Transcriptomic Profiling of the Allorecognition Response to Grafting in the Demosponge Amphimedon queenslandica
Abstract
:1. Introduction
2. Results
2.1. Physiological Responses to Grafting
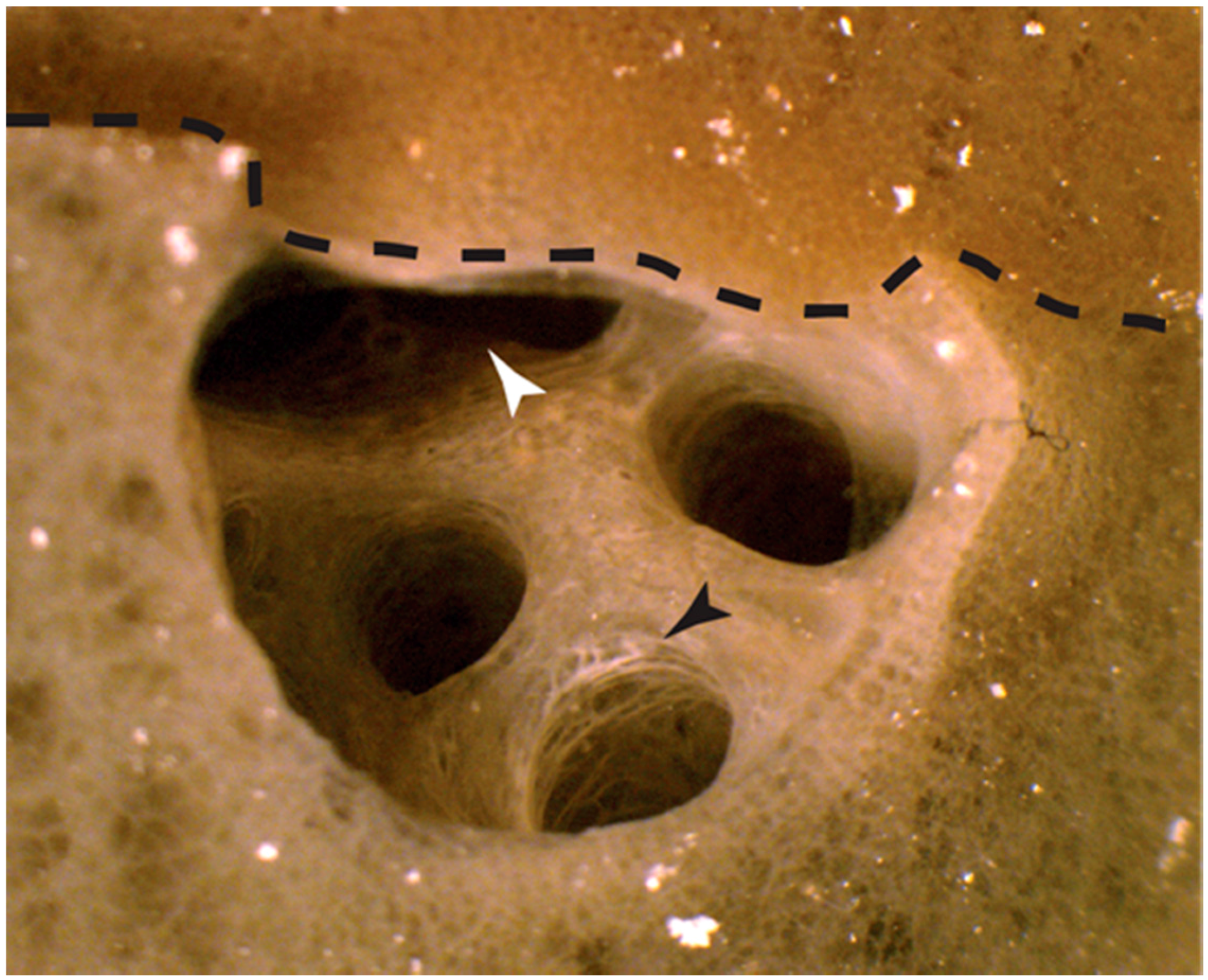
2.1.1. Autografts
2.1.2. Allografts
2.2. Transcriptome Sequencing and Statistics
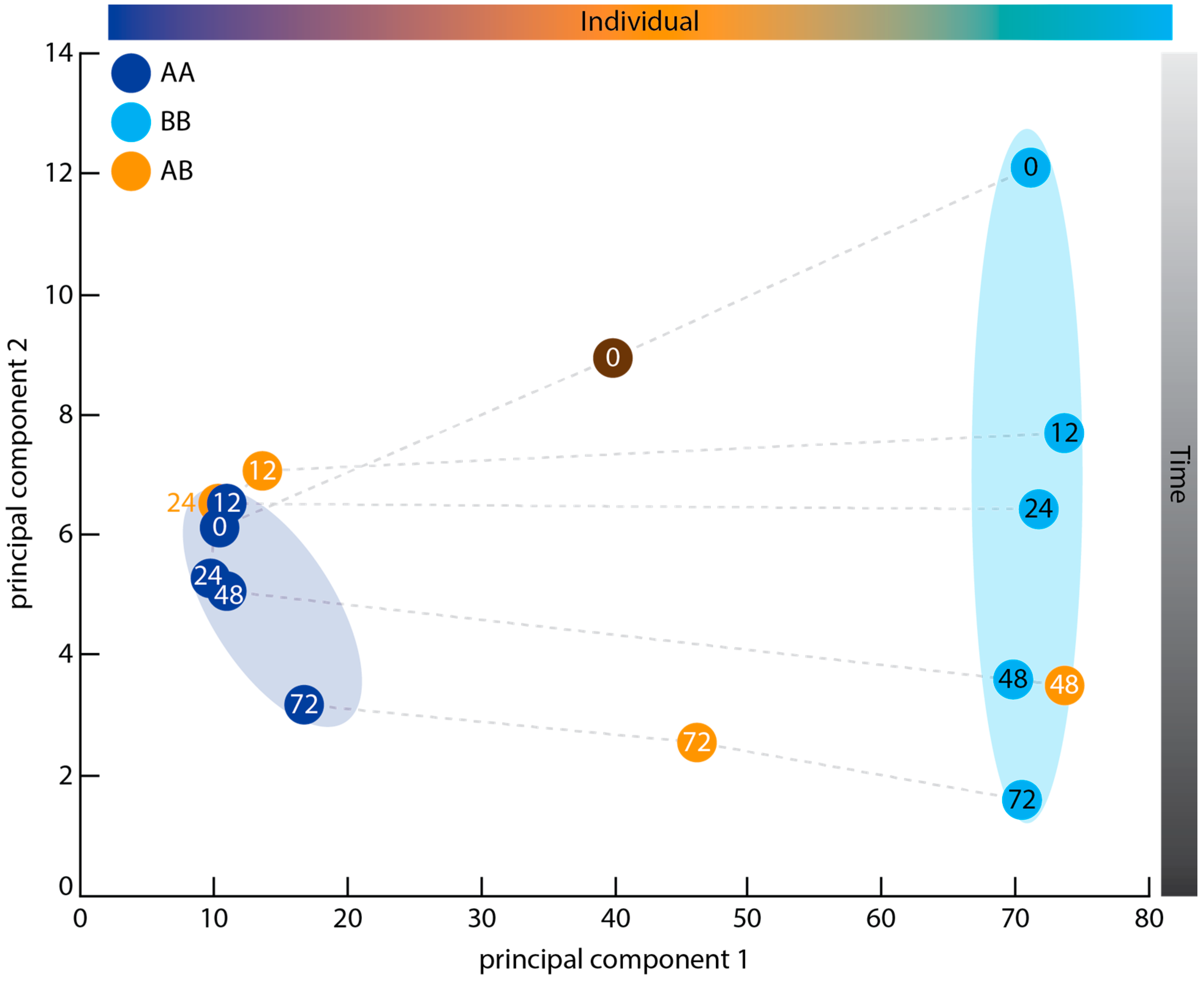
2.3. Principal Component Analysis
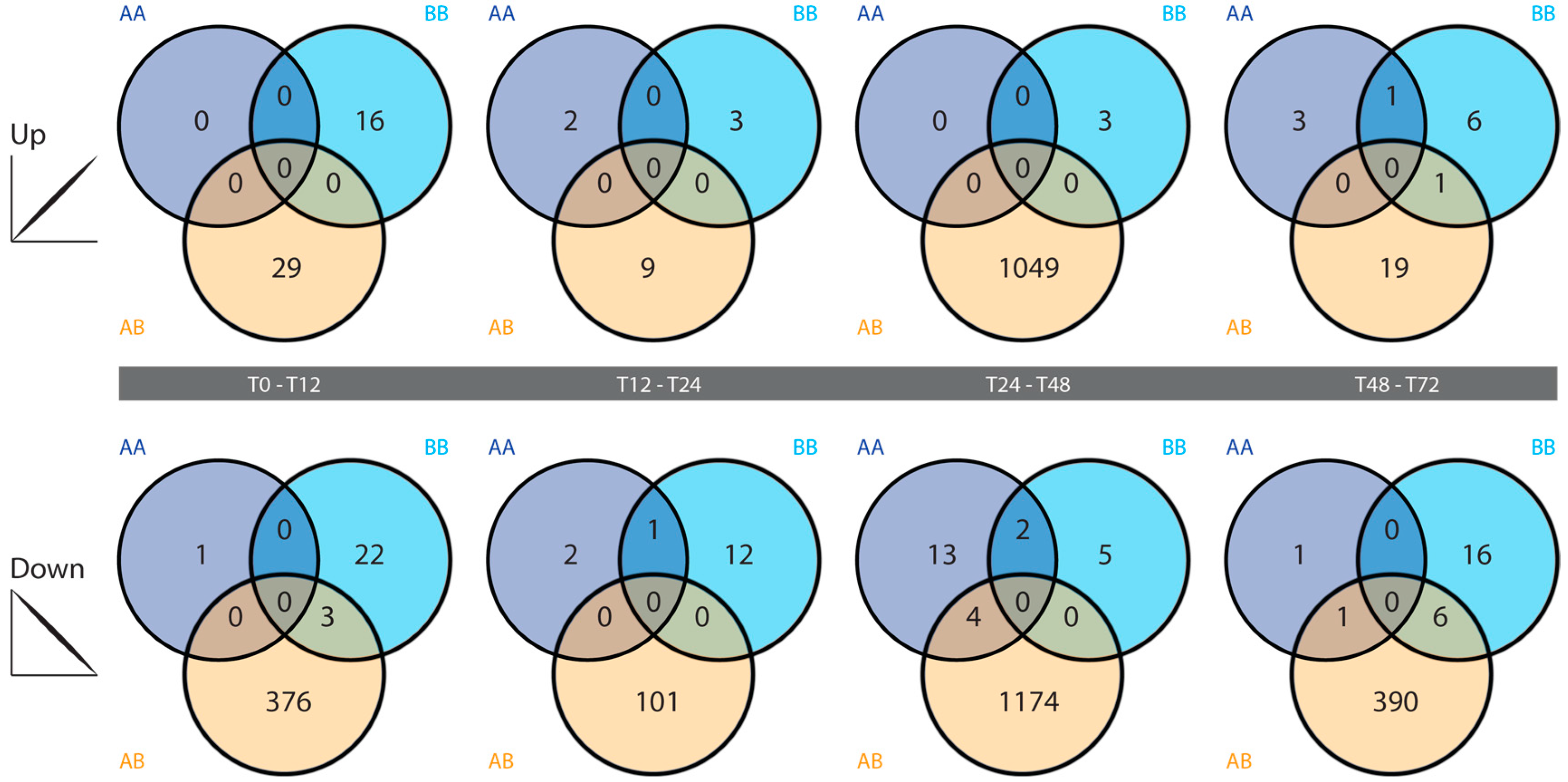
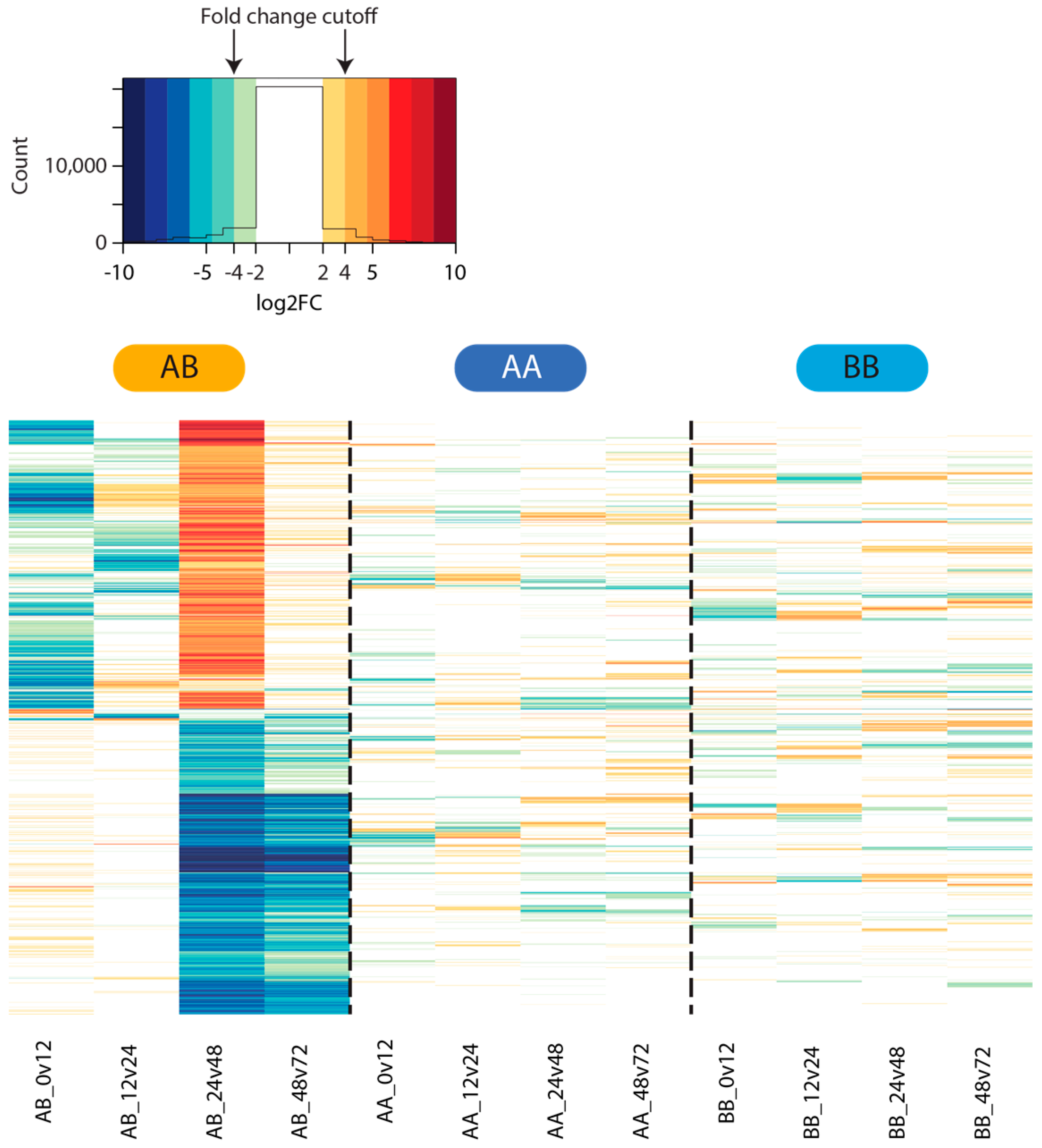
2.4. Differential Gene Expression
2.5. Gene Ontology Analysis
3. Discussion
3.1. Physiological Self and Nonself Graft Responses in Amphimedon queenslandica
3.2. Graft Transcriptome Samples Exhibit Greater Between-Individual Than Between-Time Point Variance
3.3. Differential Gene Expression Analysis
4. Materials and Methods
4.1. Grafting of Adult Sponges
4.2. RNA Extraction from Grafts
4.3. Transcriptome Sequencing
4.4. Read Mapping and Counting
4.5. Independent Filtering and Differential Gene Expression Analysis
4.6. Gene Ontology
4.7. Graphics
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yund, P.O.; Cunningham, C.W.; Buss, L.W. Recruitment and postrecruitment interactions in a colonial hydroid. Ecology 1987, 68, 971–982. [Google Scholar] [CrossRef]
- Fernàndez-Busquets, X.; Burger, M.M. The main protein of the aggregation factor responsible for species-specific cell adhesion in the marine sponge Microciona prolifera is highly polymorphic. J. Biol. Chem. 1997, 272, 27839–27847. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.T. Size and Cycle; Princeton University Press: Princeton, NJ, USA, 1966. [Google Scholar]
- Bonner, J.T. The Evolution of Complexity; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Bonner, J.T. First Signals: The Evolution of Multicellular Development; Princeton University Press: Princeton, NJ, USA, 2000. [Google Scholar]
