Briaviolides K–N, New Briarane-Type Diterpenoids from Cultured Octocoral Briareum violaceum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Identification of Isolated Briaranes
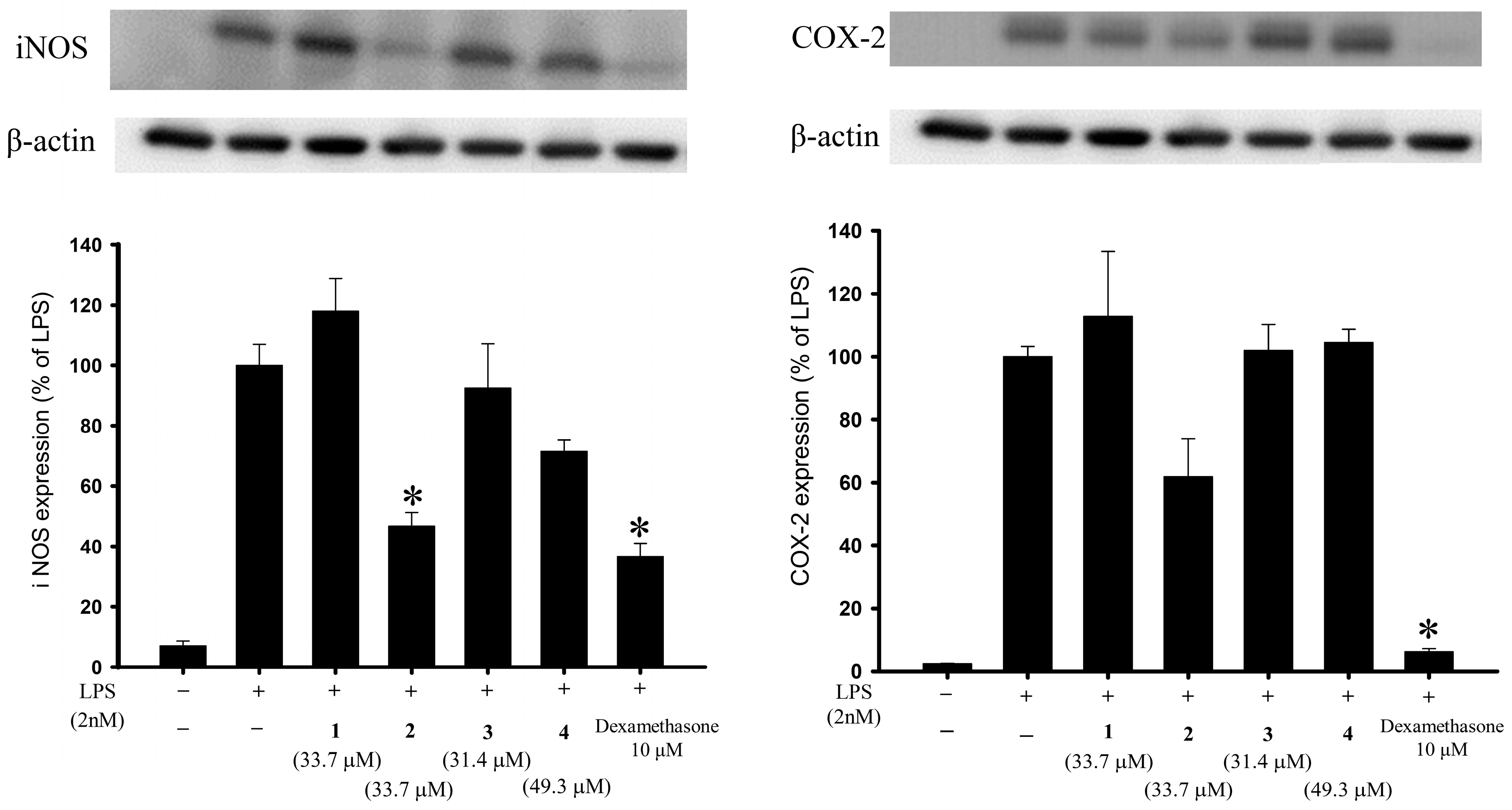
2.2. Anti-Inflammatory Activities of the Isolated Briaranes
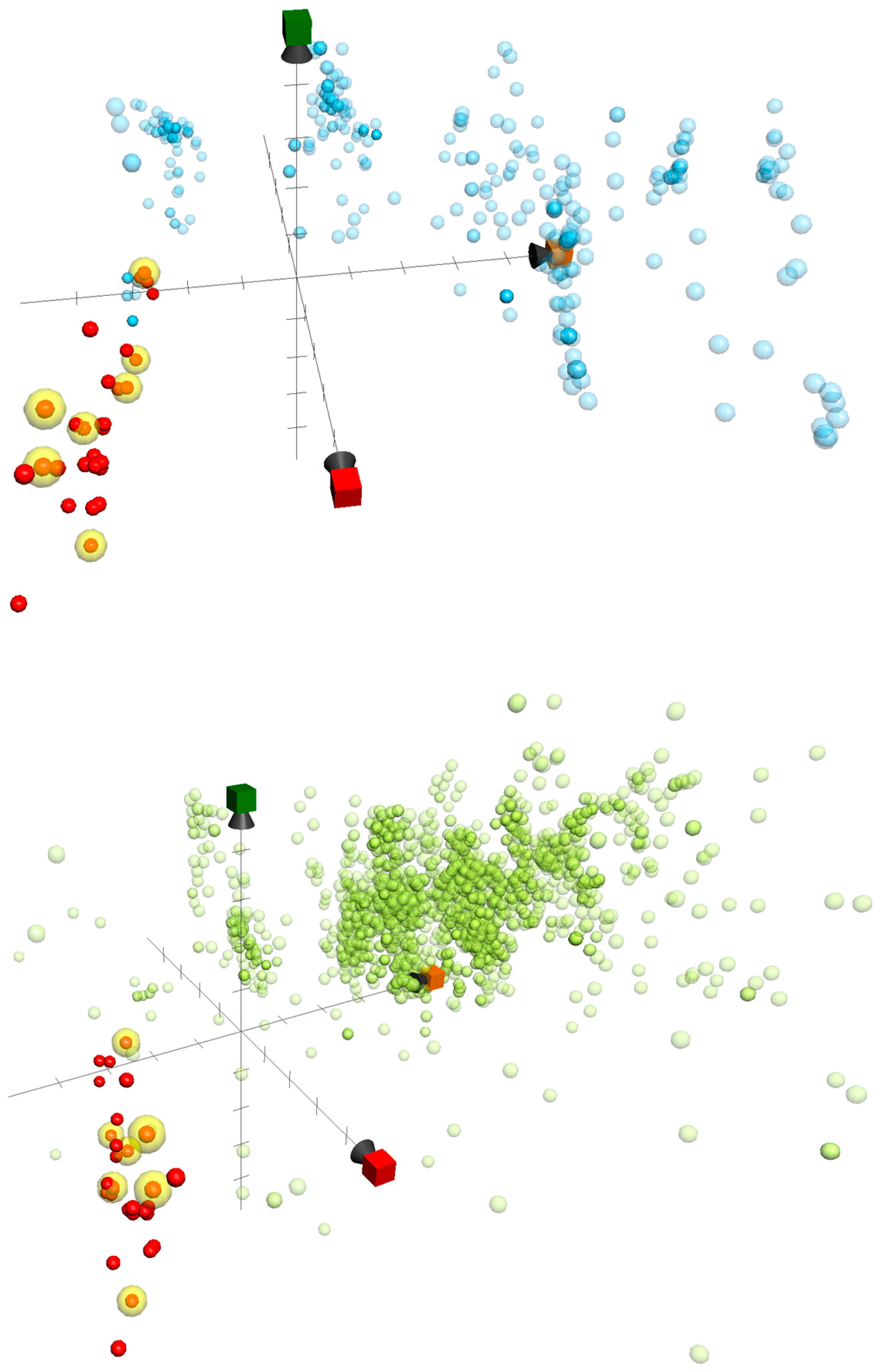
2.3. ChemGPS-NP-Based Analysis of the Active Briaranes
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Molecular Mechanics Calculations
3.5. In Vitro Anti-Inflammatory Assay
3.6. ChemGPS-NP Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Samimi-Namin, K.; van Ofwegen, L.P. Overview of the genus Briareum (Cnidaria, Octocorallia, Briareidae) in the Indo-Pacific, with the description of a new species. ZooKeys 2016, 557, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.-H.; Sung, P.-J.; Huang, L.-H.; Lee, S.-F.; Wu, T.; Chang, B.-Y.; Duh, C.-Y.; Fang, L.-S.; Soong, K.; Lee, T.-J. New cytotoxic briaran diterpenes from the Formosan gorgonian Briareum sp. J. Nat. Prod. 1996, 59, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Patrick, B.O.; Roberge, M.; Allen, T.; van Ofwegen, L.P.; Andersen, R.J. New diterpenoids from the octocoral Pachyclavularia violacea collected in Papua New Guinea. Tetrahedron 2000, 56, 9031–9037. [Google Scholar] [CrossRef]
- Iwasaki, J.; Ito, H.; Aoyagi, M.; Sato, Y.; Iguchi, K. Briarane-type diterpenoids from the Okinawan soft coral Pachyclavularia violacea. J. Nat. Prod. 2006, 69, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Iwasaki, J.; Sato, Y.; Aoyagi, M.; Iguchi, K.; Yamori, T. Marine diterepnoids with a briarane skeleton from the Okinawan soft coral Pachyclavularia violacea. Chem. Pharm. Bull. 2007, 55, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Liaw, C.-C.; Cheng, Y.-B.; Lin, Y.-S.; Kuo, Y.-H.; Hwang, T.-L.; Shen, Y.-C. New briarane diterpenoids from Taiwanese soft coral Briareum violacea. Mar. Drugs 2014, 12, 4677–4692. [Google Scholar] [CrossRef] [PubMed]
