Angucycline Glycosides from an Intertidal Sediments Strain Streptomyces sp. and Their Cytotoxic Activity against Hepatoma Carcinoma Cells
Abstract
1. Introduction
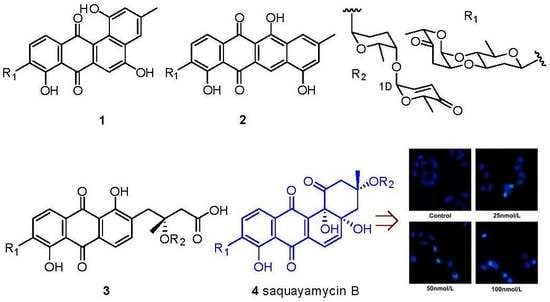
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Actinomycetes Strain
3.3. Fermentation, Extraction and Isolation
3.4. Cytotoxicity Assays, DAPI Staining Test and Flow Cytometric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rohr, J.; Thiericke, R. Angucycline group antibiotics. Nat. Prod. Rep. 1992, 9, 103–137. [Google Scholar] [CrossRef] [PubMed]
- Kharel, M.K.; Pahari, P.; Shepherd, M.D.; Tibrewal, N.; Nybo, S.E.; Shaaban, K.A.; Rohr, J. Angucyclines: biosynthesis, mode-of-action, new natural products, and synthesis. Nat. Prod. Rep. 2012, 29, 264–325. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, K.A.; Ahmed, T.A.; Leggas, M.; Rohr, J. Saquayamycins G-K, cytotoxic angucyclines from Streptomyces sp. including two analogues bearing the aminosugar rednose. J. Nat. Prod. 2012, 75, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Gui, C.; Liu, Y.N.; Zhou, Z.B.; Zhang, S.W.; Hu, Y.F.; Gu, Y.C.; Huang, H.B.; Ju, J.H. Angucycline glycosides from mangrove-derived Streptomyces diastaticus subsp. SCSIO GJ056. Mar. Drugs 2018, 16, 185. [Google Scholar] [CrossRef] [PubMed]
- Erb, A.; Luzhetskyy, A.; Hardter, U.; Bechthold, A. Cloning and sequencing of the biosynthetic gene cluster for saquayamycin Z and galtamycin B and the elucidation of the assembly of their saccharide chains. ChemBioChem 2009, 10, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.B.; Yang, T.T.; Ren, X.M.; Liu, J.; Song, Y.X.; Sun, A.J.; Ma, J.Y.; Wang, B.; Zhang, Y.; Huang, C.G.; et al. Cytotoxic angucycline class glycosides from the deep sea actinomycete Streptomyces lusitanus SCSIO LR32. J. Nat. Prod. 2012, 75, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.C.; Duan, Y.W.; Cui, Z.M.; Wang, Z.; Li, Z.X.; Zhang, Y.; Ju, J.H.; Huang, H.B. Cytotoxic rearranged angucycline glycosides from deep sea-derived Streptomyces lusitanus SCSIO LR32. J. Antibiot. 2017, 70, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Helaly, S.E.; Goodfellow, M.; Zinecker, H.; Imhoff, J.F.; Sussmuth, R.D.; Fiedler, H.P. Warkmycin, a novel angucycline antibiotic produced by Streptomyces sp. Acta 2930. J. Antibiot. 2013, 66, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Hara, C.; Tokuyama, S.; Takada, K.; Imamura, N. Saprolmycins A-E, new angucycline antibiotics active against Saprolegnia parasitica. J. Antibiot. 2012, 65, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Panchuk, R.R.; Lehka, L.V.; Terenzi, A.; Matselyukh, B.P.; Rohr, J.; Jha, A.K.; Downey, T.; Kril, I.J.; Herbacek, I.; van Schoonhoven, S.; et al. Rapid generation of hydrogen peroxide contributes to the complex cell death induction by the angucycline antibiotic landomycin E. Free Radical Bio. Med. 2017, 106, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Korynevska, A.; Heffeter, P.; Matselyukh, B.; Elbling, L.; Micksche, M.; Stoika, R.; Berger, W. Mechanisms underlying the anticancer activities of the angucycline landomycin E. Biochem. Pharmacol. 2007, 74, 1713–1726. [Google Scholar] [CrossRef] [PubMed]
