The In Vitro Anti-Tumor Activity of Phycocyanin against Non-Small Cell Lung Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Phycocyanin Induces Morphological Changes in NSCLC Cells
2.2. Phycocyanin Induces Apoptosis in NSCLC Cells
2.3. Phycocyanin Displays Anti-Migratory Effect against NSCLC Cells
2.4. Phycocyanin Inhibits Proliferation and Colony Formation Ability of NSCLC Cells
2.5. Phycocyanin Induces Cell Cycle Arrest in NSCLC Cells
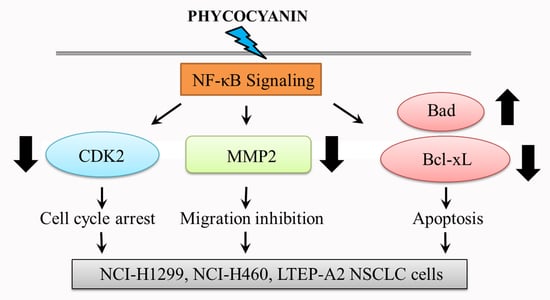
2.6. Phycocyanin Reduces NF-κB Signaling Activity in NSCLC Cells
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Cell Line and Culture Conditions
4.3. Cell Viability Assay
4.4. Cell Proliferation Assay
4.5. Colony Formation Assay
4.6. Cell Apoptosis Assay
4.7. Cell Cycle Assay
4.8. Western Blot Analysis
4.9. Quantitative RT-PCR
4.10. Wound-Healing Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amin, A.R.; Kucuk, O.; Khuri, F.R.; Shin, D.M. Perspectives for cancer prevention with natural compounds. J. Clin. Oncol. 2009, 27, 2712–2725. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.L. Soluble extract from Moringa oleifera leaves with a new anticancer activity. PLoS ONE 2014, 9, e95492. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M.F.; de Morais, R.M.; de Morais, A.M. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huang, Y.; Zhang, R.; Cai, T.; Cai, Y. Medical Application of Spirulina platensis Derived C-Phycocyanin. Evid. Based Complement. Altern. Med. 2016, 2016, 7803846. [Google Scholar]
- Ravi, M.; Tentu, S.; Baskar, G.; Rohan Prasad, S.; Raghavan, S.; Jayaprakash, P.; Jeyakanthan, J.; Rayala, S.K.; Venkatraman, G. Molecular mechanism of anti-cancer activity of phycocyanin in triple-negative breast cancer cells. BMC Cancer 2015, 15, 768. [Google Scholar] [CrossRef] [PubMed]
- Thangam, R.; Suresh, V.; Asenath Princy, W.; Rajkumar, M.; Senthilkumar, N.; Gunasekaran, P.; Rengasamy, R.; Anbazhagan, C.; Kaveri, K.; Kannan, S. C-Phycocyanin from Oscillatoria tenuis exhibited an antioxidant and in vitro antiproliferative activity through induction of apoptosis and G0/G1 cell cycle arrest. Food Chem. 2013, 140, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Ling, Q.; Cai, Z.; Wang, Y.; Zhang, Y.; Hoffmann, P.R.; Zheng, W.; Zhou, T.; Huang, Z. Selenium-Containing Phycocyanin from Se-Enriched Spirulina platensis Reduces Inflammation in Dextran Sulfate Sodium-Induced Colitis by Inhibiting NF-κB Activation. J. Agric. Food Chem. 2016, 64, 5060–5070. [Google Scholar] [CrossRef] [PubMed]
- Riss, J.; Décordé, K.; Sutra, T.; Delage, M.; Baccou, J.C.; Jouy, N.; Brune, J.P.; Oréal, H.; Cristol, J.P.; Rouanet, J.M. Phycobiliprotein C-phycocyanin from Spirulina platensis is powerfully responsible for reducing oxidative stress and NADPH oxidase expression induced by an atherogenic diet in hamsters. J. Agric. Food Chem. 2007, 55, 7962–7967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, T.; Jiang, J.; Wong, Y.S.; Yang, F.; Zheng, W. Selenium-containing allophycocyanin purified from selenium-enriched Spirulina platensis attenuates AAPH-induced oxidative stress in human erythrocytes through inhibition of ROS generation. J. Agric. Food Chem. 2011, 59, 8683–8690. [Google Scholar] [CrossRef] [PubMed]
