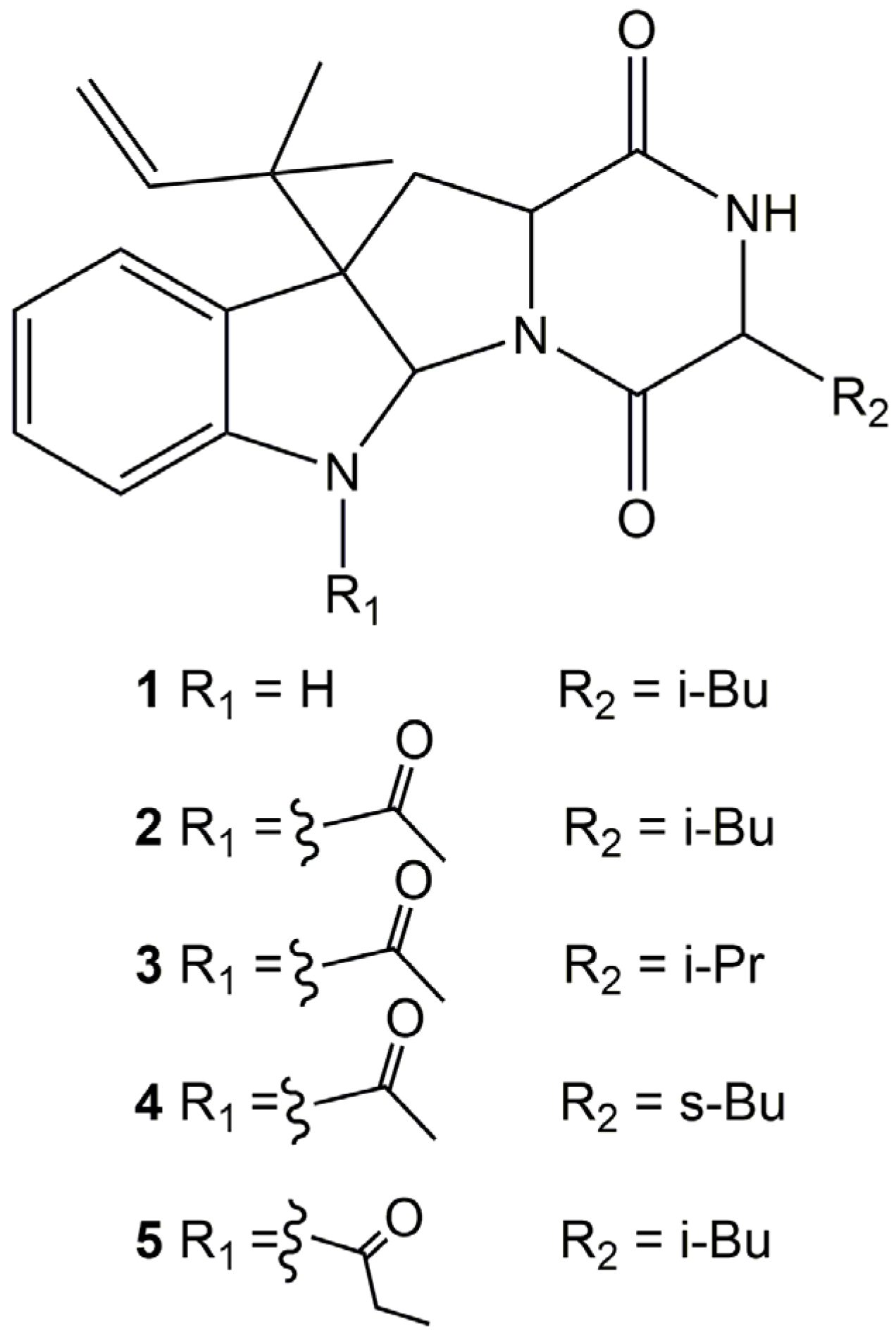
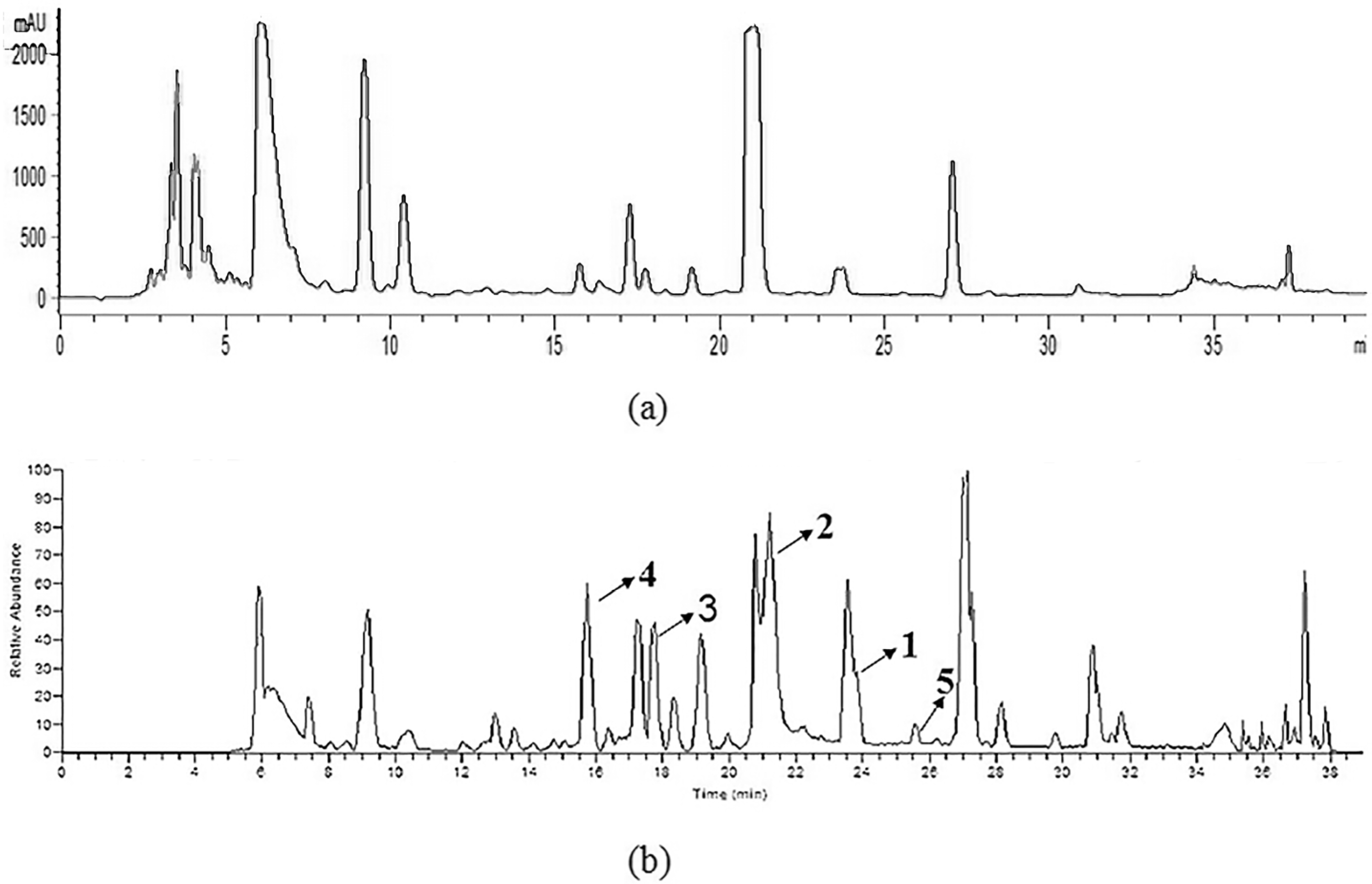
2.1. Identification of Prenylated Indole Alkaloids
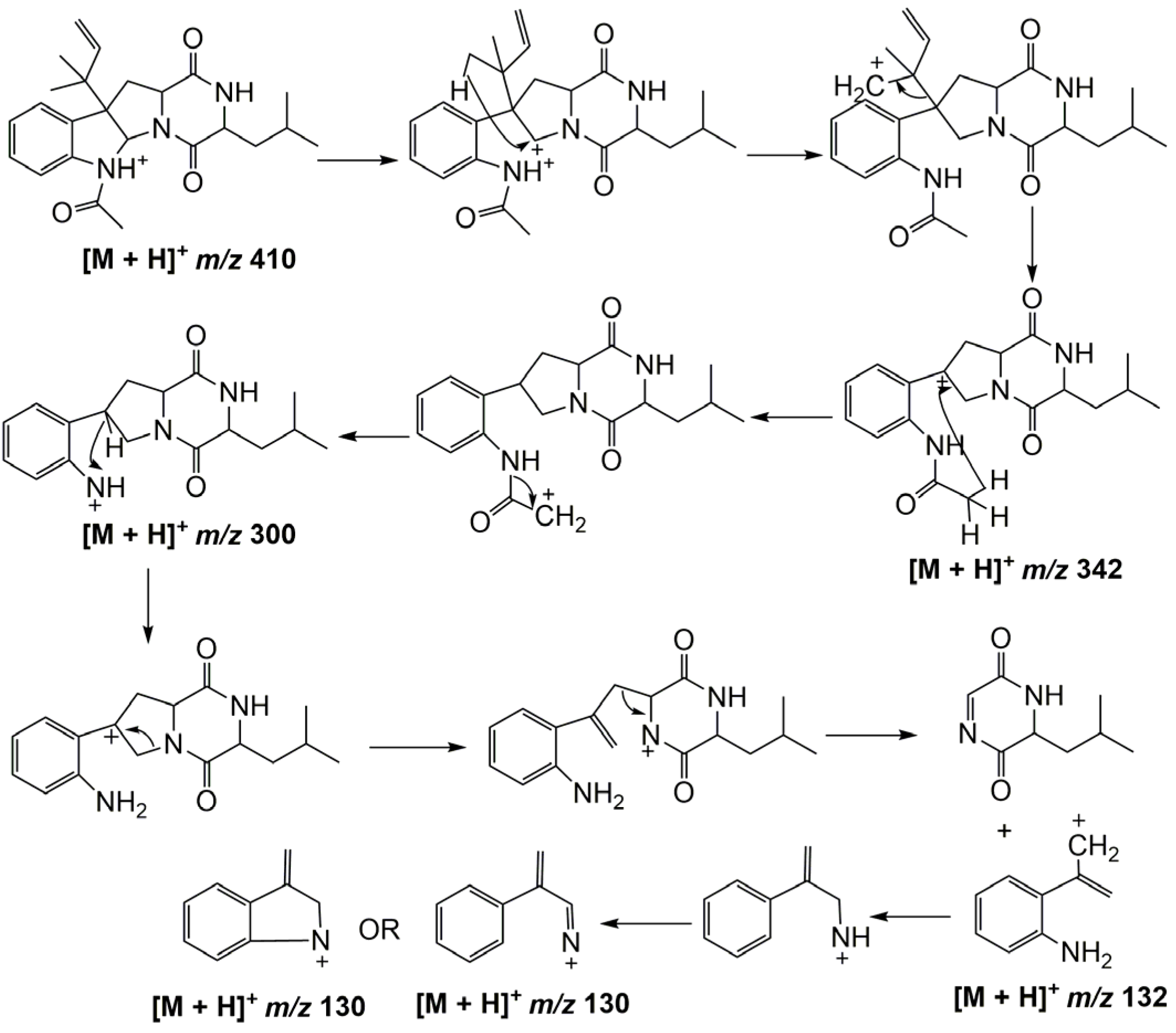
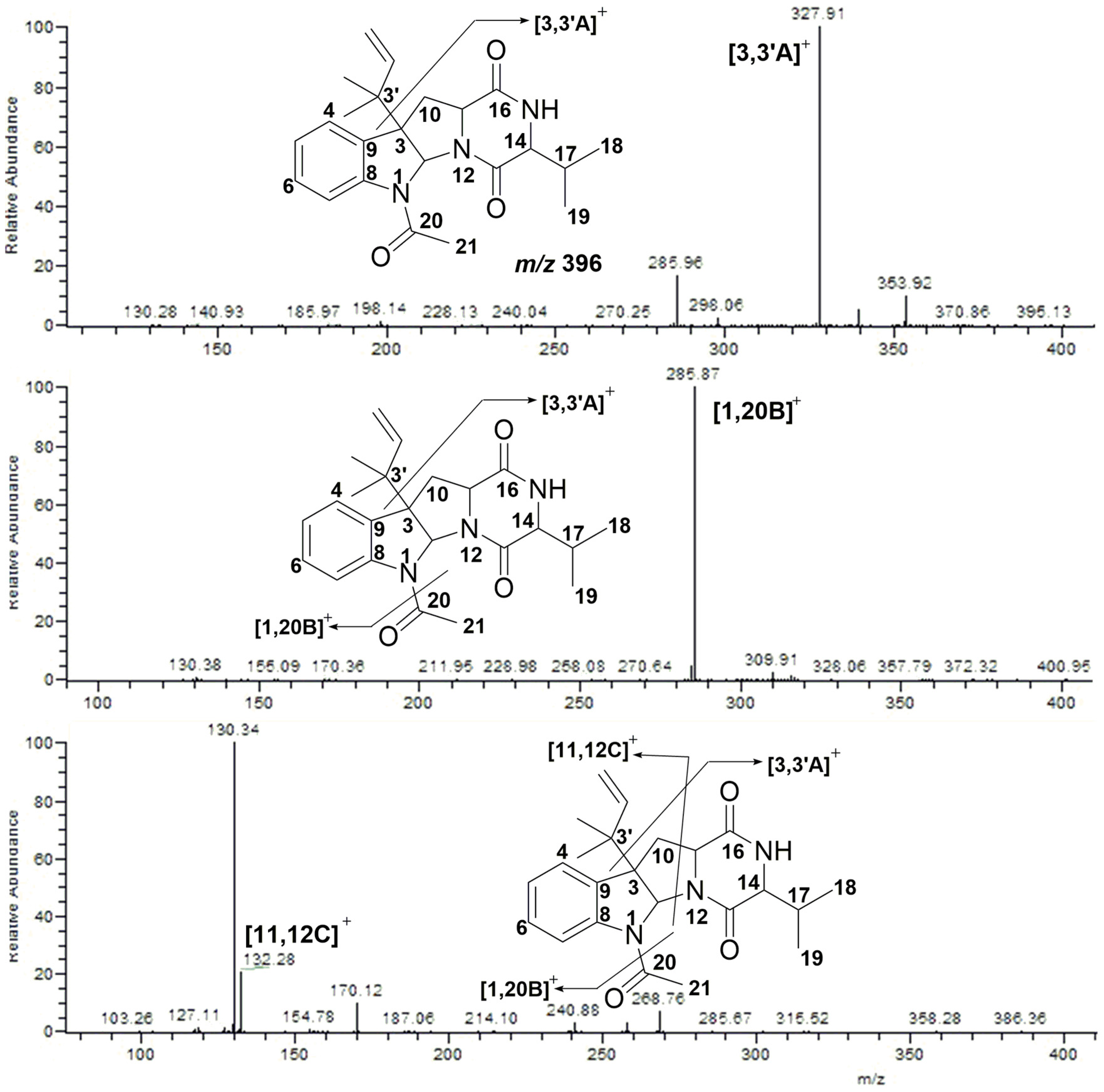
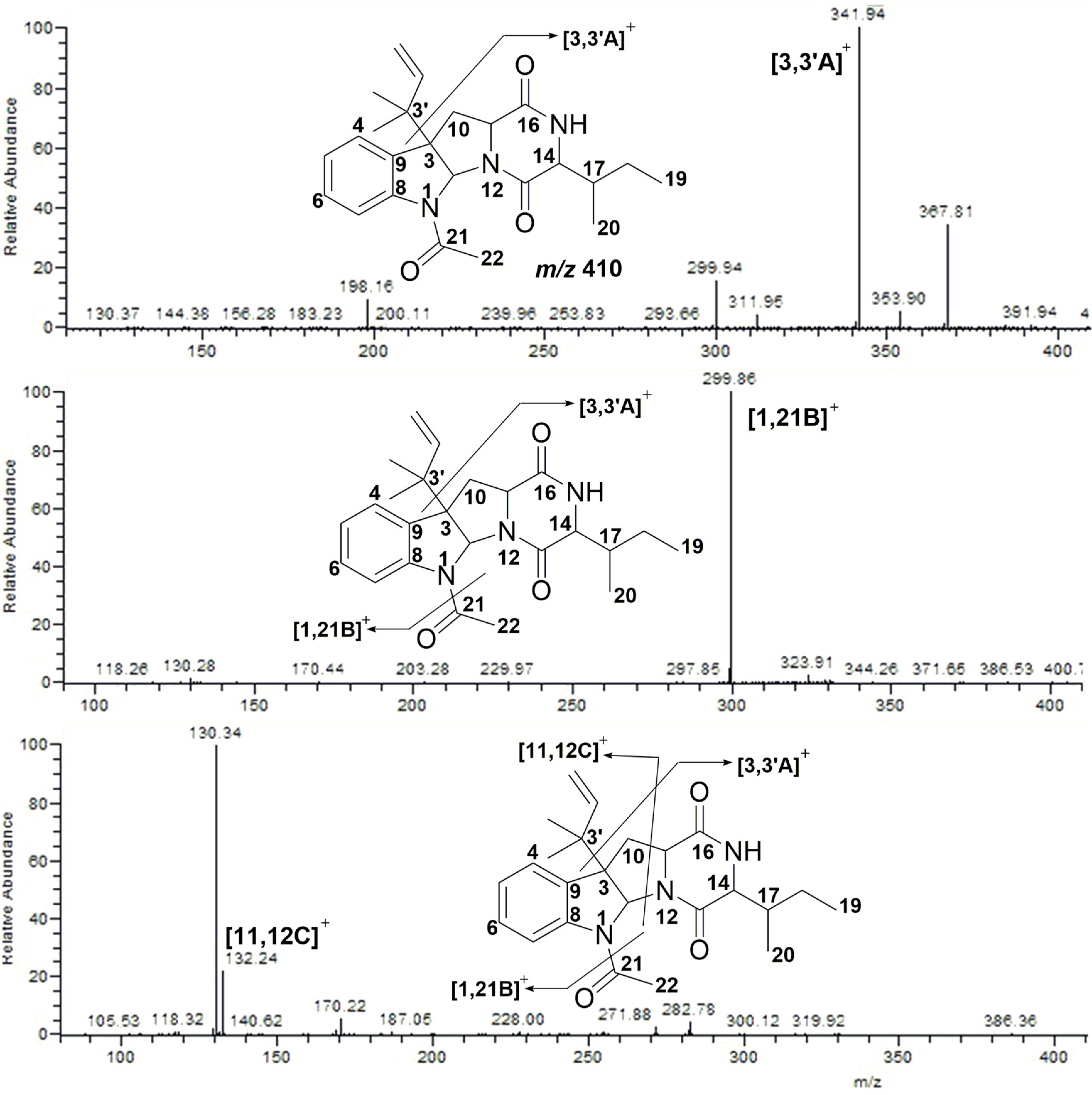
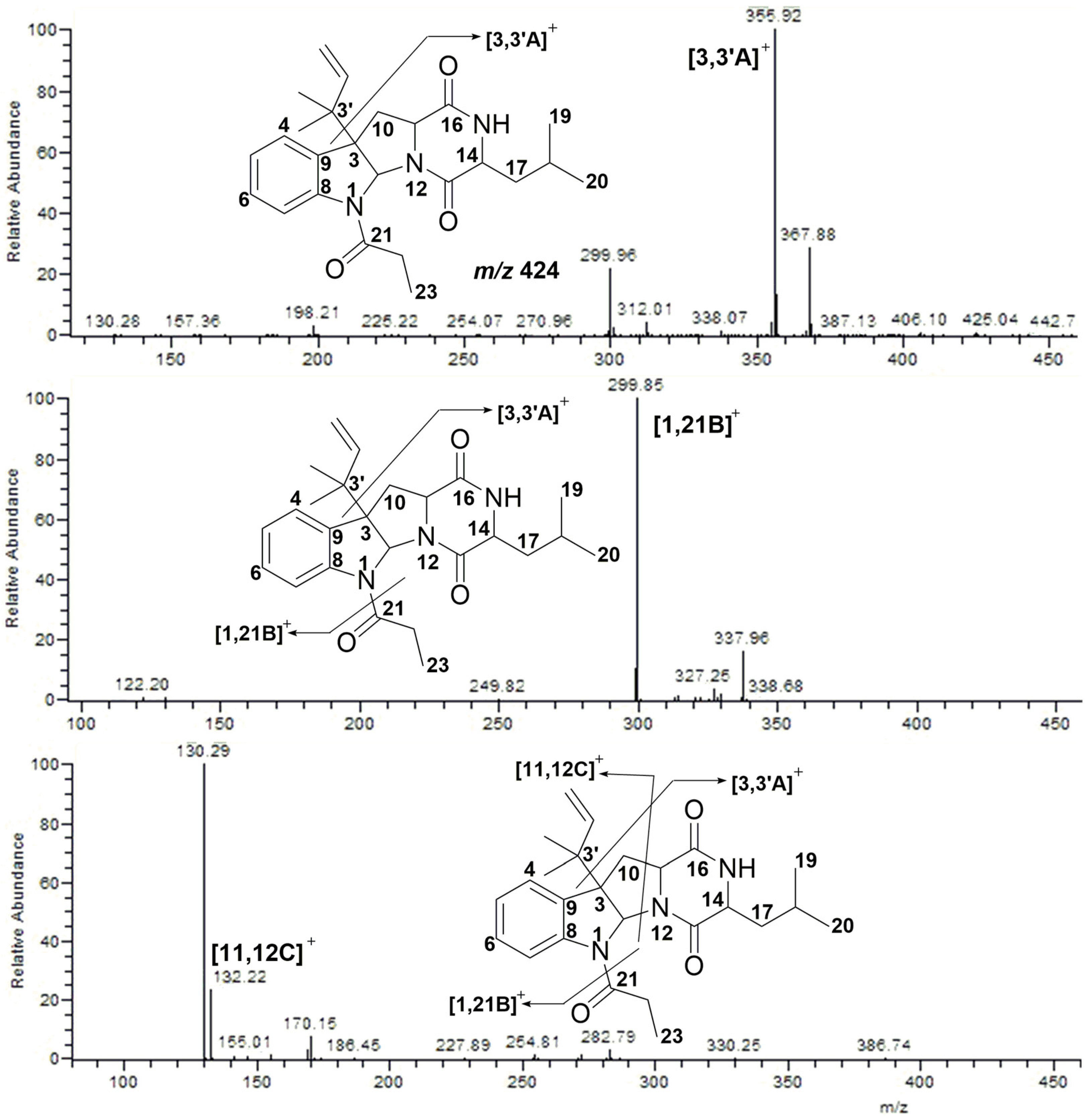
2.2. MS/MS Analysis of Compounds 1–5
2.3. Cytotoxicity Assay
3. Experimental Section
3.1. Fungus Material
3.2. Growing Biomass and Crude Extracts Preparation
3.3. Fractions Isolation
3.4. LC-MS/MS Analysis
3.5. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Giddings, L.-A. Natural products as leads to antitumor drugs. Phytochem. Rev. 2014, 13, 123–137. [Google Scholar] [CrossRef]
- Nagia, M.M.; El-Metwally, M.M.; Shaaban, M.; El-Zalabani, S.M.; Hanna, A.G. Four butyrolactones and diverse bioactive secondary metabolites from terrestrial Aspergillus flavipes MM2: Isolation and structure