- Fidan, O.; Yan, R.M.; Gladstone, G.; Zhou, T.; Zhu, D.; Zhan, J.X. New insights into the glycosylation steps in the biosynthesis of Sch47554 and Sch47555. ChemBioChem 2018, 19, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.M.; Weidenbach, S.; Rohr, J. Two cooperative glycosyltransferases are responsible for the sugar diversity of saquayamycins isolated from Streptomyces sp. KY 40-1. Acs Chem. Biol. 2017, 12, 2529–2534. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.S.; Yang, C.F.; Zhang, W.J.; Zhang, L.P.; De, B.C.; Zhu, Y.G.; Jiang, X.D.; Fang, C.Y.; Zhang, Q.B.; Yuan, C.S.; Liu, H.W.; Zhang, C.S. Molecular basis of dimer formation during the biosynthesis of benzofluorene-containing atypical angucyclines. Nat. Commun. 2018, 9, 2088. [Google Scholar] [CrossRef] [PubMed]
- Jose, P.A.; Jha, B. Intertidal marine sediment harbours Actinobacteria with promising bioactive and biosynthetic potential. Sci. Rep. 2017, 7, 10041. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.S.; Ye, W.C.; Wang, Z.T.; Che, C.T.; Zhou, R.H.; Xu, G.J.; Xu, L.S. β-Carboline alkaloids from Hypodematium squamuloso-pilosum. Phytochemistry 1998, 49, 1807–1809. [Google Scholar] [CrossRef]
- Han, X.; Hou, L.K.; Hou, J.; Zhang, Y.Y.; Li, H.Y.; Li, W.L. Heterologous expression of a VioA variant activates cryptic compounds in a marine-derived Brevibacterium strain. Mar. Drugs 2018, 16, 191. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Imoto, M.; Watanabe, Y.; Miura, K.; Dobashi, T.; Matsuda, N.; Sawa, T.; Naganawa, H.; Hamada, M.; Takeuchi, T.; et al. Saquayamycins, new aquayamycin-group antibiotics. J. Antibiot. 1985, 38, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.M.; He, J.; Jiang, J.S.; Chen, Z.; Yang, Y.N.; Zhang, P.C. NMR solution structure study of the representative component hydroxysafflor yellow A and other quinochalcone C-glycosides from Carthamus tinctorius. J. Nat. Prod. 2013, 76, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.X.; Liu, G.F.; Li, J.; Huang, H.B.; Zhang, X.; Zhang, H.; Ju, J.H. Cytotoxic and antibacterial angucycline- and prodigiosin-analogues from the deep-sea derived Streptomyces sp. SCSIO 11594. Mar. Drugs 2015, 13, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Stroch, K.; Zeeck, A.; Antal, N.; Fiedler, H.P. Retymicin, galtamycin B, saquayamycin Z and ribofuranosyllumichrome, novel secondary metabolites from Micromonospora sp. Tu 6368-II. Structure elucidation. J. Antibiot. 