Extraction of Astaxanthin and Lutein from Microalga Haematococcus pluvialis in the Red Phase Using CO2 Supercritical Fluid Extraction Technology with Ethanol as Co-Solvent
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Yield
2.2. Astaxanthin Recovery
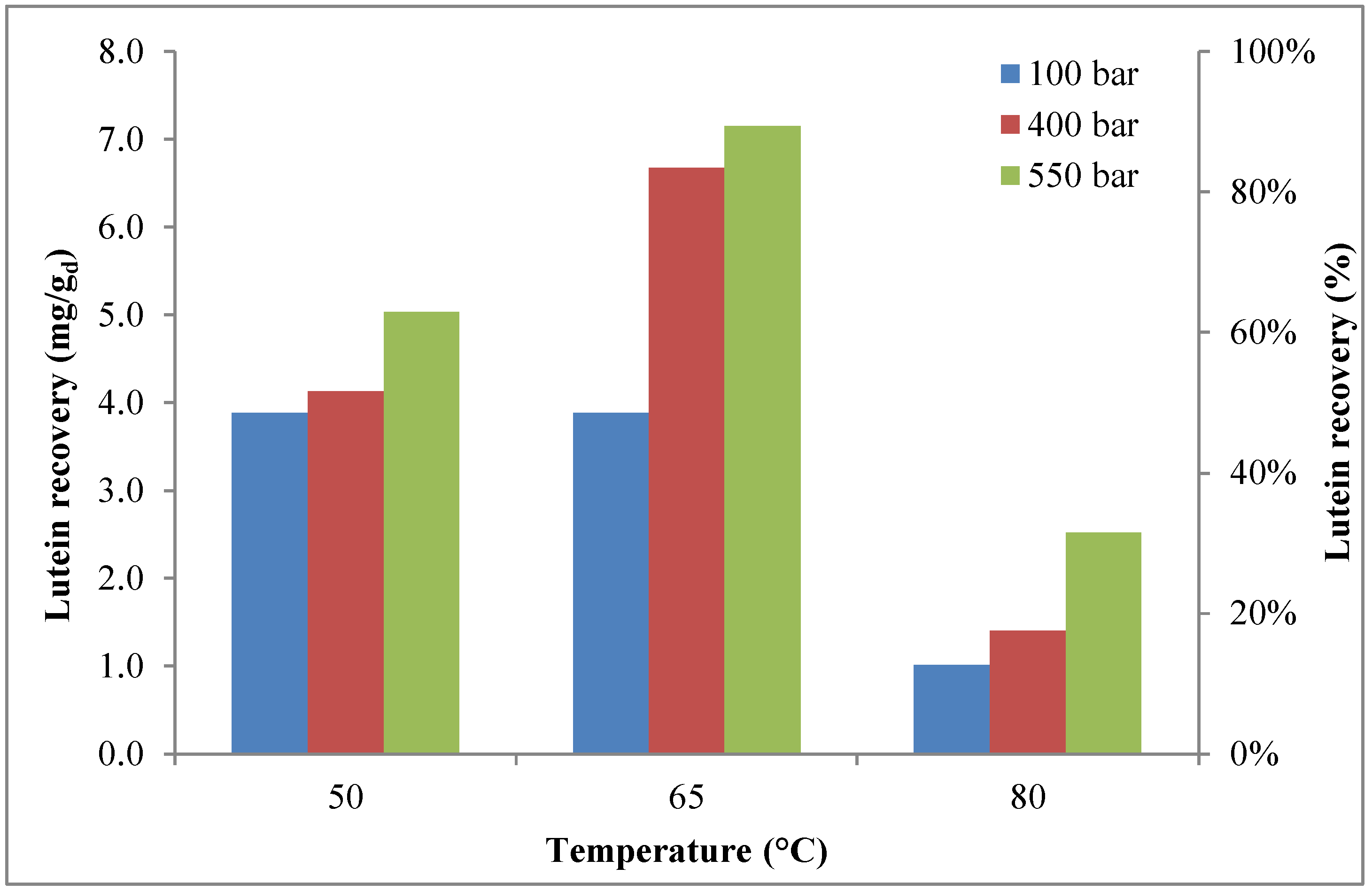
2.3. Lutein Recovery
2.4. Comparison with Literature for Recovery of Astaxanthin and Lutein Using CO2-SFE with Co-Solvent
3. Materials and Methods
3.1. Samples and Chemicals
3.2. Experimental Set-Up
3.3. Analytical Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes, R.L.; Coelho, J.P.; Fernandes, H.L.; Marrucho, I.J.; Cabral, J.M.S.; Novais, J.M.; Palavra, A.F. Applications of supercritical CO2 extraction to microalgae and plants. J. Chem. Technol. Biotechnol. 1995, 62, 53–59. [Google Scholar] [CrossRef]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Jaswir, I.; Noviendri1, D.; Hasrini, R.F.; Octavianti, F. Carotenoids: Sources, medicinal properties and their application in food and nutraceutical industry. J. Med. Plants Res. 2011, 5. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Crampon, C.; Boutin, O.; Badens, E. Supercritical carbon dioxide extraction of molecules of interest from microalgae and seaweeds. Ind. Eng. Chem. Res. 2011, 50, 8941–8953. [Google Scholar] [CrossRef]
- Mendes, R.L.; Nobre, B.P.; Cardoso, M.T.; Pereira, A.P.; Palavra, A.F. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. Inorg. Chim. Acta 2003, 356, 328–334. [Google Scholar] [CrossRef]
- Palavra, A.M.F.; Coelho, J.P.; Barroso, J.G.; Rauter, A.P.; Fareleira, J.M.N.A.; Mainar, A.; Urieta, J.S.; Nobre, B.P.; Gouveia, L.; Mendes, R.L.; et al. Supercritical carbon dioxide extraction of bioactive compounds from microalgae and volatile oils from aromatic plants. J. Supercrit. Fluids 2011, 60, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Sovová, H. Rate of the vegetable oil extraction with supercritical CO2—I. Modeling of extraction curves. Chem. Eng. Sci. 1994, 49, 409–414. [Google Scholar] [CrossRef]
- Wood, J.A.; Bernards, M.A.; Wan, W.-K.; Charpentier, P.A. Extraction of ginsenosides from North American ginseng using modified supercritical carbon dioxide. J. Supercrit. Fluids 2006, 39, 40–47. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattea, F.; Martín, Á.; Cocero, M.J. Carotenoid processing with supercritical fluids. J. Food Eng. 2009, 93, 255–265. [Google Scholar] [CrossRef]
- Smith, R.M. Supercritical fluids in separation science—The dreams, the reality and the future. J. Chromatogr. A 1999, 856, 83–115. [Google Scholar] [CrossRef]
- Temelli, F. Perspectives on supercritical fluid processing of fats and oils. J. Supercrit. Fluids 2009, 47, 583–590. [Google Scholar] [CrossRef]