- Allinger, N.L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977, 99, 8127–8134. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chen, B.-Y.; Lin, M.-R.; Hwang, T.-L.; Wang, W.-H.; Sheu, J.-H.; Wu, Y.-C. Excavatoids E and F: Discovery of two new briaranes from the cultured octocoral Briareum excavatum. Mar. Drugs 2009, 7, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Bowden, B.F.; Coll, J.C.; Vasilescu, I.M. Studies of Australian soft corals. XLVI New diterpenes from a Briareum species (Anthozoa, Octocorallia, Gorgonacea). Aust. J. Chem. 1989, 42, 1705–1726. [Google Scholar] [CrossRef]
- Sung, P.-J.; Su, J.-H.; Duh, C.-Y.; Chiang, M.Y.; Sheu, J.-H. Briaexcavatolides K–N, new briarane diterpenes from the gorgonian Briareum excavatum. J. Nat. Prod. 2001, 64, 318–323. [Google Scholar]
- Larsson, J.; Gottfries, J.; Muresan, S.; Backlund, A. ChemGPS-NP: Tuned for navigation in biologically relevant chemical space. J. Nat. Prod. 2007, 70, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.; Lovgren, A.; Kogej, T.; Muresan, S.; Gottfries, J.; Backlund, A. ChemGPS-NP(web): Chemical space navigation online. J. Comput. Aided Mol. Des. 2009, 23, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.-H.; Lu, M.-C.; Du, Y.-C.; El-Shazly, M.; Wu, T.-Y.; Hsu, Y.-M.; Henz, A.; Yang, J.-C.; Backlund, A.; Chang, F.-R.; et al. Cytotoxic lanostanoids from Poria cocos. J. Nat. Prod. 2016, 79, 2805–2813. [Google Scholar] [CrossRef] [PubMed]
- Korinek, M.; Tsai, Y.-H.; El-Shazly, M.; Lai, K.-H.; Backlund, A.; Wu, S.-F.; Lai, W.-C.; Wu, T.-Y.; Chen, S.-L.; Wu, Y.-C.; et al. Anti-allergic hydroxy fatty acids from Typhonium blumei explored through ChemGPS-NP. Front. Pharmacol. 2017, 8, 356. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Heidmarsson, S.; Olafsdottir, E.S.; Buonfiglio, R.; Kogej, T.; Omarsdottir, S. Secondary metabolites from cetrarioid lichens: Chemotaxonomy, biological activities and pharmaceutical potential. Phytomedicine 2016, 23, 441–459. [Google Scholar] [CrossRef] [PubMed]