- Buss, L.W. Somatic cell parasitism and the evolution of somatic tissue compatibility. Proc. Natl. Acad. Sci. USA 1982, 79, 5337–5341. [Google Scholar] [CrossRef] [PubMed]
- Grosberg, R.K. The evolution of allorecognition specificity in clonal invertebrates. Q. Rev. Biol. 1988, 63, 377–412. [Google Scholar] [CrossRef]
- Vaillant, M.L. Note on the vitality of a sponge of the family Corticatæ (Tethya lyncurium, Lamarck). Ann. Mag. Nat. Hist. 1869, 3, 172. [Google Scholar] [CrossRef]
- Moscona, A.A. Cell aggregation: Properties of specific cell-ligands and their role in the formation of multicellular systems. Dev. Biol. 1968, 18, 250–277. [Google Scholar] [CrossRef]
- Hildemann, W.H.; Johnson, I.S.; Jokiel, P.L. Immunocompetence in the lowest metazoan phylum: Transplantation immunity in sponges. Science 1979, 204, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Jokiel, P.L.; Hildemann, W.H.; Bigger, C.H. Frequency of intercolony graft acceptance or rejection as a measure of population structure in the sponge Callyspongia diffusa. Mar. Biol. 1982, 71, 135–139. [Google Scholar] [CrossRef]
- Buscema, M.; Van de Vyver, G. Variability of allograft rejection processes in Axinella verrucosa. Dev. Comp. Immunol. 1983, 7, 613–616. [Google Scholar] [CrossRef]
- Neigel, J.E.; Avise, J.C. The precision of histocompatibility response in clonal recognition in tropical marine sponges. Evolution 1985, 39, 724–732. [Google Scholar] [CrossRef]