- Uddin, M.S.; Sarker, M.Z.I.; Ferdosh, S.; Akanda, M.J.H.; Easmin, M.S.; Bt Shamsudin, S.H.; Yunus, K. Bin Phytosterols and their extraction from various plant matrices using supercritical carbon dioxide: A review. J. Sci. Food Agric. 2015, 95, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Shi, X.; Jiang, W. Theoretical models for supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Brunner, G. Supercritical fluids: Technology and application to food processing. J. Food Eng. 2005, 67, 21–33. [Google Scholar] [CrossRef]
- Crampon, C.; Nikitine, C.; Zaier, M.; Lépine, O.; Tanzi, C.D.; Vian, M.A.; Chemat, F.; Badens, E. Oil extraction from enriched Spirulina platensis microalgae using supercritical carbon dioxide. J. Supercrit. Fluids 2017, 119, 289–296. [Google Scholar] [CrossRef]
- Phelps, C.L.; Smart, N.G.; Wai, C.M. Past, present, and possible future applications of supercritical fluid extraction technology. J. Chem. Educ. 1996, 73, 1163. [Google Scholar] [CrossRef]
- Raventós, M.; Duarte, S.; Alarcón, R. Application and possibilities of supercritical CO2 extraction in food processing industry: An overview. Food Sci. Technol. Int. 2002, 8, 269–284. [Google Scholar] [CrossRef]
- Span, R.; Wagner, W. A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef]
- Fernández-Ronco, M.P.; De Lucas, A.; Rodríguez, J.F.; García, M.T.; Gracia, I. New considerations in the economic evaluation of supercritical processes: Separation of bioactive compounds from multicomponent mixtures. J. Supercrit. Fluids 2013, 79, 345–355. [Google Scholar] [CrossRef]
- Shilpi, A.; Shivhare, U.S.; Basu, S. Supercritical CO2 extraction of compounds with antioxidant activity from fruits and vegetables waste—A review. Focus. Mod. Food Ind. 2013, 2, 43–62. [Google Scholar]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, J.F.; Eckert, C.A. Phase equilibria for supercritical fluid process design. AIChE J. 1989, 35, 1409–1427. [Google Scholar] [CrossRef]
- Han, X.; Poliakoff, M. Continuous reactions in supercritical carbon dioxide: problems, solutions and possible ways forward. Chem. Soc. Rev. 2012, 41, 1428. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.S.; Debenedetti, P.G.; Prud’homme, R.K. Effects of process conditions on crystals obtained from supercritical mixtures. AIChE J. 1989, 35, 325–328. [Google Scholar] [CrossRef]
- Randolph, T.W. Supercritical fluid extractions in biotechnology. Trends Biotechnol. 1990, 8, 78–82. [Google Scholar] [CrossRef]
- Rozzi, N.L.; Singh, R.K. Supercritical fluids and the food industry. Compr. Rev. Food Sci. Food Saf. 2002, 1, 33–44. [Google Scholar] [CrossRef]
