New Antibacterial Phenone Derivatives Asperphenone A–C from Mangrove-Derived Fungus Aspergillus sp. YHZ-1
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Strain Isolation and Cultivation
3.3. Extraction and Isolation
3.4. Crystal Data of 1
3.5. Crystal Data of 2
3.6. Antibacterial Activity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gomes, N.G.M.; Lefranc, F.; Kijjoa, A.; Kiss, R. Can some marine-derived fungal metabolites become actual anticancer agents? Mar. Drugs 2015, 13, 3950–3991. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.J.; Meng, W.; Cao, C.; Wang, J.; Shan, W.J.; Wang, Q.G. Antibacterial and antifungal compounds from marine fungi. Mar. Drugs 2015, 13, 3479–3513. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Nikzad, S.; Kadir, H.A.; Abubakar, S.; Zandi, K. Potential antiviral agents from marine fungi: An overview. Mar. Drugs 2015, 13, 4520–4538. [Google Scholar] [CrossRef] [PubMed]
- Han, W.B.; Lu, Y.H.; Zhang, A.H.; Zhang, G.F.; Mei, Y.N.; Jiang, N.; Lei, X.; Song, Y.C.; Ng, S.W.; Tan, R.X. Curvulamine, a new antibacterial alkaloid incorporating two undescribed units from a Curvularia species. Org. Lett. 2014, 16, 5366–5369. [Google Scholar] [CrossRef] [PubMed]