- Smith, L.; Hildemann, W.H. Allograft rejection, autograft fusion and inflammatory responses to injury in Callyspongia diffusa (Porifera; Demospongia). Proc. R. Soc. Lond. B 1986, 226, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Ilan, M.; Loya, Y. Ontogenetic variation in sponge histocompatibility responses. Biol. Bull. 1990, 179, 279–286. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Steffen, R.; Lorenz, B.; Batel, R.; Kruse, M.; Krasko, A.; Müller, I.M.; Schröder, H.C. Suppression of allograft rejection in the sponge Suberites domuncula by FK506 and expression of genes encoding FK506-binding proteins in allografts. J. Exp. Biol. 2001, 204, 2197–2207. [Google Scholar] [PubMed]
- Müller, W.E.G.; Krasko, A.; Skorokhod, A.; Bünz, C.; Grebenjuk, V.A.; Steffen, R.; Batel, R.; Schröder, H.C. Histocompatibility reaction in tissue and cells of the marine sponge Suberites domuncula in vitro and in vivo: Central role of the allograft inflammatory factor 1. Immunogenetics 2002, 54, 48–58. [Google Scholar] [CrossRef] [PubMed]
- McGhee, K. The importance of life-history stage and individual variation in the allorecognition system of a marine sponge. J. Exp. Mar. Biol. Ecol. 2006, 333, 241–250. [Google Scholar] [CrossRef]
- Gauthier, M.; Degnan, B.M. Partitioning of genetically distinct cell populations in chimeric juveniles of the sponge Amphimedon queenslandica. Dev. Comp. Immunol. 2008, 32, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Fernàndez-Busquets, X.; Burger, M.M. Cell adhesion and histocompatibility in sponges. Microsc. Res. Tech. 1999, 44, 204–218. [Google Scholar] [CrossRef]
- Fernàndez-Busquets, X.; Kuhns, W.J.; Simpson, T.L.; Ho, M.; Gerosa, D.; Grob, M.; Burger, M.M. Cell adhesion-related proteins as specific markers of sponge cell types involved in allogeneic recognition. Dev. Comp. Immunol. 2002, 26, 313–323. [Google Scholar] [CrossRef]