- Karhu, E.; Isojarvi, J.; Vuorela, P.; Hanski, L.; Fallarero, A. Identification of privileged anti-chlamydial natural products by a ligand-based strategy. J. Nat. Prod. 2017, 80, 2602–2608. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chai, C.-Z.; Yan, Y.; Duan, Y.-D.; Henz, A.; Zhang, B.-L.; Backlund, A.; Yu, B.-Y. Spasmolytic mechanism of aqueous licorice extract on oxytocin-induced uterine contraction through inhibiting the phosphorylation of heat shock protein 27. Molecules 2017, 22, 1392. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-D.; Hwang, T.-L.; Lin, N.-C.; Chen, Y.-H.; Wu, Y.-C.; Sheu, J.-H.; Sung, P.-J. Briarenolides H and I: New 8-hydroxybriarane diterpenoids from a formosan octocoral Briareum sp. (Briareidae). Bull. Chem. Soc. Jpn. 2012, 85, 1531–1536. [Google Scholar] [CrossRef]
- Su, Y.-D.; Cheng, C.-H.; Chen, W.-F.; Chang, Y.-C.; Chen, Y.-H.; Hwang, T.-L.; Wen, Z.-H.; Wang, W.-H.; Fang, L.-S.; Chen, J.-J.; et al. Briarenolide J, the first 12-chlorobriarane diterpenoid from an octocoral Briareum sp. Tetrahedron Lett. 2014, 55, 6065–6067. [Google Scholar] [CrossRef]
- Su, Y.-D.; Su, T.-R.; Wen, Z.-H.; Hwang, T.-L.; Fang, L.-S.; Chen, J.-J.; Wu, Y.-C.; Sheu, J.-H.; Sung, P.-J. Briarenolides K and L, new anti-inflammatory briarane diterpenoids from an octocoral Briareum sp. (Briareidae). Mar. Drugs 2015, 13, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-D.; Wen, Z.-H.; Wu, Y.-C.; Fang, L.-S.; Chen, Y.-H.; Chang, Y.-C.; Sheu, J.-H.; Sung, P.-J. Briarenolides M–T, new briarane diterpenoids from a formosan octocoral Briareum sp. Tetrahedron 2016, 72, 944–951. [Google Scholar] [CrossRef]
