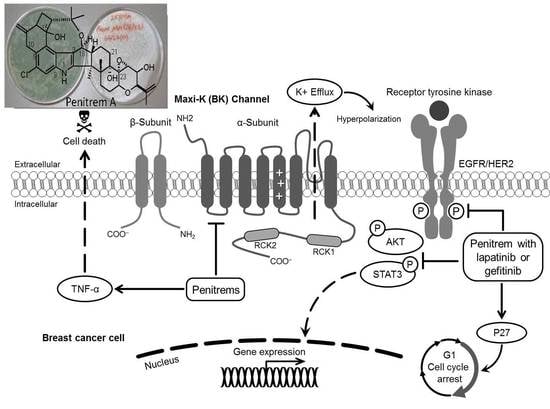
The Maxi-K (BK) Channel Antagonist Penitrem A as a Novel Breast Cancer-Targeted Therapeutic
Abstract
:1. Introduction
2. Results
2.1. Antiproliferative Effects of Penitrems in Breast Cancer Cells In Vitro
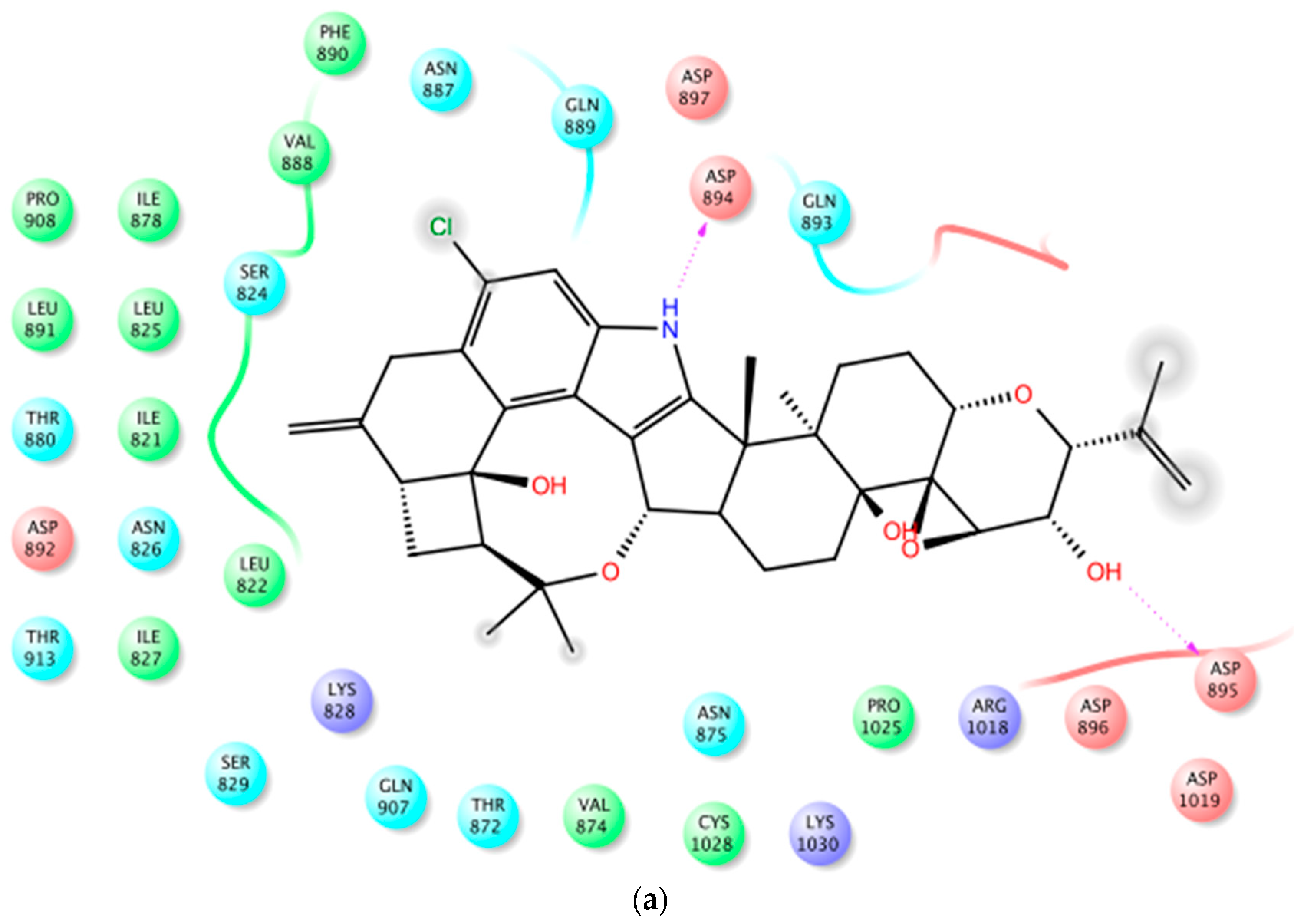
2.2. In Silico Binding of Penitrems with BK Channel
2.3. Expression of BK Channels in BC Cells and In Vitro Impact of Penitrems on Channel Expression
2.4. Effect of Penitrem A Treatment on Cell Cycle Progression in BC Cells
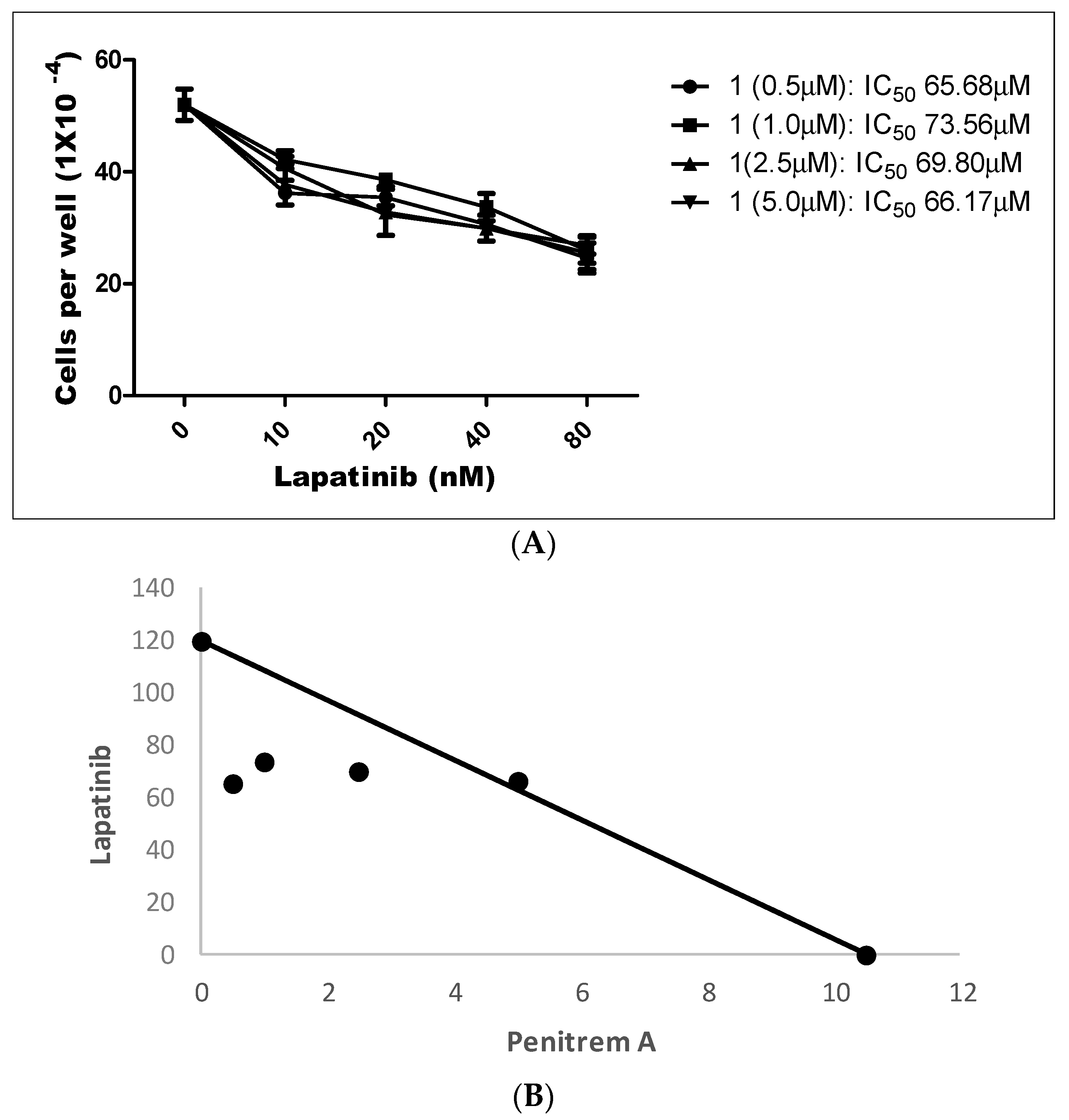
2.5. Effects of Combined Treatment of Targeted Agents and Penitrem A on the Growth of BC Cells
2.6. Effects of Combined Treatment of Targeted Agents and Penitrem A on Receptor Tyrosine Kinases (RTKs) and Their Downstream Effectors
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Docking Study
4.3. Cell Viability Assay
4.4. Western Blot Analysis
4.5. Immunocytochemistry
4.6. Analysis of Cell Cycle Progression by Flow Cytometry
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K. Breast cancer: Origins and evolution. J. Clin. Investig. 2007, 117, 3155–3163. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Glick, J.H.; Gelber, R.D.; Coates, A.S.; Thurlimann, B.; Senn, H.J.; Panel, M. Meeting highlights: International expert consensus on the primary therapy of early breast cancer 2005. Ann. Oncol. 2005, 16, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, T.; Tibshirani, R.; Parker, J.; Hastie, T.; Marron, J.S.; Nobel, A.; Deng, S.; Johnsen, H.; Pesich, R.; Geisler, S.; et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 2003, 100, 8418–8423. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.; Bedard, P.L. Luminal-B breast cancer and novel therapeutic targets. Breast Cancer Res. 2011, 13, 221. [Google Scholar] [CrossRef] [PubMed]
