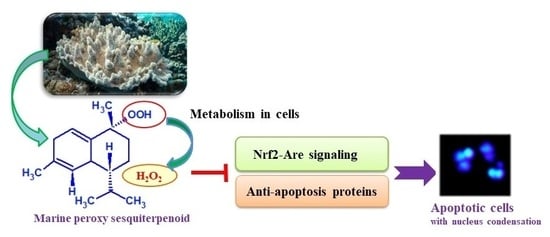
Marine Peroxy Sesquiterpenoids Induce Apoptosis by Modulation of Nrf2-ARE Signaling in HCT116 Colon Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Peroxy Sesquiterpenoids
2.2. Nrf2 Protein Expression
2.3. HO-1 Protein Expression
2.4. Bcl-xL Protein Expression
2.5. pNrf2 and pAkt Expression
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Western Blot Analysis
4.4. LC/MS Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Chen, W.; Li, Y.; Guo, Y. Terpenoids of Sinularia soft corals: Chemistry and bioactivity. Acta Pharm. Sin. B 2012, 2, 227–324. [Google Scholar] [CrossRef]
- Zhu, H.; Hua, X.X.; Gong, T.; Pang, J.; Hou, Q.; Zhu, P. Hypocreaterpenes A and B, cadinane-type sesquiterpenes from a marine-derived fungus, Hypocreales sp. Phytochem. Lett. 2013, 6, 392–396. [Google Scholar] [CrossRef]
- Yang, B.; Liao, S.; Lin, X.; Wang, J.; Liu, J.; Zhou, X.; Yang, X.; Liu, Y. New sinularianin sesquiterpenes from soft coral Sinularia sp. Mar. Drugs 2013, 11, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Su, J.H.; Huang, C.Y.; Li, P.J.; Lu, Y.; Wen, Z.H.; Kao, Y.H.; Sheu, J.H. Bioactive cadinane-type compounds from the soft coral Sinularia scabra. Arch. Pharm. Res. 2012, 35, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Xian, Y.F.; Ip, S.P.; Su, Z.R.; Su, J.Y.; He, J.J.; Xie, Q.F.; Lai, X.P.; Lin, Z.X. Anti-inflammatory activity of patchouli alcohol isolated from Pogostemonis Herba in animal models. Fitoterapia 2011, 82, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Taira, J.; Tsuchida, E.; Uehara, M.; Kinjyo, Y.; Roy, P.K.; Ueda, K. Dual biological functions of the apoptotic activity and anti-inflammatory effect by alcyonolide congeners from the Okinawan soft coral, Cespitularia sp. Bioorg. Med. Chem. Lett. 2015, 25, 4496–4499. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Ashimine, R.; Miyazato, H.; Taira, J.; Ueda, K. Endoperoxy and hydroperoxy cadinane-type sesquiterpenoids from an Okinawan soft coral, Sinularia sp. Arch. Pharmacal. Res. 2016, 39, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, H.; Taira, L.; Ueda, K. Hydrogen peroxide derived from marine peroxy sesquiterpenoids induces apoptosis in HCT116 human colon cancer cells. Bioorg. Med. Chem. Lett. 2016, 26, 4641–4644. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [Green Version]
- Mitsuishi, Y.; Taguchi, K.; Kawatani, Y.; Shibata, T.; Nukiwa, T.; Aburatani, H.; Yamamoto, M.; Motohashi, H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic Reprogramming. Cancer Cell 2012, 22, 66–79. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Samali, A.; Orrenius, S. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 2000, 29, 323–333. [Google Scholar] [CrossRef]
- Franke, T.F.; Hornik, C.P.; Segev, L.; Shostak, G.A.; Sugimoto, C. PI3K/Akt and apoptosis: Size matters. Oncogene 2003, 22, 8983–8998. [Google Scholar] [CrossRef] [PubMed]
- Taira, J.; Sonamoto, M.; Uehara, M. Dual biological functions of a cytoprotective effect and apoptosis induction by boavailable marine carotenoid fucoxanthinol through modulation of the Nrf2 activation in RAW264.7 macrophage cells. Mar. Drugs 2017, 15, 305. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taira, J.; Miyazato, H.; Ueda, K. Marine Peroxy Sesquiterpenoids Induce Apoptosis by Modulation of Nrf2-ARE Signaling in HCT116 Colon Cancer Cells. Mar. Drugs 2018, 16, 347. https://doi.org/10.3390/md16100347
Taira J, Miyazato H, Ueda K. Marine Peroxy Sesquiterpenoids Induce Apoptosis by Modulation of Nrf2-ARE Signaling in HCT116 Colon Cancer Cells. Marine Drugs. 2018; 16(10):347. https://doi.org/10.3390/md16100347
Chicago/Turabian StyleTaira, Junsei, Haruna Miyazato, and Katsuhiro Ueda. 2018. "Marine Peroxy Sesquiterpenoids Induce Apoptosis by Modulation of Nrf2-ARE Signaling in HCT116 Colon Cancer Cells" Marine Drugs 16, no. 10: 347. https://doi.org/10.3390/md16100347