- Van de Vyver, G.; Barbieux, B. Cellular aspects of allograft rejection in marine sponges of the genus Polymastia. J. Exp. Zool. 1983, 227, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, T. Rapid allogeneic recognition in the marine sponge Microciona prolifera: Implications for evolution of immune recognition. Ann. N. Y. Acad. Sci. 1994, 712, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Hildemann, W.H.; Bigger, C.H.; Johnston, I.S.; Jokiel, P.L. Characteristics of transplantation immunity in the sponge, Callyspongia diffusa. Transplantation 1980, 30, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Bigger, C.H.; Hildemann, W.H.; Jokiel, P.L.; Johnston, I.S. Afferent sensitization and efferent cytotoxicity in allogeneic tissue responses of the marine sponge Callyspongia diffusa. Transplantation 1981, 31, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.Q.; Humphreys, T. Acute cytotoxic allogeneic histoincompatibility reactions involving gray cells in the marine sponge, Callyspongia diffusa. Biol. Bull. 1996, 191, 159–167. [Google Scholar] [CrossRef]
- Van de Vyver, G. Phenomena of cellular recognition in sponges. Curr. Top. Dev. Biol. 1975, 10, 123–140. [Google Scholar] [PubMed]
- Kaye, H.; Ortiz, T. Strain specificity in a tropical marine sponge. Mar. Biol. 1981, 63, 165–173. [Google Scholar] [CrossRef]
- Humphreys, T.; Reinherz, E.L. Invertebrate immune recognition, natural immunity and the evolution of positive selection. Immunol. Today 1994, 15, 316–320. [Google Scholar] [CrossRef]
- Curtis, A.S.G.; Kerr, J.; Knowlton, N. Graft rejection in sponges. Genetic structure of accepting and rejecting populations. Transplantation 1982, 33, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Hildemann, W.H.; Linthicum, D.S. Transplantation immunity in the Palaun sponge, Xestospongia exigua. Transplantation 1981, 32, 77–80. [Google Scholar] [PubMed]