- Hon, J.D.; Singh, B.; Sahin, A.; Du, G.; Wang, J.; Wang, V.Y.; Deng, F.M.; Zhang, D.Y.; Monaco, M.E.; Lee, P. Breast cancer molecular subtypes: From TNBC to QNBC. Am. J. Cancer Res 2016, 6, 1864–1872. [Google Scholar] [PubMed]
- Toro, L.; Li, M.; Zhang, Z.; Singh, H.; Wu, Y.; Stefani, E. Maxi-K channel and cell signalling. Pflugers Arch. Eur. J. Physiol. 2014, 466, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, G.; Cui, J. BK channels: Multiple sensors, one activation gate. Front. Physiol. 2015, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Knaus, H.G.; Eberhart, A.; Glossmann, H.; Munujos, P.; Kaczorowski, G.J.; Garcia, M.L. Pharmacology and structure of high conductance calcium-activated potassium channels. Cell. Signal. 1994, 6, 861–870. [Google Scholar] [CrossRef]
- Tanaka, Y.; Meera, P.; Song, M.; Knaus, H.G.; Toro, L. Molecular constituents of maxi KCa channels in human coronary smooth muscle: Predominant alpha + beta subunit complexes. J. Physiol. 1997, 502 Pt 3, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Vetri, F.; Choudhury, M.S.; Pelligrino, D.A.; Sundivakkam, P. BKCa channels as physiological regulators: A focused review. J. Recept. Ligand Channel Res. 2014, 7, 3–13. [Google Scholar] [CrossRef]
- Ouadid-Ahidouch, H.; Roudbaraki, M.; Delcourt, P.; Ahidouch, A.; Joury, N.; Prevarskaya, N. Functional and molecular identification of intermediate-conductance Ca2+-activated K+ channels in breast cancer cells: Association with cell cycle progression. Am. J. Physiol. Cell Physiol. 2004, 287, C125–C134. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, K. Ion channels and cancer. J. Membr. Biol. 2005, 205, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Wonderlin, W.F.; Strobl, J.S. Potassium channels, proliferation and G1 progression. J. Membr. Biol. 1996, 154, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Pardo, L.A. Voltage-gated potassium channels in cell proliferation. Physiology 