- Francis, A.W. Ternary systems of liquid carbon dioxide. J. Phys. Chem. 1954, 58, 1099–1114. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Kitzberger, C.S.G.; Smânia, A.; Pedrosa, R.C.; Ferreira, S.R.S. Antioxidant and antimicrobial activities of shiitake (Lentinula edodes) extracts obtained by organic solvents and supercritical fluids. J. Food Eng. 2007, 80, 631–638. [Google Scholar] [CrossRef]
- Mendiola, J.A.; Herrero, M.; Cifuentes, A.; Ibañez, E. Use of compressed fluids for sample preparation: Food applications. J. Chromatogr. A 2007, 1152, 234–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zancan, K.C.; Marques, M.O.M.; Petenate, A.J.; Meireles, M.A.A. Extraction of ginger (Zingiber officinale roscoe) oleoresin with CO2 and co-solvents: A study of the antioxidant action of the extracts. J. Supercrit. Fluids 2001, 24, 57–76. [Google Scholar] [CrossRef]
- Food and Drug Administration. Substances Generally Recognized as Safe; Final Rule. Fed. Regist. 2016, 81, 54959–55055. [Google Scholar]
- Lang, Q.; Wai, C.M. Supercritical fluid extraction in herbal and natural product studies—A practical review. Talanta 2001, 53, 771–782. [Google Scholar] [CrossRef]
- Reverchon, E. Supercritical fluid extraction and fractionation of essential oils and related products. J. Supercrit. Fluids 1997, 10, 1–37. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Karim, A.A.; Abbas, K.A.; Norulaini, N.A.N.; Omar, A.K.M. Application of supercritical CO2 in lipid extraction—A review. J. Food Eng. 2009, 95, 240–253. [Google Scholar] [CrossRef]
- Herrero, M.; del PilarSánchez-Camargo, A.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Mäki-Arvela, P.; Hachemi, I.; Murzin, D.Y. Comparative study of the extraction methods for recovery of carotenoids from algae: Extraction kinetics and effect of different extraction parameters. J. Chem. Technol. Biotechnol. 2014, 89, 1607–1626. [Google Scholar] [CrossRef]
- Yen, H.W.; Yang, S.C.; Chen, C.H.; Chang, J.S. Supercritical fluid extraction of valuable compounds from microalgal biomass. Bioresour. Technol. 2015, 184, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Catchpole, O.; Moreno, T.; Montañes, F.; Tallon, S. Perspectives on processing of high value lipids using supercritical fluids. J. Supercrit. Fluids 2018, 134, 260–268. [Google Scholar] [CrossRef]
- Sarada, R.; Tripathi, U.; Ravishankar, G.A. Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process Biochem. 2002, 37, 623–627. [Google Scholar] [CrossRef]
- Chekanov, K.; Lobakova, E.; Selyakh, I.; Semenova, L.; Sidorov, R.; Solovchenko, A. Accumulation of astaxanthin by a new Haematococcus pluvialis strain BM1 from the white sea coastal rocks (Russia). Mar. Drugs 2014, 12, 4504–4520. [Google Scholar] [CrossRef] [PubMed]