- Su, Y.-D.; Wu, T.-Y.; Wen, Z.-H.; Su, C.-C.; Chen, Y.-H.; Chang, Y.-C.; Wu, Y.-C.; Sheu, J.-H.; Sung, P.-J. Briarenolides U–Y, new anti-inflammatory briarane diterpenoids from an octocoral Briareum sp. (Briareidae). Mar. Drugs 2015, 13, 7138–7149. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-D.; Sung, C.-S.; Wen, Z.-H.; Chen, Y.-H.; Chang, Y.-C.; Chen, J.-J.; Fang, L.-S.; Wu, Y.-C.; Sheu, J.-H.; Sung, P.-J. New 9-hydroxybriarane diterpenoids from a gorgonian coral Briareum sp. (Briareidae). Int. J. Mol. Sci. 2016, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-J.; Su, Y.-D.; Liao, Z.-J.; Wen, Z.-H.; Su, J.-H.; Wu, Y.-C.; Sung, P.-J. Briarenol B, a new polyoxygenated briarane from the octocoral Briareum excavatum. Nat. Prod. Commun. 2017, 12, 221–224. [Google Scholar]
- Chen, N.-F.; Su, Y.-D.; Hwang, T.-L.; Liao, Z.-J.; Tsui, K.-H.; Wen, Z.-H.; Wu, Y.-C.; Sung, P.-J. Briarenols C–E, new polyoxygenated briaranes from the octocoral Briareum excavatum. Molecules 2017, 22, 475. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Y.; Chen, N.-F.; Chen, W.-F.; Hung, H.-C.; Lee, H.-P.; Lin, Y.-Y.; Wang, H.-M.; Sung, P.-J.; Sheu, J.-H.; Wen, Z.-H. Sinularin from indigenous soft coral attenuates nociceptive responses and spinal neuroinflammation in carrageenan-induced inflammatory rat model. Mar. Drugs 2012, 10, 1899–1919. [Google Scholar] [CrossRef] [PubMed]
- Jean, Y.-H.; Chen, W.-F.; Sung, C.-S.; Duh, C.-Y.; Huang, S.-Y.; Lin, C.-S.; Tai, M.-H.; Tzeng, S.-F.; Wen, Z.-H. Capnellene, a natural marine compound derived from soft coral, attenuates chronic constriction injury-induced neuropathic pain in rats. Br. J. Pharmacol. 2009, 158, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Jean, Y.-H.; Chen, W.-F.; Duh, C.-Y.; Huang, S.-Y.; Hsu, C.-H.; Lin, C.-S.; Sung, C.-S.; Chen, I.-M.; Wen, Z.