- Han, W.B.; Zhang, A.H.; Deng, X.Z.; Lei, X.; Tan, RX. Curindolizine, an anti-inflammatory agent assembled via Michael addition of pyrrole alkaloids inside fungal cells. Org. Lett. 2016, 18, 1816–1819. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Xu, G.M.; Li, X.M.; Li, C.S.; Wang, B.G. Characterization of a newly isolated marine fungus Aspergillus dimorphicus for optimized production of the anti-tumor agent Wentilactones. Mar. Drugs 2015, 13, 7040–7054. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Shao, C.L.; Wu, L.Y.; Chen, M.; Wang, K.L.; Zhao, D.L.; Sun, X.P.; Chen, G.Y.; Wang, C.Y. Bioactive phenylalanine derivatives and cytochalasins from the soft coral-derived fungus, Aspergillus elegans. Mar. Drugs 2013, 11, 2054–2068. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.K.; Gai, C.J.; Cai, C.H.; Chen, L.L.; Liu, S.B.; Zeng, Y.B.; Yuan, J.Z.; Mei, W.L.; Dai, H.F. Metabolites with insecticidal activity from Aspergillus fumigatus JRJ111048 isolated from mangrove plant Acrostichum specioum endemic to Hainan island. Mar. Drugs 2017, 15, 381. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.Y.; Wei, W.; Guo, Y.; Wang, T.; Jiao, R.H.; Ng, S.W.; Tan, R.X.; Ge, H.M. Sesquiterpenoids from the mangrove-derived endophytic fungus Diaporthe sp. J. Nat. Prod. 2012, 75, 1744–1749. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Ge, H.M.; Zang, L.Y.; Bei, Y.C.; Niu, Z.Y.; Wei, W.; Feng, X.J.; Ding, S.; Ng, S.W.; Shen, P.P.; et al. Diaporine, a novel endophyte-derived regulator of macrophage differentiation. Org. Biomol. Chem. 2014, 12, 6545–6548. [Google Scholar] [CrossRef] [PubMed]
- Wuringege; Guo, Z.K.; Wei, W.; Jiao, R.H.; Yan, T.; Zang, L.Y.; Jiang, R.; Tan, R.X.; Ge, H.M. Polyketides from the plant endophytic fungus Cladosporium sp. IFB3lp-2. J. Asian Nat. Prod. Res. 2013, 15, 928–933. [Google Scholar]
- Ma, S.Y.; Xiao, Y.S.; Zhang, B.; Shao, F.L.; Guo, Z.K.; Zhang, J.J.; Jiao, R.H.; Sun, Y.; Xu, Q.; Tan, R.X.; et al. Amycolamycins A and B, two enediyne-derived compounds from a locust-associated actinomycete. Org. Lett. 2017, 19, 6208–6211. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.S.; Zhang, B.; Zhang, M.; Guo, Z.K.; Deng, X.Z.; Shi, J.; Li, W.; Jiao, R.H.; Tan, R.X.; Ge, H.M. Rifamorpholines A-E, potential antibiotics from a locust-associated actinobacteria Amycolatopsis sp. Hca4. Org. Biomol. Chem. 2017, 15, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
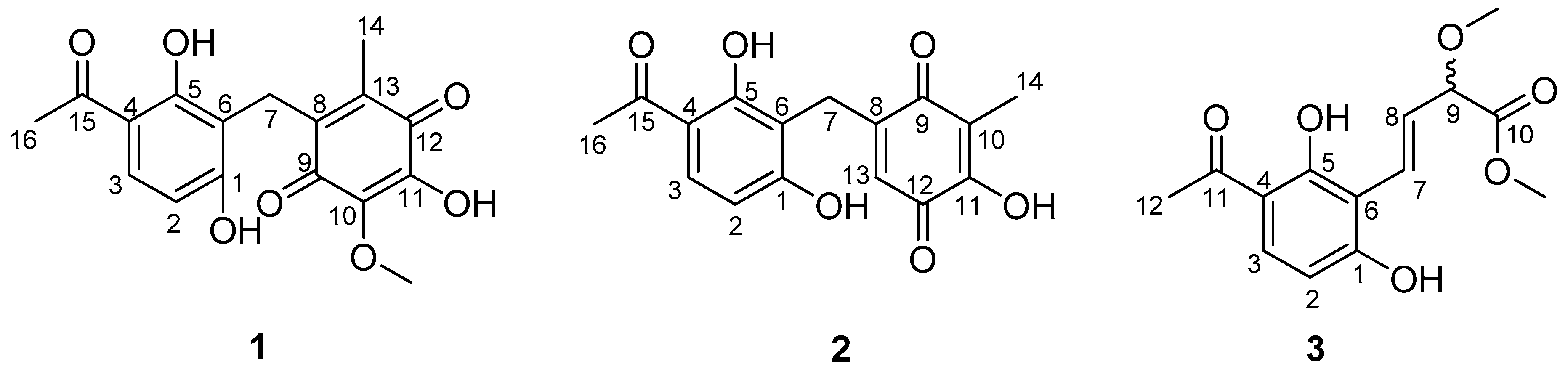