determination. Org. Med. Chem. Lett. 2012, 1, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.G.; Liu, Q.; Zhu, G.L.; Liu, H.S.; Zhu, W.M. Marine natural products sourced from marine-derived Penicillium fungi. J. Asian Nat. Prod. Res. 2016, 18, 92–115. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2010, 27, 165–237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Y.; Zhu, T.H.; Zhu, W.M. New marine natural products of microbial origin from 2010 to 2013. Chin. J. Org. Chem. 2013, 33, 1195–1234. [Google Scholar] [CrossRef]
- Wang, M.H.; Li, X.M.; Li, C.S.; Ji, N.Y.; Wang, B.G. Secondary Metabolites from Penicillium pinophilum SD-272, a marine sediment-derived fungus. Mar. Drugs 2013, 11, 2230–2238. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M. Prenylated indole derivatives from fungi: Structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat. Prod. Rep. 2010, 27, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, K.; Kawasaki, T. Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Nat. Prod. Rep. 2007, 24, 843–868. [Google Scholar] [CrossRef] [PubMed]
- An, C.Y.; Li, X.M.; Li, C.S.; Xu, G.M.; Wang, B.G. Prenylated indolediketopiperazine peroxides and related homologues from the marine sediment-derived fungus Penicillium brefeldianum SD-273. Mar. Drugs 2014, 12, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.F.; Turk, J.; Shi, Y.; Groisman, E.A. Characterization of acylphosphatidylglycerols from Salmonella typhimurium by tandem mass spectrometry with electrospray ionization. J. Am. Soc. Mass Spectrom. 2004, 15. [Google Scholar] [CrossRef] [PubMed]
- Prasain, J.K.; Ueki, M.; Stefanowicz, P.; Osada, H. Rapid screening and identification of cytochalasins by electrospray tandem mass spectrometry. J. Mass Spectrom. 