-H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from Formosan soft coral Lemnalia cervicorni. Eur. J. Pharmacol. 2008, 578, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Sheu, J.-H.; Wu, Y.-C.; Sung, P.-J. Terpenoids from octocorals of the genus Pachyclavularia. Mar. Drugs 2017, 15, 382. [Google Scholar] [CrossRef] [PubMed]

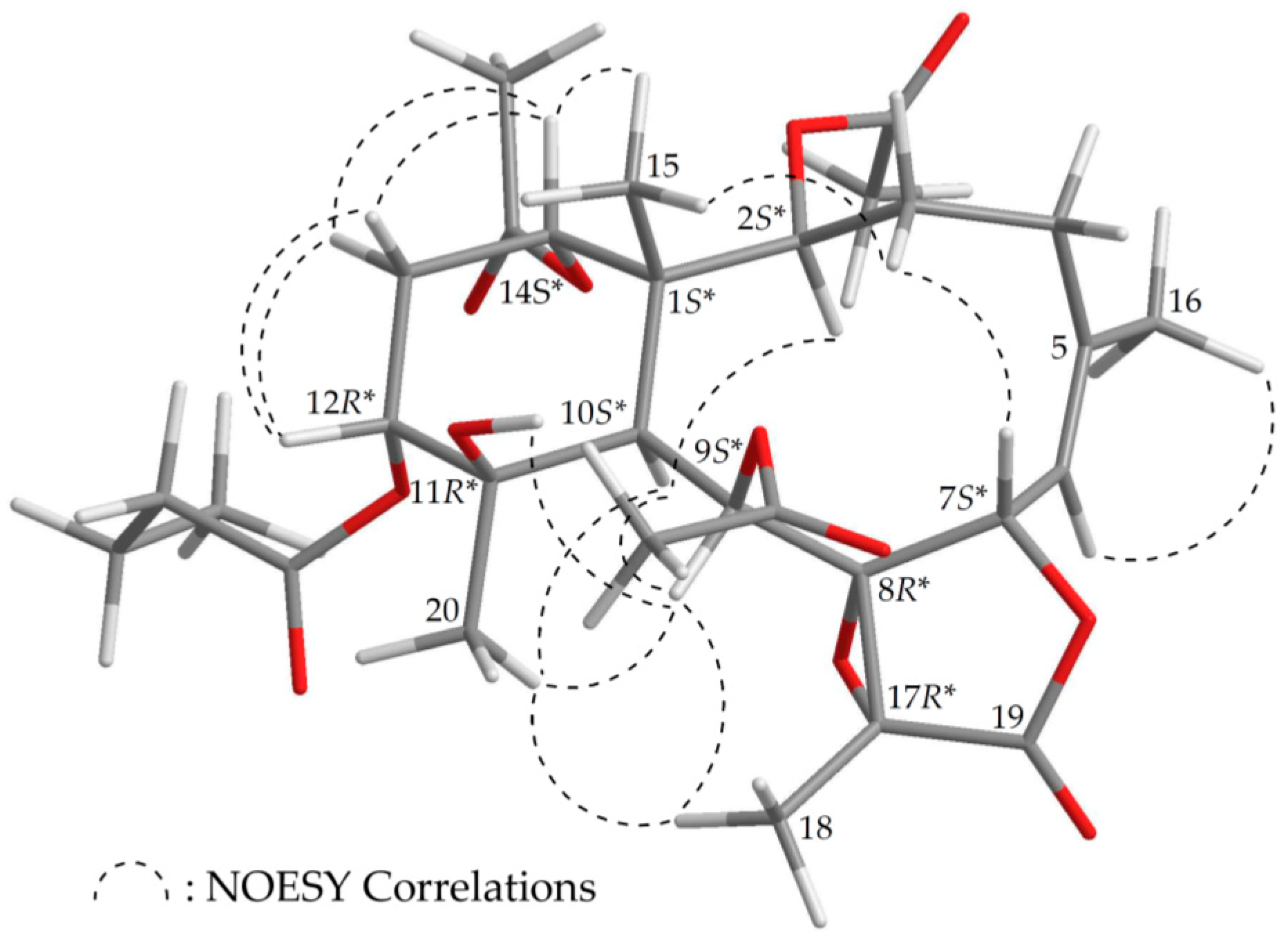

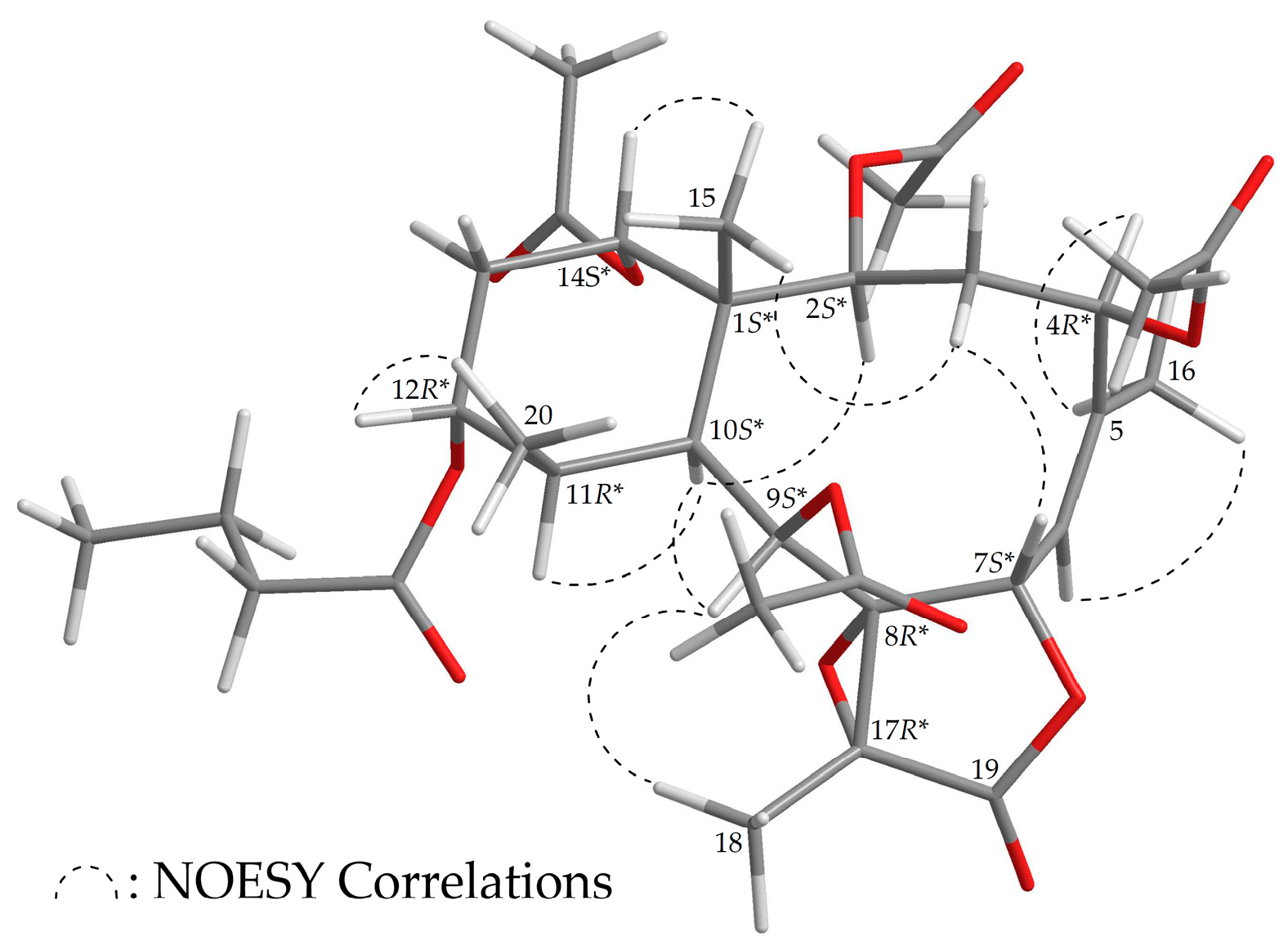
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 46.5, C | |||
| 2 | 5.17 d (8.0) | 75.4, CH | H2-3 | C-1, C-4, C-15, acetate carbonyl |
| 3α/β | 1.73 m; 2.62 ddd (15.2, 15,2, 6.0) | 32.3, CH2 | H-2, H2-4 | C-4 |
| 4/4′ | 1.95 m; 2.53 br d (15.2) | 28.7, CH2 | H2-3 | C-5, C-6 |
| 5 | 146.2, C | |||
| 6 | 5.29 d (9.2) | 117.4, CH | H-7, H3-16 | n. o. a |
| 7 | 5.37 d (9.2) | 75.1, CH | H-6 | C-5, C-6, C-19 |
| 8 | 70.7, C | |||
| 9 | 5.74 d (2.0) | 68.0, CH | H-10 | C-1, C-7, C-8, C-10, C-11, C-17, |
| acetate carbonyl | ||||
| 10 | 2.34 br s | 44.9, CH | H-9 | C-1, C-8, C-9, C-15, C-20 |
| 11 | 76.0, C | |||
| 12 | 4.85 dd (3.6, 2.4) | 75.0, CH | H2-13 | n-butyrate carbonyl |
| 13/13′ | 2.01 m; 2.19 m | 24.3, CH2 | H-12, H-14 | C-12 |
| 14 | 4.56 dd (2.8, 2.8) | 74.9, CH | H2-13 | C-1, acetate carbonyl |
| 15 | 1.28 s | 14.8, CH3 | C-1, C-2, C-10, C-14 | |
| 16 | 2.05 br s | 27.0, CH3 | H-6 | C-4, C-5, C-6 |
| 17 | 64.5, C | |||
| 18 | 1.70 s | 10.2, CH3 | C-8, C-17, C-19 | |
| 19 | 170.7, C | |||
| 20 | 1.29 s | 30.0, CH3 | C-10, C-11, C-12 | |
| OAc-2 | 170.4, C | |||
| 1.98 s | 21.5, CH3 | Acetate carbonyl | ||
| OAc-9 | 169.4, C | |||
| 2.22 s | 21.2, CH3 | Acetate carbonyl | ||
| OAc-14 | 170.6, C | |||
| 2.00 s | 21.5, CH3 | Acetate carbonyl | ||
| n-OC(O)Pr-12 | 172.6, C | |||
| 2.29 t (7.6) | 36.4, CH2 | H2-3′ | C-1′, C-3′, C-4′ | |
| 1.65 sext (7.6) | 18.3, CH2 | H2-2′, H3-4′ | C-1′, C-2′, C-4′ | |