2005, 58, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Maskey, R.P.; Helmke, E.; Laatsch, H. Himalomycin A and B: Isolation and structure elucidation of new fridamycin type antibiotics from a marine Streptomyces isolate. J. Antibiot. 2003, 56, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Herzon, S.B. The mechanism of action of (-)-lomaiviticin A. Accounts Chem. Res. 2017, 50, 2577–2588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Liu, X.; Wang, Z.; Tian, Z.H.; Xie, W.D. Viridobrunnines A and B, antimicrobial phenoxazinone alkaloids from a soil associated Streptomyces sp. Heterocycles 2015, 91, 1809–1814. [Google Scholar]
- Liu, S.S.; Wang, Y.F.; Ma, L.S.; Zheng, B.B.; Li, L.; Xie, W.D.; Li, X. 1-Oxoeudesm-11(13)-eno-12,8a-lactone induces G2/M arrest and apoptosis of human glioblastoma cells in vitro. Acta Pharmacol. Sin. 2013, 34, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.B.; Wu, L.H.; Ma, L.S.; Liu, S.S.; Li, L.; Xie, W.D.; Li, X. Telekin induces apoptosis associated with the mitochondria-mediated pathway in human hepatocellular carcinoma cells. Biol. Pharm. Bull. 2013, 36, 1118–1125. [Google Scholar] [CrossRef] [PubMed]





| No. | 1 b | 2 b | 3 c | |||
|---|---|---|---|---|---|---|
| δC type | δH, mult (J in Hz) | δC type | δH, mult (J in Hz) | δC type | δH, mult (J in Hz) | |
| 1 | 155.4 C | - | 155.9 C | - | 172.1 C | - |
| 2 | 119.4 CH | 6.96, brs | 116.2 CH | 6.95, brs | 44.6 CH2 | 2.63, d (15.0) 2.72, d (15.0) |
| 3 | 139.0 C | - | 141.8 C | - | 78.0 C | - |
| 4 | 114.2 CH | 7.26, brs | 114.2 CH | 7.52, brs | 39.0 CH2 | 3.19, d (13.4) 3.23, d (13.4) |
| 4a | 130.6 C | - | 128.2 C | - | 136.4 C | - |
| 5 | 166.4 C | - | 124.1 C | - | 140.9 CH | 7.84, d (7.8) |
| 6 | 106.4 CH | 7.46, s | 116.7 CH | 8.39, s | 119.2 CH | 7.75, d (7.8) |
| 6a | 137.3 C | - | 125.1 C | - | 132.5 C | - |
| 7 | 188.9 C | - | 187.3 C | - | 189.1 C | - |
| 7a | 114.1 C | - | 116.2 C | - | 116.3 C | - |
| 8 | 156.9 C | - | 158.4 C | - | 159.6 C | - |
| 9 | 135.0 C | - | 136.3 C | - | 138.8 C | - |
| 10 | 133.7 CH | 7.84, d (7.9) | 133.2 CH | 7.87, d (7.8) | 134.3 CH | 7.94, d (7.8) |
| 11 | 119.6 CH | 7.72, d (7.9) | 118.4 CH | 7.73, d (7.8) | 119.9 CH | 7.80, d (7.8) |
| 11a | 134.7 C | - | 132.4 C | - | 133.0 C | - |
| 12 | 182.6 C | - | 186.3 C | - | 189.2 C | - |
| 12a | 119.6 C | - | 108.8 C | - | 116.4 C | - |
| 12b | 122.1 C | - | 162.1 C | - | 162.4 C | - |
| 13 | 20.9 CH3 | 2.40, s | 21.9 CH3 | 2.40, s | 23.5 CH3 | 1.43, s |
| OH | - | 12.53, brs | - | 14.40, brs | - | 13.14, brs |
| OH | - | 12.08, brs | - | 13.41, brs | - | 13.10, brs |
| OH | - | - | 10.92, brs | - | ||
| Sugar A, β-d-olivose | ||||||
| 1A | 70.4 CH | 4.97, brd (10.5) | 70.5 CH | 4.96, brd (10.8) | 72.1 CH | 5.01, brd (10.9) |
| 2A | 35.9 CH2 | 1.63, ddd (11.6, 11.6, 10.5) 2.22, m | 35.8 CH2 | 1.61, ddd (11.7, 11.7, 10.8) 2.24, m | 37.4 CH2 | 1.60, ddd (11.6, 11.6, 10.9) 2.40, m |