- Heimann, K.; Huerlimann, R. Microalgal Classification: Major Classes and Genera of Commercial Microalgal Species. Handb. Mar. Sci. 2015, 578, 25–41. [Google Scholar] [CrossRef]
- Thana, P.; Machmudah, S.; Goto, M.; Sasaki, M.; Pavasant, P.; Shotipruk, A. Response surface methodology to supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2008, 99, 3110–3115. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, J.O.; Perrut, M.; Majewski, W. Extraction of Astaxantine and phycocyanine from microalgae with supercritical carbon dioxide. J. Chem. Eng. Data 2003, 48, 827–830. [Google Scholar] [CrossRef]
- Cheng, X.; Qi, Z.B.; Burdyny, T.; Kong, T.; Sinton, D. Low pressure supercritical CO2 extraction of astaxanthin from Haematococcus pluvialis demonstrated on a microfluidic chip. Bioresour. Technol. 2018, 250, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Kwan, T.A.; Kwan, S.E.; Peccia, J.; Zimmerman, J.B. Selectively biorefining astaxanthin and triacylglycerol co-products from microalgae with supercritical carbon dioxide extraction. Bioresour. Technol. 2018, 269, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Nobre, B.; Marcelo, F.; Passos, R.; Beirão, L.; Palavra, A.; Gouveia, L.; Mendes, R. Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol. 2006, 223, 787–790. [Google Scholar] [CrossRef]
- Machmudah, S.; Shotipruk, A.; Goto, M.; Sasaki, M.; Hirose, T. Extraction of astaxanthin from Haematococcus pluvialis using supercritical CO2 and ethanol as entrainer. Ind. Eng. Chem. Res. 2006, 45, 3652–3657. [Google Scholar] [CrossRef]
- Kitada, K.; Machmudah, S.; Sasaki, M.; Goto, M.; Nakashima, Y.; Kumamoto, S.; Hasegawa, T. Supercritical CO2 extraction of pigment components with pharmaceutical importance from Chlorella vulgaris. J. Chem. Technol. Biotechnol. 2009, 84, 657–661. [Google Scholar] [CrossRef]
- Ruen-Ngam, D.; Shotipruk, A.; Pavasant, P.; Machmudah, S.; Goto, M. Selective extraction of lutein from alcohol treated Chlorella vulgaris by supercritical CO2. Chem. Eng. Technol. 2012, 35, 255–260. [Google Scholar] [CrossRef]
- Yen, H.W.; Chiang, W.C.; Sun, C.H. Supercritical fluid extraction of lutein from Scenedesmus cultured in an autotrophical photobioreactor. J. Taiwan Inst. Chem. Eng. 2012, 43, 53–57. [Google Scholar] [CrossRef]
- Sanzo, G.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical carbon dioxide extraction of astaxanthin, lutein, and fatty acids from Haematococcus pluvialis microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef] [PubMed]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, T.M.; Johnson, D.M.; McNally, M.P.; Fahmy, T.M.; Paulaitis, M.E. Modifier effects in the supercritical fluid extraction of solutes from clay, soil, and plant materials. Anal. Chem. 1993, 65, 1462–1469. [Google Scholar] [CrossRef]