- Grice, L.F.; Gauthier, M.E.A.; Roper, K.E.; Fernàndez-Busquets, X.; Degnan, S.M.; Degnan, B.M. Origin and evolution of the sponge aggregation factor gene family. Mol. Biol. Evol. 2017, 34, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Simakov, O.; Chapman, J.; Fahey, B.; Gauthier, M.E.A.; Mitros, T.; Richards, G.S.; Conaco, C.; Dacre, M.; Hellsten, U.; et al. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature 2010, 466, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Buscema, M.; Van de Vyver, G. Cellular aspects of alloimmune reactions in sponges of the genus Axinella I. Axinella verrucosa and Axinella damicornis. J. Exp. Zool. 1984, 229, 7–17. [Google Scholar] [CrossRef]
- Fernàndez-Busquets, X.; Burger, M.M. Circular proteoglycans from sponges: First members of the spongican family. Cell. Mol. Life Sci. 2003, 60, 88–112. [Google Scholar] [CrossRef] [PubMed]
- Sabella, C.; Faszewski, E.; Himic, L.; Colpitts, K.M.; Kaltenbach, J.; Burger, M.M.; Fernàndez-Busquets, X. Cyclosporin A suspends transplantation reactions in the marine sponge Microciona prolifera. J. Immunol. 2007, 179, 5927–5935. [Google Scholar] [CrossRef] [PubMed]
- Cadavid, L.F.; Powell, A.E.; Nicotra, M.L.; Moreno, M.; Buss, L.W. An invertebrate histocompatibility complex. Genetics 2004, 167, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Fernàndez-Busquets, X.; Gerosa, D.; Hess, D.; Burger, M.M. Accumulation in marine sponge grafts of the mRNA encoding the main proteins of the cell adhesion system. J. Biol. Chem. 1998, 273, 29545–29553. [Google Scholar] [CrossRef] [PubMed]