2004, 19, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.; Ousingsawat, J.; Simon, R.; Schraml, P.; Gasser, T.C.; Mihatsch, M.J.; Kunzelmann, K.; Bubendorf, L. KCNMA1 gene amplification promotes tumor cell proliferation in human prostate cancer. Oncogene 2007, 26, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Foller, M.; Lang, K.S.; Lang, P.A.; Ritter, M.; Gulbins, E.; Vereninov, A.; Huber, S.M. Ion channels in cell proliferation and apoptotic cell death. J. Membr. Biol. 2005, 205, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Ousingsawat, J.; Spitzner, M.; Schreiber, R.; Kunzelmann, K. Upregulation of colonic ion channels in APC (Min/+) mice. Pflugers Arch. Eur. J. Physiol. 2008, 456, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Spitzner, M.; Ousingsawat, J.; Scheidt, K.; Kunzelmann, K.; Schreiber, R. Voltage-gated K+ channels support proliferation of colonic carcinoma cells. FASEB J. 2007, 21, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kraft, R.; Krause, P.; Jung, S.; Basrai, D.; Liebmann, L.; Bolz, J.; Patt, S. BK channel openers inhibit migration of human glioma cells. Pflugers Arch. Eur. J. Physiol. 2003, 446, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Sontheimer, H. An unexpected role for ion channels in brain tumor metastasis. Exp. Biol. Med. 2008, 233, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion channels and the hallmarks of cancer. Trends Mol. Med. 2010, 16, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Knaus, H.G.; McManus, O.B.; Lee, S.H.; Schmalhofer, W.A.; Garcia-Calvo, M.; Helms, L.M.; Sanchez, M.; Giangiacomo, K.; Reuben, J.P.; Smith, A.B., 3rd; et al. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry 1994, 33, 5819–5828. [Google Scholar] [CrossRef] [PubMed]
