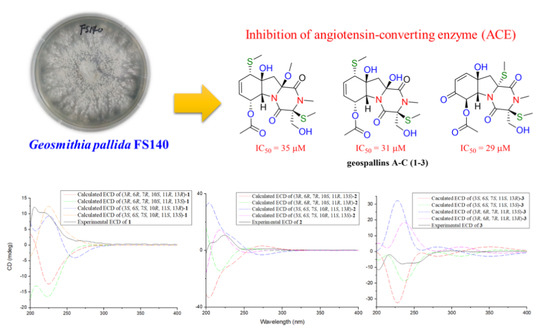
Geospallins A–C: New Thiodiketopiperazines with Inhibitory Activity against Angiotensin-Converting Enzyme from a Deep-Sea-Derived Fungus Geosmithia pallida FS140
Abstract
:1. Introduction
2. Results
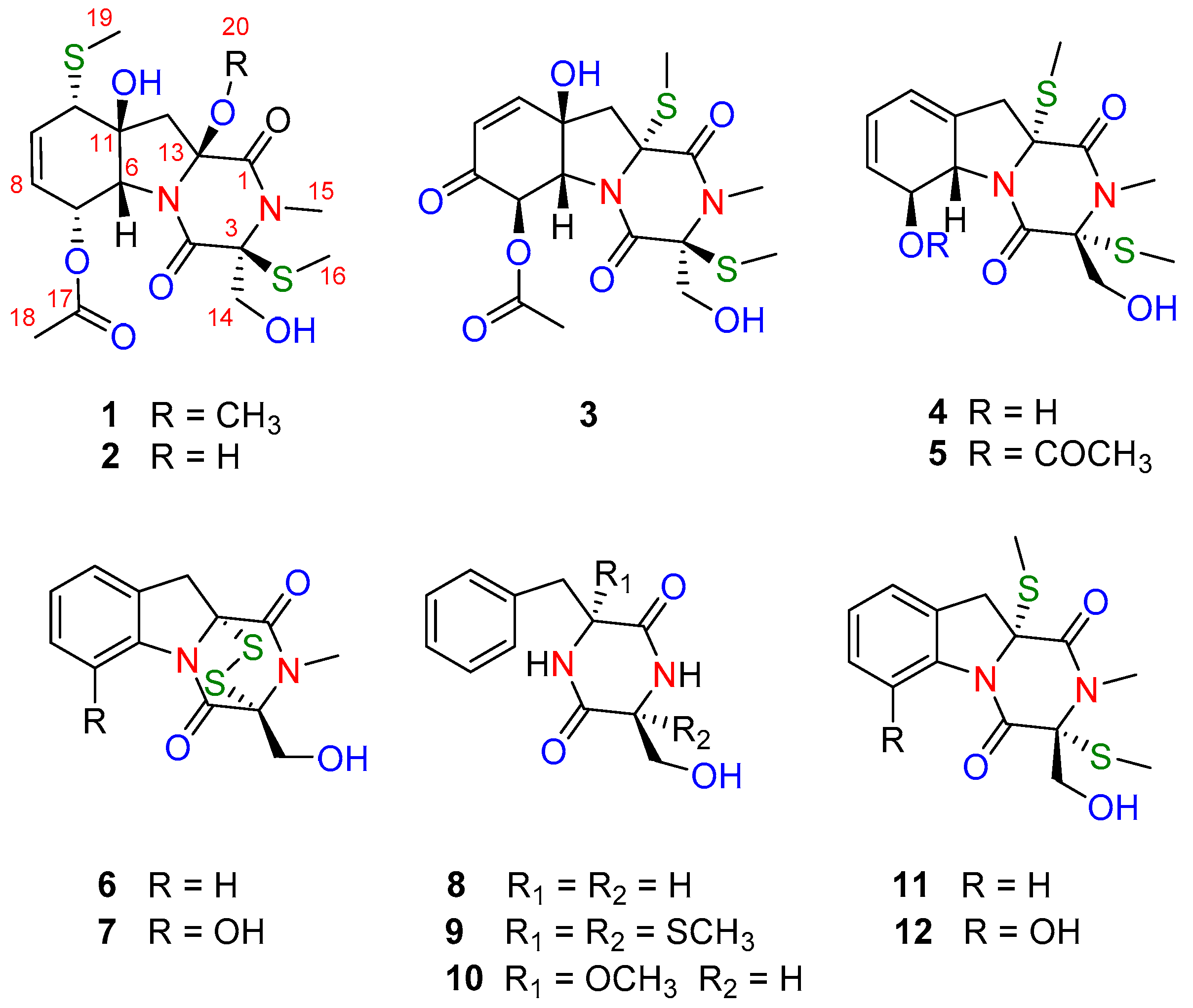
2.1. Identification of New Compounds
2.2. Angiotensin-Converting Enzyme (ACE) Inhibitory Assay
2.3. α-Glucosidase Inhibitory Activity Assay
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material and Identification
3.3. Fermentation, Extraction, and Isolation
3.4. Quantum Chemical ECD Calculation
3.5. Angiotensin-Converting Enzyme (ACE) Inhibitory Assay
3.6. α-Glucosidase Inhibitory Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2016, 36, 382–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhou, X.; Xu, T.; Yang, X.; Liu, Y. Diketopiperazines from marine organisms. Chem. Biodivers. 2010, 7, 2809–2829. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, K.E.; Khan, Z.; Giani, J.F.; Cao, D.Y.; Bernstein, E.A.; Shen, X.Z. Angiotensin-converting enzyme in innate and adaptive immunity. Nat. Rev. Nephrol. 2018, 14, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Yang, Z.; Nishizawa, T.; Mori, T.; Saito, K. Top-down targeted metabolomics teveals a sulfur-containing metabolite with inhibitory activity against angiotensin-converting enzyme in Asparagus officinalis. J. Nat. Prod. 2015, 78, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Liu, H.X.; Chen, Y.C.; Tan, H.B.; Guo, H.; Xu, L.Q.; Li, S.N.; Huang, Z.L.; Li, H.H.; Gao, X.X.; Zhang, W.M. Highly substituted benzophenone aldehydes and eremophilane derivatives from the deep-sea derived fungus Phomopsis lithocarpus FS508. Mar. Drugs 2018, 16, 329. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tan, H.; Chen, Y.; Li, S.; Huang, Z.; Guo, H.; Li, H.; Gao, X.; Liu, H.; Zhang, M.W. Lithocarpins A–D: Four tenellone-macrolide conjugated [4 + 2] hetero-adducts from the deep-sea derived fungus Phomopsis lithocarpus FS508. Org. Chem. Front. 2018, 5, 1792–1797. [Google Scholar] [CrossRef]
- Fan, Z.; Sun, Z.-H.; Liu, Z.; Chen, Y.-C.; Liu, H.-X.; Li, H.-H.; Zhang, W.M. Dichotocejpins A–C: New diketopiperazines from a deep-sea-derived fungus Dichotomomyces cejpii FS110. Mar. Drugs 2016, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Gordon, W.K.; David, J.R.; Mark, A.S.; Ratnaker, R.T. Biosynthesis of bisdethiobis(methylthio)gliotoxin, a new metabolite of Gliocladium deliquescens. J. Chem. Soc. Perk. Trans. 1 1980, 1, 119–121. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Kalinovskii, A.I.; Pivkin, M.V.; Dmitrenok, P.S.; Kuznetsova, T.A. Alkaloids from the marine isolate of the fungus Aspergillus fumigatus. Chem. Nat. Compd. 2005, 41, 236–238. [Google Scholar] [CrossRef]