| 0.95 t (7.6) | 13.7, CH3 | H2-3′ | C-2′, C-3′ | |
| OH-11 | 2.11 s | C-10, C-11, C-12, C-20 |
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 47.3, C | |||
| 2 | 5.23 d (8.4) | 75.0, CH | H2-3 | C-1, C-4, C-14, C-15, acetate carbonyl |
| 3α/β | 1.69 m; 2.64 ddd (15.2, 15.2, 5.6) | 31.4, CH2 | H-2, H2-4 | C-2 |
| 4/4′ | 1.93 m; 2.49 br d (15.2) | 28.4, CH2 | H2-3 | C-5 |
| 5 | 144.3, C | |||
| 6 | 5.20 d (8.0) | 118.6, CH | H-7, H3-16 | C-5, C-8 |
| 7 | 5.19 d (8.0) | 75.0, CH | H-6 | C-6, C-8 |
| 8 | 70.7, C | |||
| 9 | 5.83 s | 67.4, CH | H-10 | C-1, C-7, C-8, C-10, C-11, C-17, acetate carbonyl |
| 10 | 2.41 s | 45.5, CH | H-9 | C-1, C-2, C-8, C-12, C-14, C-15, C-20 |
| 11 | 73.5, C | |||
| 12 | 4.80 dd (2.8, 2.4) | 73.8, CH | H2-13 | C-10, C-11, C-14, C-20, n-butyrate carbonyl |
| 13/13′ | 1.96 m; 2.26 m | 25.8, CH2 | H-12, H-14 | C-11, C-12, C-14 |
| 14 | 4.68 dd (2.8, 2.8) | 74.2, CH | H2-13 | C-1, C-10, C-15, acetate carbonyl |
| 15 | 1.22 s | 14.3, CH3 | C-1, C-2, C-10, C-14 | |
| 16 | 1.99 s | 27.2, CH3 | H-6 | C-4, C-5, C-6 |
| 17 | 66.3, C | |||
| 18 | 1.77 s | 10.4, CH3 | C-8, C-17, C-19 | |
| 19 | 170.4, C | |||
| 20 | 1.22 s | 23.2, CH3 | C-10, C-11, C-12 | |
| OAc-2 | 170.1, C | |||
| 1.98 s | 21.4, CH3 | Acetate carbonyl | ||
| OAc-9 | 168.2, C | |||
| 2.22 s | 21.2, CH3 | Acetate carbonyl | ||
| OAc-14 | 170.1, C | |||
| 1.99 s | 21.5, CH3 | Acetate carbonyl | ||
| n-OC(O)Pr-12 | 172.5, C | |||
| 2.33 t (7.2) | 36.3, CH2 | H2-3′ | C-1′, C-3′, C-4′ | |
| 1.67 sext (7.2) | 18.3, CH2 | H2-2′, H3-4′ | C-1′, C-2′, C-4′ | |
| 0.97 t (7.2) | 13.7, CH3 | H2-3′ | C-2′, C-3′ | |
| OH-11 | 1.96 s | C-11, C-12, C-20 |
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 46.1, C | |||
| 2 | 5.07 d (7.5) | 73.0, CH | H2-3 | C-1, C-3, C-4, C-15, |
| acetate carbonyl | ||||
| 3α/β | 2.02 (15.0, 7.5, 5.5); 2.89 dd (15.0, 13.0) | 37.7, CH2 | H-2, H-4 | C-2, C-4, C-5 |