- Coiret, G.; Borowiec, A.S.; Mariot, P.; Ouadid-Ahidouch, H.; Matifat, F. The antiestrogen tamoxifen activates BK channels and stimulates proliferation of MCF-7 breast cancer cells. Mol. Pharmacol. 2007, 71, 843–8451. [Google Scholar] [CrossRef] [PubMed]
- Valverde, M.A.; Rojas, P.; Amigo, J.; Cosmelli, D.; Orio, P.; Bahamonde, M.I.; Mann, G.E.; Vergara, C.; Latorre, R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science 1999, 285, 1929–1931. [Google Scholar] [CrossRef] [PubMed]
- Steyn, P.S.; Vleggaar, R. Tremorgenic mycotoxins. Prog. Chem. Org. Nat. Prod. 1985, 48, 1–80. [Google Scholar]
- Smith, M.M.; Warren, V.A.; Thomas, B.S.; Brochu, R.M.; Ertel, E.A.; Rohrer, S.; Schaeffer, J.; Schmatz, D.; Petuch, B.R.; Tang, Y.S.; et al. Nodulisporic acid opens insect glutamate-gated chloride channels: Identification of a new high affinity modulator. Biochemistry 2000, 39, 5543–5554. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.A.; Ayoub, N.M.; Foudah, A.I.; Gissendanner, C.R.; Meyer, S.A.; El Sayed, K.A. Indole diterpene alkaloids as novel inhibitors of the Wnt/beta-catenin pathway in breast cancer cells. Eur. J. Med. Chem. 2013, 70, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.A.; Houssen, W.E.; Gissendanner, C.R.; Orabi, K.Y.; Foudah, A.I.; El Sayed, K.A. Bioguided discovery and pharmacophore modeling of the mycotoxic indole diterpene alkaloids penitrems as breast cancer proliferation, migration, and invasion inhibitors. Med. Chem. Commun. 2013, 4, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Y.; Ye, S.; Jiang, Y. Structure of the gating ring from the human large-conductance Ca2+-gated K+ channel. Nature 2010, 466, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Lallet-Daher, H.; Roudbaraki, M.; Bavencoffe, A.; Mariot, P.; Gackiere, F.; Bidaux, G.; Urbain, R.; Gosset, P.; Delcourt, P.; Fleurisse, L.; et al. Intermediate-conductance Ca2+-activated K+ channels (IKCa1) regulate human prostate cancer cell proliferation through a close control of calcium entry. Oncogene 2009, 28, 1792–1806. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Leonetti, M.D.; Pico, A.R.; Hsiung, Y.; MacKinnon, R. Structure of the human BK channel Ca2+-activation apparatus at 3.0 Å resolution. Science 2010, 329, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Shi, J.; Ma, Z.; Krishnamoorthy, G.; Sieling, F.; Zhang, G.; Horrigan, F.T.; Cui, J. Participation of the S4 voltage sensor in the Mg2+-dependent activation of large conductance (BK) K+ channels. Proc. Natl. Acad. Sci. USA 2003, 100, 10488–10493. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Rapin, A.M.; Holmstrand, E.C.; Cox, D.H. Elimination of the BK(Ca) channel’s high-affinity Ca2+ sensitivity. J. Gen. Physiol. 2002, 120, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Wonderlin, W.F.; Woodfork, K.A.; Strobl, J.S. Changes in membrane potential during the progression of MCF-7 human mammary tumor cells through the cell cycle. J. Cell. Physiol. 1995, 165, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Ouadid-Ahidouch, H.; Le Bourhis, X.; Roudbaraki, M.; Toillon, R.A.; Delcourt, P.; Prevarskaya, N. Changes in the K+ current-density of MCF-7 cells during progression through the cell cycle: Possible involvement of a h-ether.a-gogo K+ channel. Recept. Channels 2001, 7, 345–356. [Google Scholar] [PubMed]
- Wegman, E.A.; Young, J.A.; Cook, D.I. A 23-pS Ca2+-activated K+ channel in MCF-7 human breast carcinoma cells: An apparent correlation of channel incidence with the rate of cell proliferation. J. Physiol. 1991, 417, 562–570. [Google Scholar] [CrossRef]
- Fiers, W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991, 285, 199–212. [Google Scholar] [CrossRef]