2002, 37, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Brombacher, S.; Edmonds, S.; Volmer, D.A. Studies on azaspiracid biotoxins. II. Mass spectral behavior and structural elucidation of azaspiracid analogs. Rapid Commun. Mass Spectrom. 2002, 16, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Pergantis, S.A.; Wangkarn, S.; Francesconi, K.A.; Thomas-Oates, J.E. Identification of arsenosugars at the picogram level using nanoelectrospray quadrupole time-of-flight mass spectrometry. Anal. Chem. 2000, 72, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Stefanowicz, P.; Prasain, J.K.; Yeboah, K.F.; Konishi, Y. Detection and partial structure elucidation of basic taxoids from Taxus wallichiana by electrospray ionization tandem mass spectrometry. Anal. Chem. 2001, 73, 3583–3589. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Hao, C.; Sun, W.; Liu, Z.; Liu, S. Rapid analysis of steroidal saponin mixture using electrospray ionization mass spectrometry combined with sequential tandem mass spectrometry. Rapid Commun. Mass Spectrom. 1998, 12, 589–594. [Google Scholar] [CrossRef]
- Fang, S.; Hao, C.; Liu, Z.; Song, F.; Liu, S. Application of electrospray ionization mass spectrometry combined with sequential tandem mass spectrometry techniques for the profiling of steroidal saponin mixture extracted from Tribulus terrestris. Planta Med. 1999, 65, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Sprogoee, K.; Manniche, S.; Larsen, T.O.; Christophersen, C. Janoxepin and brevicompanine B: Antiplasmodial metabolites from the fungus Aspergillus janus. Tetrahedron 2005, 61, 8718–8721. [Google Scholar] [CrossRef]
- Arai, K.; Kimura, K.; Mshiroda, T.; Yamamoto, Y. Structures of Fructigenines A and B, new alkaloids isolated from Penicillium fructigenum Takeuchi. Chem. Pharm. Bull. 1989, 37, 2937–2939. [Google Scholar] [CrossRef]