- Chafer, A.; Fornari, T.; Berna, A.; Stateva, R.P. Solubility of quercetin in supercritical CO2+ ethanol as a modifier: Measurements and thermodynamic modelling. J. Supercrit. Fluids 2004, 32, 89–96. [Google Scholar] [CrossRef]
- Krichnavaruk, S.; Shotipruk, A.; Goto, M.; Pavasant, P. Supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis with vegetable oils as co-solvent. Bioresour. Technol. 2007, 99, 5556–5560. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, A.; Roberts, P.; Aravena, R.; Valle, J. Supercritical extraction of astaxanthin from H. pluvialis using ethanol-modified CO2. Experiments and modeling. In Proceedings of the 11th International Conference of Eng Food, Athens, Greece, 22–26 May 2011. [Google Scholar]
- Pan, J.L.; Wang, H.M.; Chen, C.Y.; Chang, J.S. Extraction of astaxanthin from Haematococcus pluvialis by supercritical carbon dioxide fluid with ethanol modifier. Eng. Life Sci. 2012, 12, 638–647. [Google Scholar] [CrossRef]
- Reyes, F.A.; Mendiola, J.A.; Ibañez, E.; Del Valle, J.M. Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J. Supercrit. Fluids 2014, 92, 75–83. [Google Scholar] [CrossRef]
- Ruen-ngam, D.; Shotipruk, A.; Pavasant, P. Comparison of extraction methods for recovery of astaxanthin from Haematococcus pluvialis. Sep. Sci. Technol. 2011, 46, 64–70. [Google Scholar] [CrossRef]
- Vasapollo, G.; Longo, L.; Rescio, L.; Ciurlia, L. Innovative supercritical CO2 extraction of lycopene from tomato in the presence of vegetable oil as co-solvent. J. Supercrit. Fluids 2004, 29, 87–96. [Google Scholar] [CrossRef]
- Li, Y.; Miao, F.; Geng, Y.; Lu, D.; Zhang, C.; Zeng, M. Accurate quantification of astaxanthin from Haematococcus crude extract spectrophotometrically. Chin. J. Oceanol. Limnol. 2012, 30, 627–637. [Google Scholar] [CrossRef]
- Vidhyavathi, R.; Venkatachalam, L.; Kamath, B.S.; Sarada, R.; Ravishankar, G.A. Differential expression of carotenogenic genes and associated changes in pigment profile during regeneration of Haematococcus pluvialis cysts. Appl. Microbiol. Biotechnol. 2007, 75, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Rimauro, J.; Casella, P.; Cerbone, A.; Larocca, V.; Chianese, S.; Karatza, D.; Mehariya, S.; Ferraro, A.; Hristoforou, E.; et al. Extraction of astaxanthin from microalga Haematococcus pluvialis in red phase by using generally recognized as safe solvents and accelerated extraction. J. Biotechnol. 2018, 283, 51–61. [Google Scholar] [CrossRef] [PubMed]




| Operative Conditions | Total Extraction Yield (mg/g) (Ethanol Flow Rate = 1 mL/min) | Total Extraction Yield (mg/g) (Without Co-Solvent) | |
|---|---|---|---|
| Extraction Time = 80 min | |||