- Nemoto-Kawamura, C.; Hirahashi, T.; Nagai, T.; Yamada, H.; Katoh, T.; Hayashi, O. Phycocyanin enhances secretary IgA antibody response and suppresses allergic IgE antibody response in mice immunized with antigen-entrapped biodegradable microparticles. J. Nutr. Sci. Vitaminol. 2004, 50, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Bharathiraja, S.; Seo, H.; Manivasagan, P.; Santha Moorthy, M.; Park, S.; Oh, J. In Vitro Photodynamic Effect of Phycocyanin against Breast Cancer Cells. Molecules 2016, 21, 1470. [Google Scholar] [CrossRef] [PubMed]
- Pardhasaradhi, B.V.; Ali, A.M.; Kumari, A.L.; Reddanna, P.; Khar, A. Phycocyanin-mediated apoptosis in AK-5 tumor cells involves down-regulation of Bcl-2 and generation of ROS. Mol. Cancer Ther. 2003, 2, 1165–1170. [Google Scholar] [PubMed]
- Ying, J.; Wang, J.; Ji, H.; Lin, C.; Pan, R.; Zhou, L.; Song, Y.; Zhang, E.; Ren, P.; Chen, J.; et al. Transcriptome analysis of phycocyanin inhibitory effects on SKOV-3 cell proliferation. Gene 2016, 585, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.K.; Sanyal, S.N. Targeting angiogenic pathway for chemoprevention of experimental colon cancer using C-phycocyanin as cyclooxygenase-2 inhibitor. Biochem. Cell Biol. 2014, 92, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Gantar, M.; Dhandayuthapani, S.; Rathinavelu, A. Phycocyanin induces apoptosis and enhances the effect of topotecan on prostate cell line LNCaP. J. Med. Food 2012, 15, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Baudelet, P.H.; Gagez, A.L.; Bérard, J.B.; Juin, C.; Bridiau, N.; Kaas, R.; Thiéry, V.; Cadoret, J.P.; Picot, L. Antiproliferative activity of Cyanophora paradoxa pigments in melanoma, breast and lung cancer cells. Mar. Drugs 2013, 11, 4390–4406. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gao, M.H.; Lv, C.Y.; Yang, P.; Yin, Q.F. Study of the synergistic effects of all-transretinoic acid and C-phycocyanin on the growth and apoptosis of A549 cells. Eur. J. Cancer Prev. 2016, 25, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gao, M.H.; Chu, X.M.; Teng, L.; Lv, C.Y.; Yang, P.; Yin, Q.F. The synergistic antitumor effects of all-trans retinoic acid and C-phycocyanin on the lung cancer A549 cells in vitro and in vivo. Eur. J. Pharmacol. 2015, 749, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Bingula, R.; Dupuis, C.; Pichon, C.; Berthon, J.Y.; Filaire, M.; Pigeon, L.; Filaire, E. Study of the Effects of Betaine and/or C-Phycocyanin on the Growth of Lung Cancer A549 Cells In Vitro and In Vivo. J. Oncol. 2016, 2016, 8162952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, L.; Chen, J.; Liang, J.; Xu, Y.; Li, Z.; Chen, F.; Du, D. Knockdown of Diaph1 expression inhibits migration and decreases the expression of MMP2 and MMP9 in human glioma cells. Biomed. Pharmacother. 2017, 96, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Oakes, V.; Wang, W.; Harrington, B.; Lee, W.J.; Beamish, H.; Chia, K.M.; Pinder, A.; Goto, H.; Inagaki, M.; Pavey, S.; et al. Cyclin A/Cdk2 regulates Cdh1 and claspin during late S/G2 phase of the cell cycle. Cell Cycle 2014, 13, 3302–3311. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.; Odajima, J.; Hunt, S.L.; Dubus, P.; Ortega, S.; Malumbres, M.; Barbacid, M. Cdk2 is dispensable for cell cycle inhibition and tumor suppression mediated by p27Kip1 and p21Cip1. Cancer Cell 2005, 7, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Yu, J.; Zhao, X.; Song, F.; Feng, X. Expression of P57(kip2) and cyslinE proteins in human pancreatic cancer. Chin. Med. J. 2003, 116, 944–946. [Google Scholar] [PubMed]