| 4 | 5.08 dd (13.0, 5.5) | 73.0, CH | H2-3 | C-5, C-6, acetate carbonyl |
| 5 | 143.7, C | |||
| 6 | 5.40 d (9.0) | 123.1, CH | H-7 | C-4 |
| 7 | 5.57 d (9.0) | 73.7, CH | H-6 | C-6 |
| 8 | 70.3, C | |||
| 9 | 4.98 br s | 72.5, CH | n. o. b | C-1, C-7, C-8, C-11, C-17, |
| acetate carbonyl | ||||
| 10 | 2.64 dd (5.0, 1.5) | 37.3, CH | H-11 | C-1, C-8, C-11 |
| 11 | 2.04 m | 42.0, CH | H-10, H-12, H3-20 | n. o. |
| 12 | 4.83 ddd (3.5, 3.0, 3.0) | 71.4, CH | H-11, H2-13 | n. o. |
| 13α/β | 1.94 br d (16.0); 2.09 ddd (16.0, 3.0, 3.0) | 25.4, CH2 | H-12, H-14 | n. o. |
| 14 | 4.75 dd (3.0, 3.0) | 73.9, CH | H2-13 | Acetate carbonyl |
| 15 | 1.23 s | 15.4, CH3 | C-1, C-10, C-14 | |
| 16 | 2.17 s | 25.4, CH3 | C-4, C-5, C-6 | |
| 17 | 64.5, C | |||
| 18 | 1.62 s | 10.8, CH3 | C-8, C-17, C-19 | |
| 19 | 170.5, C | |||
| 20 | 1.11 d (7.5) | 15.0, CH3 | C-10, C-11, C-12 | |
| OAc-2 | 170.1, C a | |||
| 2.05 s a | 21.1, CH3 | Acetate carbonyl | ||
| OAc-4 | 170.0, C a | |||
| 2.02 s a | 21.1, CH3 | Acetate carbonyl | ||
| OAc-9 | 168.3, C | |||
| 2.24 s | 21.4, CH3 | Acetate carbonyl | ||
| OAc-14 | 170.0, C a | |||
| 2.00 s a | 21.1, CH3 | Acetate carbonyl | ||
| n-OC(O)Pr-12 | 173.0, C | |||
| 2.26 t (7.5) | 36.5, CH2 | H2-3′ | C-1′, C-3′ C-4′ | |
| 1.64 sext (7.5) | 18.5, CH2 | H2-2′, H3-4′ | C-1′, C-2′ C-4′ | |
| 0.96 t (7.5) | 13.7, CH3 | H2-3′ | C-2′, C-3′ |
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 43.3, C | |||
| 2 | 4.83 d (8.4) | 82.3, CH | H2-3 | C-4, C-10, C-14, acetate carbonyl |
| 3/3′ | 2.27 m, 1.87 m | 22.7, CH2 | H-2, H2-4 | C-1, C-2 |
| 4/4′ | 1.92 m, 2.54 dd (14.8, 4.4) | 24.7, CH2 | H2-3 | C-2, C-5 |
| 5 | 143.1, C | |||
| 6 | 5.34 d (8.8) | 121.0, CH | H-7 | C-4 |
| 7 | 5.65 d (8.8) | 73.7, CH | H-6 | C-5 |
| 8 | 70.9, C | |||
| 9 | 3.73 dd (10.0, 6.0) | 68.5, CH | H-10, OH-9 | C-8, C-11 |