| CO2 Flow Rate = 3.62 g/min | |||
| T (°C) | P (bar) | ||
| 50 | 100 | 209.99 | 0.1 |
| 50 | 400 | 235.47 | 132.4 |
| 50 | 550 | 241.64 | 234.8 |
| 65 | 100 | 241.62 | 4.8 |
| 65 | 400 | 292.70 | 277.1 |
| 65 | 550 | 280.78 | 184.6 |
| 80 | 100 | 207.67 | 10.6 |
| 80 | 400 | 253.03 | 158.7 |
| 80 | 550 | 222.57 | 59.4 |
| CO2 Flow Rate = 3.62 g/min; Extraction Time = 80 min | ||||||
|---|---|---|---|---|---|---|
| T (°C) | P (bar) | |||||
| 100 | 400 | 550 | 100 | 400 | 550 | |
| Cumulative Recovery with Co-Solvent (%) (Ethanol flow rate = 1 mL/min) | Cumulative Recovery without Co-Solvent (%) | |||||
| 50 | 24.5 | 27.8 | 27.9 | <0.01 | 91.6 | 94.5 |
| 65 | 38.3 | 74.2 | 92.4 | 0.05 | 79.8 | 35.2 |
| 80 | 8.4 | 10.1 | 14.5 | 5.7 | 72.7 | 13.5 |
| CO2 Flow Rate = 3.62 g/min | ||||||||
|---|---|---|---|---|---|---|---|---|
| T and P | Astaxanthin Purity (%) with Co-Solvent | Astaxanthin Purity (%) without Co-Solvent | ||||||
| 20 min | 40 min | 60 min | 80 min | 20 min | 40 min | 60 min | 80 min | |
| 50 °C 100 bar | 0.86 | 10.33 | 5.13 | 4.85 | 0 | 0 | 0 | 0 |
| 50 °C 400 bar | 2.71 | 1.34 | 1.55 | 1.84 | 11.81 | 29.41 | 32.96 | 32.48 |
| 50 °C 550 bar | 2.33 | 1.21 | 4.82 | 8.60 | 7.30 | 17.31 | 22.57 | 26.67 |
| 65 °C 100 bar | 3.28 | 2.01 | 0.53 | 1.18 | 0 | 0 | 0 | 0 |
| 65 °C 400 bar | 5.24 | 4.39 | 0.01 | 0.01 | 4.96 | 20.86 | 28.53 | 29.46 |
| 65 °C 550 bar | 6.67 | 0.00 | 0.40 | 0.08 | 2.42 | 15.21 | 17.75 | 27.14 |
| 80 °C 100 bar | 0.18 | 0.89 | 2.86 | 1.96 | 0 | 0 | 0 | 0 |
| 80 °C 400 bar | 0.72 | 18.11 | 0.06 | 0.08 | 7.70 | 13.32 | 29.11 | 34.23 |
| 80 °C 550 bar | 1.02 | 14.03 | 0.03 | 0.19 | 2.21 | 8.03 | 19.63 | 22.15 |
| CO2 Flow Rate = 3.62 g/min; Extraction Time = 80 min | ||||||
|---|---|---|---|---|---|---|
| T (°C) | P (bar) | |||||
| 100 | 400 | 550 | 100 | 400 | 550 | |
| Cumulative Recovery with Co-Solvent (%) (Ethanol Flow Rate = 1 mL/min) | Cumulative Recovery without Co-Solvent (%) | |||||
| 50 | 50.5 | 53.7 | 57.5 | 0.1 | 44.5 | 50.9 |
| 65 | 50.4 | 86.6 | 92.9 | 0 | 33.9 | 37.7 |
| 80 | 13.2 | 18.2 | 32.8 | 1.6 | 17.3 | 1.2 |
| CO2 Flow Rate = 3.62 g/min | ||||||||
|---|---|---|---|---|---|---|---|---|
| T and P | Lutein Purity (%) with Co-Solvent | Lutein Purity (%) without Co-Solvent | ||||||
| 20 min | 40 min | 60 min | 80 min | 20 min | 40 min | 60 min | 80 min | |
| 50 °C 100 bar | 0.61 | 2.88 | 1.94 | 1.90 | 0 | 0 | 0 | 0 |
| 50 °C 400 bar | 1.44 | 0.93 | 1.04 | 1.09 | 2.27 | 3.89 | 6.36 | 7.54 |
| 50 °C 550 bar | 1.18 | 5.93 | 3.51 | 0.33 | 1.58 | 2.83 | 2.04 | 4.63 |
| 65 °C 100 bar | 1.08 | 0.98 | 0.29 | 0.44 | 0 | 0 | 0 | 0 |
| 65 °C 400 bar | 1.36 | 6.78 | 0.06 | 0.03 | 0.93 | 0.96 | 1.52 | 3.62 |
| 65 °C 550 bar | 1.73 | 16.57 | 0.35 | 0.28 | 1.64 | 0.68 | 2.31 | 2.80 |