- Yamamoto, Y.; Gaynor, R.B. IkappaB kinases: Key regulators of the NF-kappaB pathway. Trends Biochem. Sci. 2004, 29, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J. Preventive therapy for cancer. Lancet Oncol. 2017, 18, e472–e482. [Google Scholar] [CrossRef]
- Goyal, S.; Gupta, N.; Chatterjee, S.; Nimesh, S. Natural Plant Extracts as Potential Therapeutic Agents for the Treatment of Cancer. Curr. Top. Med. Chem. 2017, 17, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Pandey, M.K.; Aggarwal, B.B. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-kappaB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IkappaBalpha kinase activation. Mol. Pharmacol. 2007, 71, 1703–1714. [Google Scholar] [PubMed]
- Tan, S.; Yang, B.; Liu, J.; Xun, T.; Liu, Y.; Zhou, X. Penicillixanthone A, a marine-derived dual-coreceptor antagonist as anti-HIV-1 agent. Nat. Prod. Res. 2017, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Castro-Carvalho, B.; Ramos, A.A.; Prata-Sena, M.; Malhao, F.; Moreira, M.; Gargiulo, D.; Dethoup, T.; Buttachon, S.; Kijjoa, A.; Rocha, E. Marine-derived Fungi Extracts Enhance the Cytotoxic Activity of Doxorubicin in Nonsmall Cell Lung Cancer Cells A459. Pharmacogn. Res. 2017, 9, S92–S98. [Google Scholar]
- Sawadogo, W.R.; Boly, R.; Cerella, C.; Teiten, M.H.; Dicato, M.; Diederich, M. A Survey of Marine Natural Compounds and Their Derivatives with Anti-cancer Activity Reported in 2012. Molecules 2015, 20, 7097–7142. [Google Scholar] [CrossRef] [PubMed]
- Bechelli, J.; Coppage, M.; Rosell, K.; Liesveld, J. Cytotoxicity of algae extracts on normal and malignant cells. Leuk. Res. Treatment 2011, 2011, 373519. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.Q.; Wang, Y.J.; Yin, Q.F.; Liu, G.X.; Liu, H.H.; Huang, Y.J.; Li, B. Phycocyanin: A Potential Drug for Cancer Treatment. J. Cancer 2017, 8, 3416–3429. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Gao, X.; Carter, C.L.; Liu, Z.R. The recombinant beta subunit of C-phycocyanin inhibits cell proliferation and induces apoptosis. Cancer Lett. 2007, 247, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Ristic, B.; Bhutia, Y.D.; Ganapathy, V. Cell-surface G-protein-coupled receptors for tumor-associated metabolites: A direct link to mitochondrial dysfunction in cancer. Biochim. Biophys. Acta 2017, 1868, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Peyressatre, M.; Prével, C.; Pellerano, M.; Morris, M.C. Targeting cyclin-dependent kinases in human cancers: From small molecules to Peptide inhibitors. Cancers 2015, 7, 179–237. [Google Scholar] [CrossRef] [PubMed]
- Subhashini, J.; Mahipal, S.V.; Reddy, M.C.; Mallikarjuna Reddy, M.; Rachamallu, A.; Reddanna, P. Molecular mechanisms in C-Phycocyanin induced apoptosis in human chronic myeloid leukemia cell line-K562. Biochem. Pharmacol. 2004, 68, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Gao, B.; Gao, Y.; Yang, X.; Cheng, X.; Ou, Y. Phycocyanin Inhibits Tumorigenic Potential of Pancreatic Cancer Cells: Role of Apoptosis and Autophagy. Sci. Rep. 2016, 6, 34564. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Wang, X.; Wang, Y.; Zhou, T.; Bai, Y.; Li, Y.C.; Huang, B. Photosensitization of phycocyanin extracted from Microcystis in human hepatocellular carcinoma cells: Implication of mitochondria-dependent apoptosis. J. Photochem. Photobiol. B. 2012, 117, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.D.; Yang, J.; Li, Q.; Zhang, Y.; Qi, M.; Fan, S.M.; Hayashi, T.; Tashiro, S.; Onodera, S.; Ikejima, T. Activation of p53 contributes to pseudolaric acid B-induced senescence in human lung cancer cells in vitro. Acta Pharmacol. Sin. 2016, 37, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Galluzzi, L.; Castedo, M.; Kroemer, G. Mitotic catastrophe: A mechanism for avoiding genomic instability. Nat. Rev. Mol. Cell Biol. 2011, 12, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Kwon, S.B.; Bak, Y.; Lee, S.K.; Yoon, D.Y. BCI induces apoptosis via generation of reactive oxygen species and activation of intrinsic mitochondrial pathway in H1299 lung cancer cells. Sci. China Life Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.C.; Chiang, T.H.; Wang, J.S.; Lin, L.J.; Chao, W.C.; Chen, B.H.; Lu, J.F. Flavonoids from Gynostemma pentaphyllum Exhibit Differential Induction of Cell Cycle Arrest in H460 and A549 Cancer Cells. Molecules 2014, 19, 17663–17681. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. NF-κB in cancer therapy. Arch. Toxicol. 2015, 89, 711–731. [Google Scholar] [CrossRef] [PubMed]
- Nishanth, R.P.; Ramakrishna, B.S.; Jyotsna, R.G.; Roy, K.R.; Reddy, G.V.; Reddy, P.K.; Reddanna, P. C-Phycocyanin inhibits MDR1 through reactive oxygen species and cyclooxygenase-2 mediated pathways in human hepatocellular carcinoma cell line. Eur. J. Pharmacol. 2010, 649, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Ding, G.B.; Yang, P.; Zhang, L.; Wu, H.; Li, H.; Li, Z. Migration Suppression of Small Cell Lung Cancer by Polysaccharides from Nostoc commune Vaucher. J. Agric. Food Chem. 2016, 64, 6277–6285. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.G.; Vignjevic, D.M. Modes of cancer cell invasion and the role of the microenvironment. Curr. Opin. Cell Biol. 2015, 36, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.C.; Lin, Y.Y.; Yang, S.Y.; Weng, Y.T.; Tsai, Y.T. Antimelanogenic effect of c-phycocyanin through modulation of tyrosinase expression by upregulation of ERK and downregulation of p38 MAPK signaling pathways. J. Biomed. Sci. 2011, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-κB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.T.; Wang, N. Correlation between MMP-2 and NF-κB expression of intracranial aneurysm. Asian Pac. J. Trop. Med. 2013, 6, 570–573. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, S.; Yan, Y.; Li, S.; Zhao, L.; Zhang, C.; Liu, L.; Wang, C. The In Vitro Anti-Tumor Activity of Phycocyanin against Non-Small Cell Lung Cancer Cells. Mar. Drugs 2018, 16, 178. https://doi.org/10.3390/md16060178
Hao S, Yan Y, Li S, Zhao L, Zhang C, Liu L, Wang C. The In Vitro Anti-Tumor Activity of Phycocyanin against Non-Small Cell Lung Cancer Cells. Marine Drugs. 2018; 16(6):178. https://doi.org/10.3390/md16060178
Chicago/Turabian StyleHao, Shuai, Yan Yan, Shuang Li, Lei Zhao, Chan Zhang, Liyun Liu, and Chengtao Wang. 2018. "The In Vitro Anti-Tumor Activity of Phycocyanin against Non-Small Cell Lung Cancer Cells" Marine Drugs 16, no. 6: 178. https://doi.org/10.3390/md16060178
APA StyleHao, S., Yan, Y., Li, S., Zhao, L., Zhang, C., Liu, L., & Wang, C. (2018). The In Vitro Anti-Tumor Activity of Phycocyanin against Non-Small Cell Lung Cancer Cells. Marine Drugs, 16(6), 178. https://doi.org/10.3390/md16060178