- Wiens, M.; Krasko, A.; Blumbach, B.; Müller, I.M.; Müller, W.E.G. Increased expression of the potential proapoptotic molecule DD2 and increased synthesis of leukotriene B4 during allograft rejection in a marine sponge. Cell. Death Differ. 2000, 7, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Tepsuporn, S.; Kaltenbach, J.C.; Kuhns, W.J.; Burger, M.M.; Fernàndez-Busquets, X. Apoptosis in Microciona prolifera allografts. Biol. Bull. 2003, 205, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Grice, L.F.; Degnan, B.M. How to build an allorecognition system: A guide for prospective multicellular organisms. In Evolutionary Transitions to Multicellular Life; Ruiz-Trillo, I., Nedelcu, A.M., Eds.; Springer: Dordrecht, The Netherlands, 2015; Volume 2, pp. 395–424. [Google Scholar]
- Oren, M.; Paz, G.; Douek, J.; Rosner, A.; Fishelson, Z.; Goulet, T.L.; Henckel, K.; Rinkevich, B. ‘Rejected’ vs. ‘rejecting’ transcriptomes in allogeneic challenged colonial urochordates. Mol. Immunol. 2010, 47, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Leys, S.P.; Larroux, C.; Gauthier, M.; Adamska, M.; Fahey, B.; Richards, G.S.; Degnan, S.M.; Degnan, B.M. Isolation of Amphimedon developmental material. Cold Spring Harb. Protoc. 2008, 12. [Google Scholar] [CrossRef] [PubMed]
- FastQC: A Quality Control Tool for High throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 28 November 2012).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Valverde, S.L.; Calcino, A.D.; Degnan, B.M. Deep developmental transcriptome sequencing uncovers numerous new genes and enhances gene annotation in the sponge Amphimedon queenslandica. BMC Genom. 2015, 16, 387. [Google Scholar] [CrossRef] [PubMed]