| 80 °C 100 bar | 0.33 | 0.58 | 0.50 | 0.43 | 2.33 | 0.92 | 0.63 | 0.67 |
| 80 °C 400 bar | 0.39 | 5.17 | 0.16 | 0.28 | 0.83 | 0.59 | 1.25 | 1.44 |
| 80 °C 550 bar | 0.76 | 4.89 | 0.08 | 0.08 | 0 | 0.32 | 1.62 | 1.66 |
| Optimum Extraction Conditions | Carotenoid Recovery e | Reference | ||||||
|---|---|---|---|---|---|---|---|---|
| Biomass Loading (g) | CO2 Flow Rate (g/min) | Co-Solvent a | Pre-Treatment | Pb (bar) | Tc (°C) | td (h) | ||
| n.a. § | 100 µL·min−1 | 20% (v/v) ethanol | Hydrotermal | 80 | 55 | 15 min | Astaxanthin 98.3% | [50] |
| n.a. § | 100 µL·min−1 | 20% (v/v) olive oil | Hydrotermal | 80 | 55 | 15 min | Astaxanthin 98.6% | |
| 7 | 1.41 g/min | 5% (v/v) ethanol | Drying | 400 | 70 | 4 | Astaxanthin 77.9% | [53] |
| 2 | 1.41 g/min | 10% (v/v) ethanol | Freeze drying and ball milling | 300 | 60 | - | Astaxanthin >90%; Lutein >90% | [52] |
| 6 | 1.41 g/min | 10% (v/v) olive oil | Drying | 400 | 70 | 5 | Asthaxanthin 36% | [61] |
| 240 | 7.8 g/min | 2.3 mL/g sample ethanol | Freeze drying (powder form) | 435 | 65 | 3.5 | Astaxanthin 87.42% | [66] |
| 1.38 | 3.62 g/min | 12.5% (v/v) ethanol | Ball milling | 550 | 65 | 1.33 (20 min for Astaxanthin; 40 min for Lutein) # | Astaxanthin 92.4%; Lutein 92.9% | This study |
| Operative Conditions | ||
|---|---|---|
| T (°C) | P (bar) | HPR Biomass Loading (g) |
| 50 | 100 | 1.43 |
| 50 | 400 | 1.43 |
| 50 | 550 | 1.37 |
| 65 | 100 | 1.36 |
| 65 | 400 | 1.36 |
| 65 | 550 | 1.38 |
| 80 | 100 | 1.35 |
| 80 | 400 | 1.38 |
| 80 | 550 | 1.34 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molino, A.; Mehariya, S.; Iovine, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Chianese, S.; Musmarra, D. Extraction of Astaxanthin and Lutein from Microalga Haematococcus pluvialis in the Red Phase Using CO2 Supercritical Fluid Extraction Technology with Ethanol as Co-Solvent. Mar. Drugs 2018, 16, 432. https://doi.org/10.3390/md16110432
Molino A, Mehariya S, Iovine A, Larocca V, Di Sanzo G, Martino M, Casella P, Chianese S, Musmarra D. Extraction of Astaxanthin and Lutein from Microalga Haematococcus pluvialis in the Red Phase Using CO2 Supercritical Fluid Extraction Technology with Ethanol as Co-Solvent. Marine Drugs. 2018; 16(11):432. https://doi.org/10.3390/md16110432
Chicago/Turabian StyleMolino, Antonio, Sanjeet Mehariya, Angela Iovine, Vincenzo Larocca, Giuseppe Di Sanzo, Maria Martino, Patrizia Casella, Simeone Chianese, and Dino Musmarra. 2018. "Extraction of Astaxanthin and Lutein from Microalga Haematococcus pluvialis in the Red Phase Using CO2 Supercritical Fluid Extraction Technology with Ethanol as Co-Solvent" Marine Drugs 16, no. 11: 432. https://doi.org/10.3390/md16110432