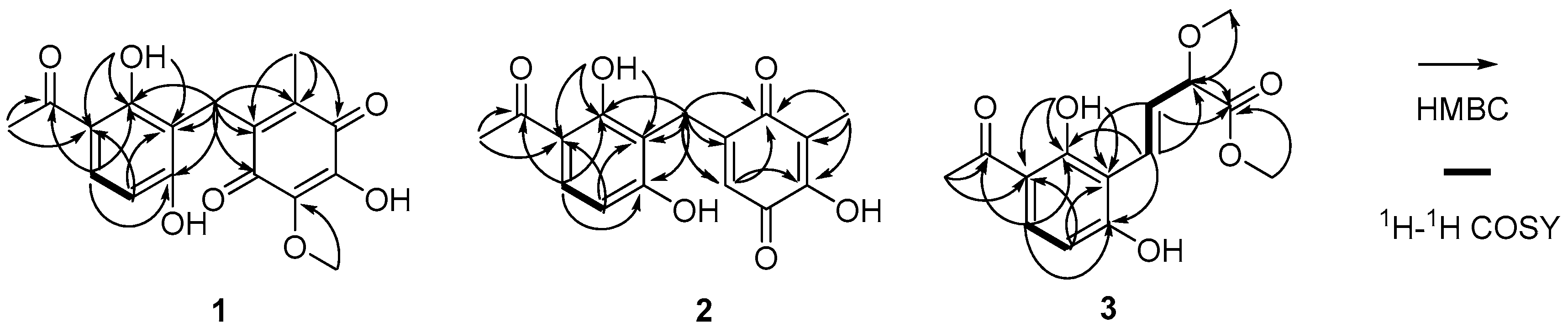
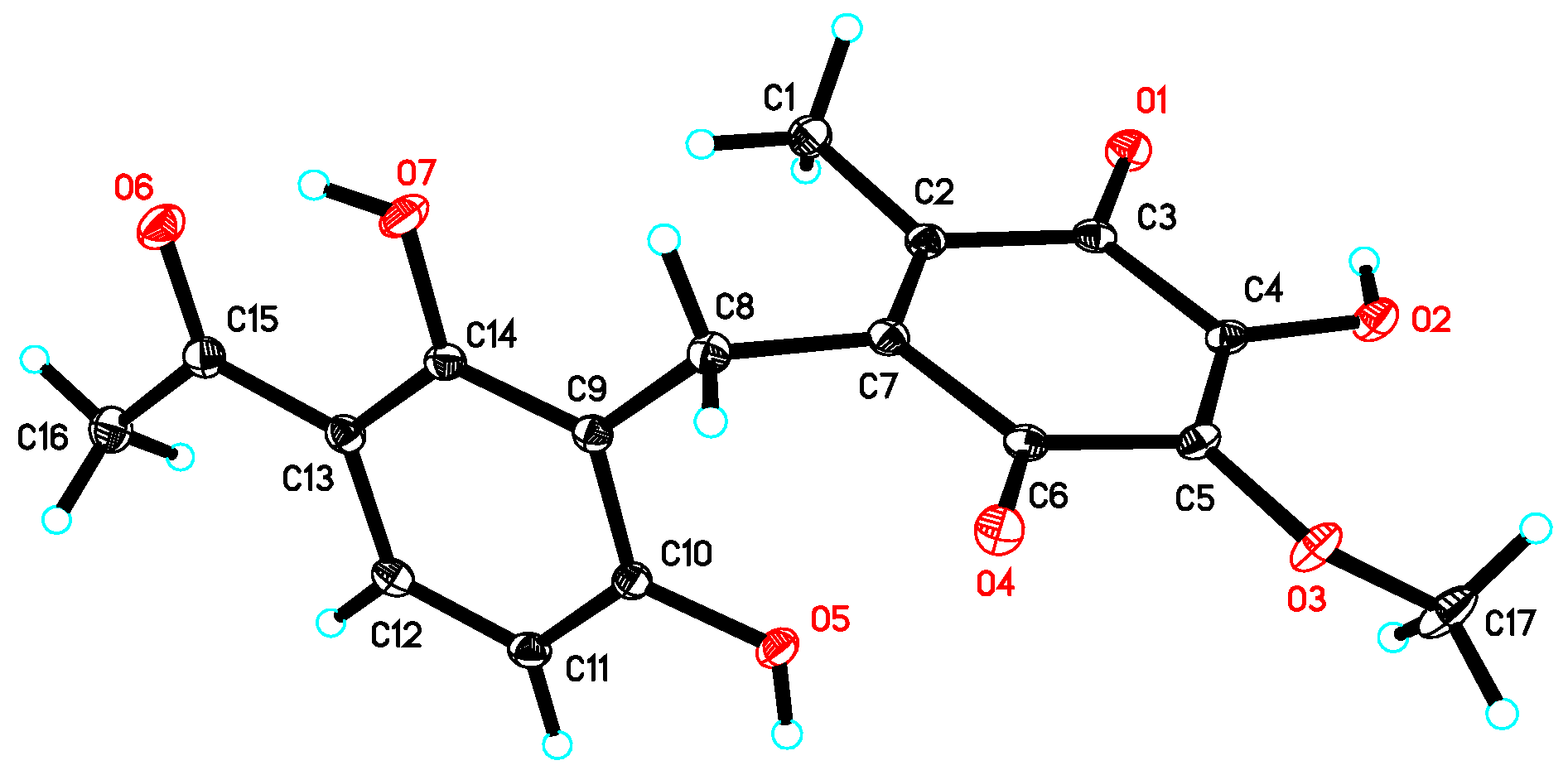
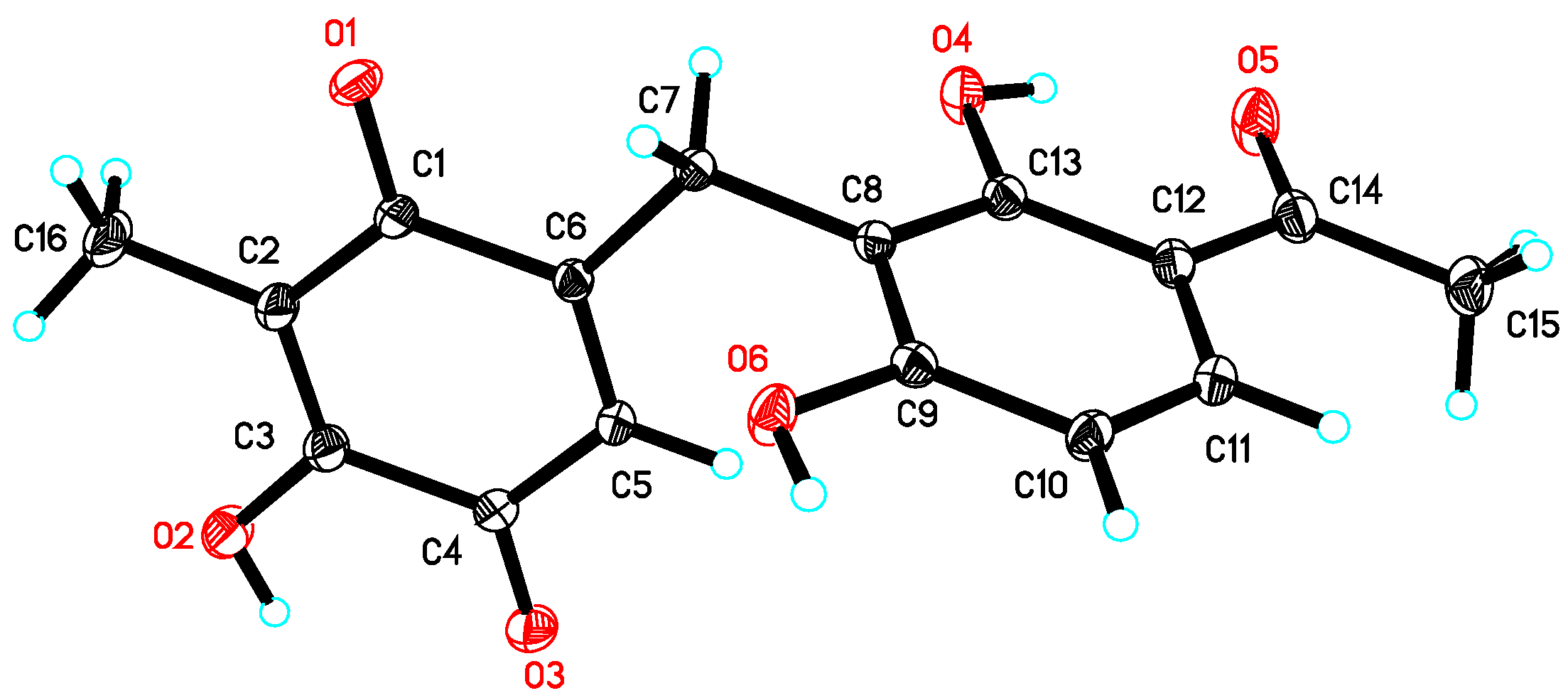
| No. | 1 a | 2 a | 3 b | |||
|---|---|---|---|---|---|---|
| δH, Mult. (J in Hz) | δC, Type | δH, Mult. (J in Hz) | δC, Type | δH, Mult. (J in Hz) | δC, Type | |
| 1 | 162.7, C | 162.3, C | 163.3, C | |||
| 1-OH | 10.25 c, brs | 10.75, brs | ||||
| 2 | 6.42, d (8.8) | 107.8, CH | 6.52, d (8.8) | 107.4, CH | 6.55, d (8.6) | 108.3, CH |
| 3 | 7.67, d (8.8) | 131.8, CH | 7.77, d (8.8) | 132.1, CH | 7.71, d (8.6) | 132.8, CH |
| 4 | 112.8, C | 112.5, C | 113.8, C | |||
| 5 | 162.4, C | 162.7, C | 164.4, C | |||
| 5-OH | 13.21, s | 13.09, s | 13.73, s | |||
| 6 | 112.0, C | 109.5, C | 111.4, C | |||
| 7 | 3.70, s | 19.4, CH2 | 3.61, d (2.0) | 22.0, CH2 | 7.07, dd (16.2, 0.8) | 124.1, CH |
| 8 | 136.0 d, C | 148.5, C | 6.78, dd (16.2, 7.0) | 128.6, CH | ||
| 9 | 182.7, C | 187.2, C | 4.42, dd (7.0, 0.8) | 83.3, CH | ||
| 9-OMe | 3.37, s | 57.0, CH3 | ||||
| 10 | 138.8, C | 116.9, C | 171.7, C | |||
| 10-OMe | 3.78, s | 60.3, CH3 | 3.70, s | 52.0, CH3 | ||
| 11 | 143.3, C | 153.8, C | 204.3, C | |||
| 11-OH | 10.85 c, br s | 10.75, brs | ||||
| 12 | 185.1, C | 183.0, C | 2.56, s | 26.3, CH3 | ||