- Anavy, L.; Levin, M.; Khair, S.; Nakanishi, N.; Fernandez-Valverde, S.L.; Degnan, B.M.; Yanai, I. BLIND ordering of large-scale transcriptomic developmental timecourses. Development 2014, 141, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Bourgon, R.; Gentleman, R.; Huber, W. Independent filtering increases detection power for high-throughput experiments. Proc. Natl. Acad. Sci. USA 2010, 107, 9546–9551. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, R.; Varey, V.; Huber, W.; Hahne, F. genefilter: Methods for Filtering Genes from High-Throughput Experiments. 2017. Available online: http://bioconductor.org/packages/release/bioc/html/genefilter.html (accessed on 9 August 2014).
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Smyth, G.K. Moderated statistical tests for assessing differences in tag abundance. Bioinformatics 2007, 23, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Smyth, G.K. Small-sample estimation of negative binomial dispersion, with applications to SAGE data. Biostatistics 2007, 9, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.J.; Chen, Y.; Smyth, G.K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012, 40, 4288–4297. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Götz, S. Blast2GO: A comprehensive suite for functional analysis in plant genomics. Int. J. Plant Genom. 2008, 2008, 619832. [Google Scholar] [CrossRef] [PubMed]
- Maere, S.; Heymans, K.; Kuiper, M. BiNGO: A Cytoscape plugin to assess overrepresentation of Gene Ontology categories in Biological Networks. Bioinformatics 2005, 21, 3448–3449. [Google Scholar] [CrossRef] [PubMed]
- Supek, F.; Bošnjak, M.; Škunca, N.; Šmuc, T. REVIGO Summarizes and Visualizes Long Lists of Gene Ontology Terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, J.C. VENNY. An Interactive Tool for Comparing Lists with Venn’s Diagrams. 2007–2015. Available online: http://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 9 October 2013).