| 10 | 3.49 dd (10.0, 3.6) | 34.6, CH | H-9, H-11 | n. o. a |
| 11 | 2.16 m | 35.7, CH | H-10, H-12, H3-20 | n. o. |
| 12 | 3.92 dd (6.0, 1.6) | 68.2, CH | H-11, H-13 | n. o. |
| 13 | 5.90 dd (10.0, 6.0) | 126.5, CH | H-12, H-14 | n. o. |
| 14 | 5.37 d (10.0) | 138.1, CH | H-13 | C-10, C-12 |
| 15 | 1.20 s | 19.2, CH3 | C-1, C-2, C-10, C-14 | |
| 16 | 1.78 s | 23.4, CH3 | C-4, C-5, C-6 | |
| 17 | 58.5, C | |||
| 18 | 1.60 s | 9.4, CH3 | C-8, C-17, C-19 | |
| 19 | 172.6, C | |||
| 20 | 0.90 d (7.2) | 13.4, CH3 | H-11 | C-10, C-11, C-12 |
| OAc-2 | 167.4, C | |||
| 2.23 s | 20.8, CH3 | Acetate carbonyl | ||
| OH-9 | 4.92 d (6.0) | H-9 | C-8, C-10 |
| Compound | iNOS | COX-2 |
|---|---|---|
| Expression (% of LPS Group) | Expression (% of LPS Group) | |
| Control | 7.08 ± 1.55 | 2.41 ± 0.13 |
| LPS | 100 ± 6.96 | 100 ± 3.26 |
| 1 | 118.02 ± 10.81 | 112.77 ± 20.69 |
| 2 | 46.68 ± 4.56 | 61.81 ± 12.14 |
| 3 | 92.49 ± 14.67 | 101.95 ± 8.22 |
| 4 | 71.49 ± 3.78 | 104.51 ± 4.22 |
| DEX a | 36.52 ± 4.53 | 6.18 ± 1.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.-H.; Lai, K.-H.; Su, Y.-D.; Chang, Y.-C.; Peng, B.-R.; Backlund, A.; Wen, Z.-H.; Sung, P.-J. Briaviolides K–N, New Briarane-Type Diterpenoids from Cultured Octocoral Briareum violaceum. Mar. Drugs 2018, 16, 75. https://doi.org/10.3390/md16030075
Xu J-H, Lai K-H, Su Y-D, Chang Y-C, Peng B-R, Backlund A, Wen Z-H, Sung P-J. Briaviolides K–N, New Briarane-Type Diterpenoids from Cultured Octocoral Briareum violaceum. Marine Drugs. 2018; 16(3):75. https://doi.org/10.3390/md16030075
Chicago/Turabian StyleXu, Jing-Hao, Kuei-Hung Lai, Yin-Di Su, Yu-Chia Chang, Bo-Rong Peng, Anders Backlund, Zhi-Hong Wen, and Ping-Jyun Sung. 2018. "Briaviolides K–N, New Briarane-Type Diterpenoids from Cultured Octocoral Briareum violaceum" Marine Drugs 16, no. 3: 75. https://doi.org/10.3390/md16030075