- Yin, W.B.; Xie, X.L.; Matuschek, M.; Li, S.M. Reconstruction of pyrrolo[2,3-b]indoles carrying an α-configured reverse C3-dimethylallyl moiety by using recombinant enzymes. Org. Biomol. Chem. 2010, 8, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yang, X.; Zhu, T.; Wang, F.; Xiao, X.; Park, H.; Gu, Q. Diketopiperazine alkaloids from a deep ocean sediment derived fungus Penicillium sp. Chem. Pharm. Bull. 2009, 57, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Miyako, K.; Go, S.; Hiroyuki, K.; Jun, U.; Masao, C.; Shozo, F.; Tsuyoshi, K.; Yasuo, K. Brevicompanines A and B: New plant growth regulators produced by the fungus, Penicillium brevicompactum. J. Chem. Soc. Perkin Trans. 1998, 17, 2823–2826. [Google Scholar]
- Pang, S.Q.; Wang, G.Q.; Lin, J.S.; Diao, Y.; Xu, R.A. Cytotoxic activity of the alkaloids from Broussonetia papyrifera fruits. Pharm. Biol. 2014, 52, 1315–1319. [Google Scholar] [CrossRef] [PubMed]

| Compounds | Inhibition Rates against Hepa Lclc7 Cells | ||
|---|---|---|---|
| 20 µg/mL | 20 nM c | 700 nM c | |
| crude extracts | 76% | - | - |
| 1 | - | <10% | - |
| 2 | - | <10% | 85% |
| cisplatin b | IC50 = 10.7 nM | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Ding, W.; Ma, Z. Mass Spectrometric Characteristics of Prenylated Indole Derivatives from Marine-Derived Penicillium sp. NH-SL. Mar. Drugs 2017, 15, 86. https://doi.org/10.3390/md15030086
Ding H, Ding W, Ma Z. Mass Spectrometric Characteristics of Prenylated Indole Derivatives from Marine-Derived Penicillium sp. NH-SL. Marine Drugs. 2017; 15(3):86. https://doi.org/10.3390/md15030086
Chicago/Turabian StyleDing, Hui, Wanjing Ding, and Zhongjun Ma. 2017. "Mass Spectrometric Characteristics of Prenylated Indole Derivatives from Marine-Derived Penicillium sp. NH-SL" Marine Drugs 15, no. 3: 86. https://doi.org/10.3390/md15030086