- Liang, W.L.; Le, X.; Li, H.J.; Yang, X.L.; Chen, J.X.; Xu, J.; Liu, H.L.; Wang, L.Y.; Wang, K.T.; Hu, K.C.; Yang, D.P.; Lan, W.J. Exploring the chemodiversity and biological activities of the secondary metabolites from the marine fungus Neosartorya pseudofischeri. Mar. Drugs 2014, 12, 5657–5676. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Takada, K.; Takemoto, Y.; Yoshida, M.; Nogi, Y.; Okada, S.; Matsunaga, S. Gliotoxin analogues from a marine-derived fungus, Penicillium sp., and their cytotoxic and histone methyltransferase inhibitory activities. J. Nat. Prod. 2012, 75, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Forseth, R.R.; Fox, E.M.; Chung, D.; Howlett, B.J.; Keller, N.P.; Schroeder, F.C. Identification of cryptic products of the gliotoxin gene cluster using NMR-based comparative metabolomics and a model for gliotoxin biosynthesis. J. Am. Chem. Soc. 2011, 133, 9678–9681. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Palasarn, S.; Rachtawee, P.; Vimuttipong, S.; Kongsaeree, P. Unique diketopiperazine dimers from the insect pathogenic fungus Verticillium hemipterigenum BCC 1449. Org. Lett. 2005, 7, 2257–2260. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Zhu, T.J.; Fan, G.T.; Liu, H.B.; Fang, Y.C.; Gu, Q.Q.; Zhu, W.M. Three new dioxopiperazine metabolites from a marine-derived fungus Aspergillus fumigatus Fres. Nat. Prod. Res. 2010, 24, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Orlikova, B.; Ji, S.; Nesaei-Mosaferan, D.; König, G.M.; Diederich, M. Epipolythiodiketopiperazines from the marine derived fungus Dichotomomyces cejpii with NF-κB inhibitory potential. Mar. Drugs 2015, 13, 4949–4966. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16W, Revision A.03. Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Centeno, J.M.; Burguete, M.C.; Castelló-Ruiz, M.; Enrique, M.; Vallés, S.; Salom, J.B.; Torregrosa, G.; Marcos, J.F.; Alborch, E.; Manzanares, P. Lactoferricin-related peptides with inhibitory effects on ACE-dependent vasoconstriction. J. Agric. Food. Chem. 2006, 54, 5323–5329. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yang, X.W.; Wang, R.F. Bio-assay guided isolation and identification of α-glucosidase inhibitors from the leaves of Aquilaria sinensis. Phytochemistry 2011, 72, 242–247. [Google Scholar] [CrossRef] [PubMed]
 ), and NOESY (
), and NOESY (  ) correlations for compounds 1 and 2.