- Carswell, E.A.; Old, L.J.; Kassel, R.L.; Green, S.; Fiore, N.; Williamson, B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl. Acad. Sci. USA 1975, 72, 3666–3670. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ji, G.; Wang, L.; Ren, H.; Xi, L. Activation of ERK1/2 and TNF-alpha production are regulated by calcium/calmodulin signaling pathway during Penicillium marneffei infection within human macrophages. Microb. Pathog. 2016, 93, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, K.; Furuya, K.; Maeno, T.; Edwards, C.; Oka, T. Oscillating activity of a calcium-activated K+ channel in normal and cancerous mammary cells in culture. J. Membr. Biol. 1991, 119, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.; Nickerson, N.K.; Nam, S.; Allen, K.T.; Gilmore, J.L.; Nephew, K.P.; Riese, D.J. EGFR signaling in breast cancer: Bad to the bone. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2010; Volume 21, pp. 951–960. [Google Scholar]
- Aaronson, S.A. Growth factors and cancer. Science 1991, 254, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Goda, A.A.; Naguib, K.M.; Mohamed, M.M.; Amra, H.A.; Nada, S.A.; Abdel-Ghaffar, A.B.; Gissendanner, C.R.; El Sayed, K.A. Astaxanthin and docosahexaenoic acid reverse the toxicity of the maxi-K (BK) channel antagonist mycotoxin penitrem A. Mar. Drugs 2016, 14, 208. [Google Scholar] [CrossRef] [PubMed]
- Sings, H.; Singh, S. Tremorgenic and nontremorgenic 2,3-fused indole diterpenoids. Alkaloids Chem. Biol. 2003, 60, 51–163. [Google Scholar] [PubMed]
- Chu, I.M.; Hengst, L.; Slingerland, J.M. The CDK inhibitor p27 in human cancer: Prognostic potential and relevance to anticancer therapy. Nat. Rev. Cancer 2008, 8, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Tallarida, R.J. An overview of drug combination analysis with isobolograms. J. Pharmacol. Exp. Ther. 2006, 319, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Matsuoka, Y.; Funahashi, A.; Kitano, H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol. Syst. Biol. 2005, 1. [Google Scholar] [CrossRef] [PubMed]