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. gplots: Various R Programming Tools for Plotting Data. 2005. Available online: https://CRAN.R-project.org/package=gplots (accessed on 14 November 2014).
| Time Point | Self | Nonself |
|---|---|---|
| 12 hpg 1 | 4/2/2 2 | 0/0/4 |
| 24 hpg | 6/1/1 | 2/2/0 |
| 48 hpg | 8/0/0 | 0/0/4 |
| 72 hpg | 8/0/0 | 0/0/4 |
| Library | Total Bases | Read Count | Trimmed Read Count | GC (%) | Q20 (%) | Q30 (%) |
|---|---|---|---|---|---|---|
| Donor A | 2,809,914,132 | 27,820,932 | 26,228,938 | 42.1 | 96.3 | 91.2 |
| Donor B | 2,663,155,678 | 26,367,878 | 25,007,728 | 41.9 | 96.6 | 91.7 |
| T12 AA | 2,435,185,144 | 24,110,744 | 22,538,612 | 41.4 | 95.9 | 90.6 |
| T12 BB | 2,494,153,186 | 24,694,586 | 23,222,990 | 41.9 | 96.2 | 91.1 |
| T12 AB | 2,682,231,144 | 26,556,744 | 24,878,304 | 40.1 | 96.3 | 91.2 |
| T24 AA | 2,229,386,534 | 22,073,134 | 20,895,012 | 41.5 | 96.5 | 91.5 |
| T24 BB | 2,249,828,934 | 22,275,534 | 20,912,980 | 41.1 | 96.2 | 91.0 |
| T24 AB (A) | 2,109,872,022 | 20,889,822 | 19,686,010 | 42.1 | 96.3 | 91.0 |
| T24 AB (B) | 2,006,084,624 | 19,862,224 | 18,581,488 | 42.0 | 95.9 | 90.5 |
| T24 AB (C) | 1,762,637,456 | 17,451,856 | 16,136,524 | 41.7 | 95.4 | 89.5 |
| T48 AA | 2,475,671,196 | 24,511,596 | 23,032,818 | 41.5 | 96.2 | 91.1 |
| T48 BB | 2,356,682,894 | 23,333,494 | 21,971,450 | 40.9 | 96.4 | 91.3 |
| T48 AB | 2,041,949,926 | 20,217,326 | 19,135,330 | 43.1 | 96.4 | 91.2 |
| T72 AA | 2,277,699,076 | 22,551,476 | 21,139,356 | 41.1 | 96.1 | 90.9 |
| T72 BB | 2,421,596,402 | 23,976,202 | 22,378,750 | 42.1 | 95.8 | 90.3 |
| T72 AB | 2,477,912,992 | 24,533,792 | 23,158,336 | 42.0 | 96.4 | 91.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grice, L.F.; Degnan, B.M. Transcriptomic Profiling of the Allorecognition Response to Grafting in the Demosponge Amphimedon queenslandica. Mar. Drugs 2017, 15, 136. https://doi.org/10.3390/md15050136
Grice LF, Degnan BM. Transcriptomic Profiling of the Allorecognition Response to Grafting in the Demosponge Amphimedon queenslandica. Marine Drugs. 2017; 15(5):136. https://doi.org/10.3390/md15050136
Chicago/Turabian StyleGrice, Laura F., and Bernard M. Degnan. 2017. "Transcriptomic Profiling of the Allorecognition Response to Grafting in the Demosponge Amphimedon queenslandica" Marine Drugs 15, no. 5: 136. https://doi.org/10.3390/md15050136