) correlations for compounds 1 and 2.

 ), and NOESY (
), and NOESY (  ) correlations for geospallin C (3).
) correlations for geospallin C (3).
| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| 1H | 13C | 1H | 13C | 1H | 13C | |
| 1 | 163.5 | 166.1 | 165.0 | |||
| 3 | 76.8 | 77.9 | 70.8 | |||
| 4 | 167.6 | 167.6 | 162.6 | |||
| 6 | 4.23, d (1.8) | 70.5 | 4.32, d (1.8) | 70.5 | 4.96, d (11.0) | 69.8 |
| 7 | 5.48, d (5.6) | 65.2 | 5.32, d (3.2) | 66.4 | 5.80, d (11.0) | 75.1 |
| 8 | 6.00, m | 127.4 | 5.96, d (3.2) | 127.4 | 191.6 | |
| 9 | 6.00, m | 137.2 | 5.96, d (3.2) | 137.2 | 6.09, d (10.3) | 125.4 |
| 10 | 3.66, m | 51.8 | 3.65, s | 51.3 | 6.98, d (10.3) | 150.5 |
| 11 | 81.0 | 81.4 | 75.1 | |||
| 12 | 2.54, m 1.86, d (14.2) | 42.3 | 2.28, d (8.5) 2.12, d (14.2) | 45.6 | 3.07, d (14.9) 2.95, d (14.9) | 49.3 |
| 13 | 90.6 | 86.3 | 69.1 | |||
| 14 | 3.93, dd (11.7, 6.7) 3.68, m | 62.8 | 3.81, m 3.71, dd (10.9, 3.6) | 62.2 | 4.18, dd (11.5, 6.0) 3.74, dd (11.5, 4.7) | 62.6 |
| N-Me | 2.99, s | 28.6 | 2.99, s | 29.1 | 2.99, s | 28.8 |
| SMe-3 | 1.76, s | 11.0 | 1.80, d (3.6) | 10.6 | 2.16, s | 13.0 |
| OAc-7 | 169.4 | 169.4 | 168.9 | |||
| 2.05, s | 20.6 | 2.05, s | 20.6 | 2.07, s | 20.4 | |
| SMe-10 | 2.26, s | 16.1 | 2.26, s | 16.1 | ||
| OMe-13 | 3.15, s | 52.1 | 2.18, s | 14.6 | ||
| 11-OH | 5.60, s | 5.68, s | 6.00, s | |||
| 13-OH | 6.04, brs | |||||
| 14-OH | 5.52, s | 6.64, brs | 5.34, t (5.4) | |||
| Compounds | IC50 (µM) |
|---|---|
| 1 | 35 ± 5.2 |
| 2 | 31 ± 3.3 |
| 3 | 29 ± 3.3 |
| Captopril | 0.041 ± 0.005 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.-H.; Gu, J.; Ye, W.; Wen, L.-X.; Lin, Q.-B.; Li, S.-N.; Chen, Y.-C.; Li, H.-H.; Zhang, W.-M. Geospallins A–C: New Thiodiketopiperazines with Inhibitory Activity against Angiotensin-Converting Enzyme from a Deep-Sea-Derived Fungus Geosmithia pallida FS140. Mar. Drugs 2018, 16, 464. https://doi.org/10.3390/md16120464
Sun Z-H, Gu J, Ye W, Wen L-X, Lin Q-B, Li S-N, Chen Y-C, Li H-H, Zhang W-M. Geospallins A–C: New Thiodiketopiperazines with Inhibitory Activity against Angiotensin-Converting Enzyme from a Deep-Sea-Derived Fungus Geosmithia pallida FS140. Marine Drugs. 2018; 16(12):464. https://doi.org/10.3390/md16120464
Chicago/Turabian StyleSun, Zhang-Hua, Jiangyong Gu, Wei Ye, Liang-Xi Wen, Qi-Bin Lin, Sai-Ni Li, Yu-Chan Chen, Hao-Hua Li, and Wei-Min Zhang. 2018. "Geospallins A–C: New Thiodiketopiperazines with Inhibitory Activity against Angiotensin-Converting Enzyme from a Deep-Sea-Derived Fungus Geosmithia pallida FS140" Marine Drugs 16, no. 12: 464. https://doi.org/10.3390/md16120464