- Shattuck, D.L.; Miller, J.K.; Carraway, K.L., 3rd; Sweeney, C. Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Res. 2008, 68, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Mohyeldin, M.M.; Busnena, B.A.; Akl, M.R.; Dragoi, A.M.; Cardelli, J.A.; El Sayed, K.A. Novel c-Met inhibitory olive secoiridoid semisynthetic analogs for the control of invasive breast cancer. Eur. J. Med. Chem. 2016, 118, 299–315. [Google Scholar] [CrossRef] [PubMed]

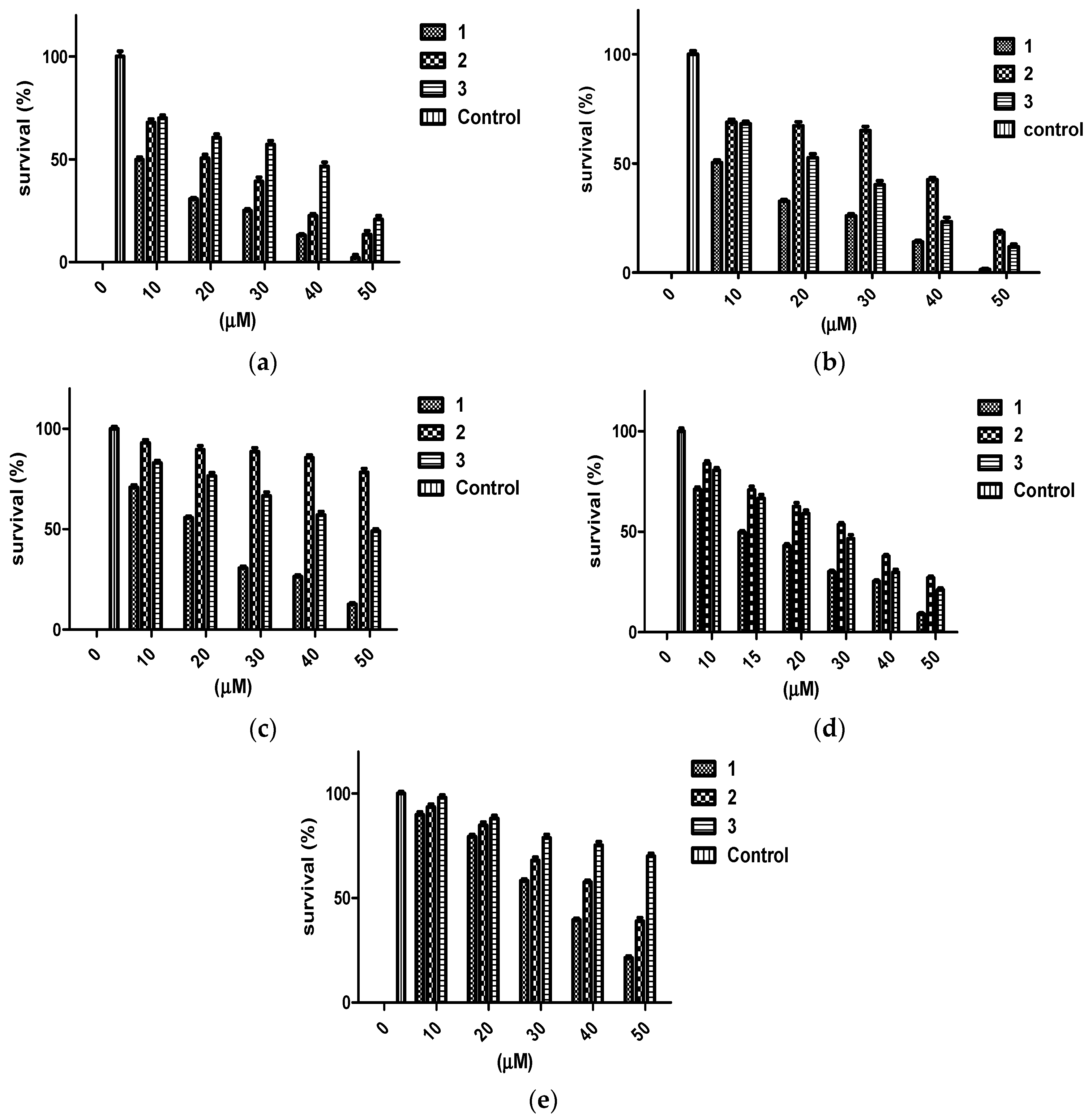

















| Compound | MCF-12A | CRL-2765 | MDA-MB-231 | BT-474 | SK-BR-3 |
|---|---|---|---|---|---|
| 1 | 33.7 | 22.6 | 9.8 | 10.3 | 15.1 |
| 2 | 44.0 | 67.8 | 20.3 | 31.8 | 36.7 |
| 3 | 78.8 | 48.2 | 37.8 | 22.4 | 27.1 |
| IC50/Combination Index (CI) Values | ||||
|---|---|---|---|---|
| LP | LP + 1 (0.5 μM) | LP + 1 (1.0 μM) | LP + 1 (2.5 μM) | LP + 1 (5.0 μM) |
| 123.0 nM | 65.68 nM/0.58 | 73.56 nM/0.70 | 69.80 nM/0.81 | 66.17 nM/1.03 |
| GF | GF + 1 (0.5 μM) | GF + 1 (1.0 μM) | GF + 1 (2.5 μM) | GF + 1 (5.0 μM) |
| 302.4 nM | 124.77 nM/0.46 | 176.78 nM/0.68 | 130.84 nM/0.68 | 114.42 nM/0.87 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goda, A.A.; Siddique, A.B.; Mohyeldin, M.; Ayoub, N.M.; El Sayed, K.A. The Maxi-K (BK) Channel Antagonist Penitrem A as a Novel Breast Cancer-Targeted Therapeutic. Mar. Drugs 2018, 16, 157. https://doi.org/10.3390/md16050157
Goda AA, Siddique AB, Mohyeldin M, Ayoub NM, El Sayed KA. The Maxi-K (BK) Channel Antagonist Penitrem A as a Novel Breast Cancer-Targeted Therapeutic. Marine Drugs. 2018; 16(5):157. https://doi.org/10.3390/md16050157
Chicago/Turabian StyleGoda, Amira A., Abu Bakar Siddique, Mohamed Mohyeldin, Nehad M. Ayoub, and Khalid A. El Sayed. 2018. "The Maxi-K (BK) Channel Antagonist Penitrem A as a Novel Breast Cancer-Targeted Therapeutic" Marine Drugs 16, no. 5: 157. https://doi.org/10.3390/md16050157