| 3A | 75.7 CH | 3.85, ddd (11.6, 9.0, 4.4) | 75.7 CH | 3.86, ddd (11.7, 9.0, 4.3) | 77.4 CH | 3.88, ddd (11.6, 8.9, 4.4) |
| 4A | 73.6 CH | 3.51, dd (9.0, 9.0) | 73.6 CH | 3.51, dd (9.0, 9.0) | 75.1 CH | 3.58, dd (8.9, 8.9) |
| 5A | 73.5 CH | 3.59, m | 73.5 CH | 3.60, m | 75.1 CH | 3.62, m |
| 6A | 17.4 CH3 | 1.26, d (6.0) | 17.4 CH3 | 1.27, d (6.0) | 17.9 CH3 | 1.34, d (5.8) |
| Sugar B, α-l-cinerulose B | ||||||
| 1B | 90.5 CH | 5.22, d (2.6) | 90.2 CH | 5.23, d (2.4) | 92.2 CH | 5.26, d (2.8) |
| 2B | 70.8 CH | 4.34, m | 70.8 CH | 4.35, m | 72.3 CH | 4.33, m |
| 3B | 39.6 CH2 | 2.47, dd (17.4, 2.6) 2.90, dd (17.4, 2.6) | 39.8 CH2 | 2.47, dd (17.3, 3.4) 2.91, dd (17.4, 2.6) | 40.6 CH2 | 2.53, dd (17.3, 3.6) 2.84, dd (17.3, 2.7) |
| 4B | 208.7 C | - | 208.7 C | - | 208.5 C | - |
| 5B | 76.9 CH | 4.72, q (6.6) | 76.9 CH | 4.72, q (6.6) | 78.2 CH | 4.76, q (6.8) |
| 6B | 16.0 CH3 | 1.24, d (6.6) | 16.0 CH3 | 1.25, d (6.6) | 16.5 CH3 | 1.26, d (6.8) |
| Sugar C, α-l-rhodinose | ||||||
| 1C | 92.0 CH | 5.20, brs | ||||
| 2C | 26.2 CH2 | 1.40, m 1.95, m | ||||
| 3C | 25.3 CH2 | 1.90, m 2.10, m | ||||
| 4C | 77.4 | 3.65, m | ||||
| 5C | 67.0 CH | 4.09, m | ||||
| 6C | 17.5 CH3 | 1.10, d (6.6) | ||||
| Sugar D, α-l-aculose | ||||||
| 1D | 96.0 CH | 5.31, d (3.5) | ||||
| 2D | 145.2 CH | 7.03, dd (10.2, 3.5) | ||||
| 3D | 127.2 CH | 6.02, d (10.2) | ||||
| 4D | 197.3 C | - | ||||
| 5D | 71.0 CH | 4.56, q (6.8) | ||||
| 6D | 15.5 CH3 | 1.27, d (6.8) | ||||
| Compounds | Cell Lines | |||
|---|---|---|---|---|
| LO2 | HepG-2 | SMMC-7721 | plc-prf-5 | |
| 1 | >40 | >40 | >40 | >40 |
| 2 | >40 | >40 | >40 | >40 |
| 3 | >40 | >40 | >40 | >40 |
| 4 | 0.343 ± 0.081 | 0.135 ± 0.056 | 0.033 ± 0.005 | 0.244 ± 0.001 |
| Doxorubicin | 2.26 ± 0.16 | 0.919 ± 0.599 | 0.706 ± 0.004 | 1.03 ± 0.99 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, A.; Qu, X.; Liu, F.; Li, X.; Li, E.; Xie, W. Angucycline Glycosides from an Intertidal Sediments Strain Streptomyces sp. and Their Cytotoxic Activity against Hepatoma Carcinoma Cells. Mar. Drugs 2018, 16, 470. https://doi.org/10.3390/md16120470
Peng A, Qu X, Liu F, Li X, Li E, Xie W. Angucycline Glycosides from an Intertidal Sediments Strain Streptomyces sp. and Their Cytotoxic Activity against Hepatoma Carcinoma Cells. Marine Drugs. 2018; 16(12):470. https://doi.org/10.3390/md16120470
Chicago/Turabian StylePeng, Aihong, Xinying Qu, Fangyuan Liu, Xia Li, Erwei Li, and Weidong Xie. 2018. "Angucycline Glycosides from an Intertidal Sediments Strain Streptomyces sp. and Their Cytotoxic Activity against Hepatoma Carcinoma Cells" Marine Drugs 16, no. 12: 470. https://doi.org/10.3390/md16120470
APA StylePeng, A., Qu, X., Liu, F., Li, X., Li, E., & Xie, W. (2018). Angucycline Glycosides from an Intertidal Sediments Strain Streptomyces sp. and Their Cytotoxic Activity against Hepatoma Carcinoma Cells. Marine Drugs, 16(12), 470. https://doi.org/10.3390/md16120470