| 13 | 144.5 d, C | 5.76, t (2.0) | 127.1, CH | |||
| 14 | 1.73, s | 11.6, CH3 | 1.82, s | 8.15, CH3 | ||
| 15 | 203.8, C | 203.4, C | ||||
| 16 | 2.52, s | 26.6, CH3 | 2.55, s | 26.2, CH3 | ||
| Compounds | S. aureus | B. subtilis | S. pyogenes | M. luteus |
|---|---|---|---|---|
| 1 | 64.0 | 64.0 | 64.0 | 32.0 |
| 2 | 32.0 | 64.0 | 32.0 | 32.0 |
| Ampicillin | 4.0 | 8.0 | 2.0 | 1.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.-K.; Zhou, Y.-Q.; Han, H.; Wang, W.; Xiang, L.; Deng, X.-Z.; Ge, H.-M.; Jiao, R.-H. New Antibacterial Phenone Derivatives Asperphenone A–C from Mangrove-Derived Fungus Aspergillus sp. YHZ-1. Mar. Drugs 2018, 16, 45. https://doi.org/10.3390/md16020045
Guo Z-K, Zhou Y-Q, Han H, Wang W, Xiang L, Deng X-Z, Ge H-M, Jiao R-H. New Antibacterial Phenone Derivatives Asperphenone A–C from Mangrove-Derived Fungus Aspergillus sp. YHZ-1. Marine Drugs. 2018; 16(2):45. https://doi.org/10.3390/md16020045
Chicago/Turabian StyleGuo, Zhi-Kai, Yi-Qin Zhou, Hao Han, Wen Wang, Lang Xiang, Xin-Zhao Deng, Hui-Ming Ge, and Rui-Hua Jiao. 2018. "New Antibacterial Phenone Derivatives Asperphenone A–C from Mangrove-Derived Fungus Aspergillus sp. YHZ-1" Marine Drugs 16, no. 2: 45. https://doi.org/10.3390/md16020045




