Sorbicillasins A–B and Scirpyrone K from a Deep-Sea-Derived Fungus, Phialocephala sp. FL30r
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
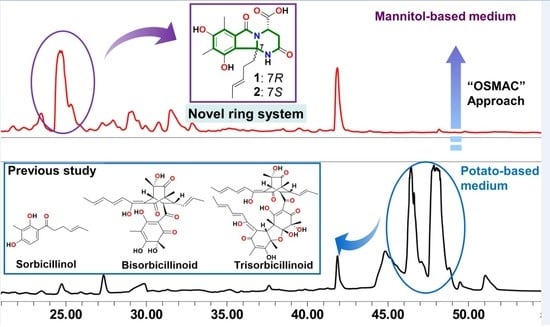
3.3. Fermentation and Extraction
3.4. Isolation
3.5. Biological Assay
3.6. Computation Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hoffmeister, D.; Keller, N.P. Natural products of filamentous fungi: Enzymes, genes, and their regulation. Nat. Prod. Rep. 2007, 24, 393–416. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.F.; Somoza, A.D.; Keller, N.P.; Wang, C.C.C. Advances in Aspergillus secondary metabolite research in the post-genomic era. Nat. Prod. Rep. 2012, 29, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Eze, P.M.; Hoefert, S.P.; Janiak, C.; Hartmann, R.; Okoye, F.B.C.; Esimone, C.O.; Orfali, R.S.; Dai, H.; Liu, Z.; et al. Substituted l-tryptophan-l-phenyllactic acid conjugates produced by an endophytic fungus Aspergillus aculeatus using an OSMAC approach. RSC Adv. 2018, 8, 7863–7872. [Google Scholar] [CrossRef]
- Meng, L.H.; Li, X.M.; Liu, Y.; Xu, G.M.; Wang, B.G. Antimicrobial alkaloids produced by the mangrove endophyte Penicillium brocae MA-231 using the OSMAC approach. RSC Adv. 2017, 7, 55026–55033. [Google Scholar] [CrossRef]
- Yuan, C.; Guo, Y.H.; Wang, H.Y.; Ma, X.J.; Jiang, T.; Zhao, J.L.; Zou, Z.M.; Ding, G. Allelopathic polyketides from an endolichenic fungus Myxotrichum sp. by using OSMAC strategy. Sci. Rep. UK 2016, 6, 19350. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, Z.; Zhu, T.; Gu, Q.; Li, D. Penicyclones A-E, antibacterial polyketides from the deep sea-derived fungus Penicillium sp. F23-2. J. Nat. Prod. 2015, 78, 2699–2703. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, F.; Xiao, X.; Fang, Y.; Zhu, T.; Gu, Q.; Zhu, W. Trisorbicillinone A, a novel sorbicillin trimer, from a deep ocean sediment derived fungus, Phialocephala sp. FL30r. Tetrahedron Lett. 2007, 48, 5235–5238. [Google Scholar] [CrossRef]
- Li, D.; Wang, F.; Cai, S.; Zeng, X.; Xiao, X.; Gu, Q.; Zhu, W. Two new bisorbicillinoids isolated from a deep-sea fungus, Phialocephala sp. FL30r. J. Antibiot. 2007, 60, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cai, S.; Zhu, T.; Wang, F.; Xiao, X.; Gu, Q. Three new sorbicillin trimers, trisorbicillinones B, C, and D, from a deep ocean sediment derived fungus, Phialocephala sp. FL30r. Tetrahedron 2010, 66, 5101–5106. [Google Scholar] [CrossRef]
- Li, D.H.; Cai, S.X.; Zhu, T.J.; Wang, F.P.; Xiao, X.; Gu, Q.Q. New cytotoxic metabolites from a deep-sea-derived fungus, Phialocephala sp., strain FL30r. Chem. Biodivers. 2011, 8, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Washida, K.; Abe, N.; Sugiyama, Y.; Hirota, A. Novel DPPH radical scavengers, demethylbisorbibutenolide and trichopyrone, from a fungus. Biosci. Biotechnol. Biochem. 2007, 71, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, F.; Miao, C.; Liu, K.; Li, Q.; Qin, S.; Zhao, L.; Ding, Z. Antifungal metabolites from the rhizospheric Penicillium sp. YIM PH 30003 associated with Panax notoginseng. Phytochem. Lett. 2015, 11, 249–253. [Google Scholar] [CrossRef]
- Tian, J.F.; Yu, R.J.; Li, X.X.; Gao, H.; Guo, L.D.; Tang, J.S.; Yao, X.S. 1H and 13C NMR spectral assignments of 2-pyrone derivatives from an endophytic fungus of Sarcosomataceae. Magn. Reson. Chem. 2015, 53, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Rosen, R.T.; Ho, C.T. 2,2-Diphenyl-1-picrylhydrazyl radical-scavenging active components from Polygonum multiflorum Thunb. J. Agric. Food Chem. 1999, 47, 2226–2228. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wang, X.; Xu, D.; Fu, X.; Zhang, X.; Lai, D.; Zhou, L.; Zhang, G. Sorbicillinoids from fungi and their bioactivities. Molecules 2016, 21, 715. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Lang, G.; Gulder, T.A.M.; Tsuruta, H.; Muhlbacher, J.; Maksimenka, K.; Steffens, S.; Schaumann, K.; Stohr, R.; Wiese, J.; et al. The first sorbicillinoid alkaloids, the antileukemic sorbicillactones A and B, from a sponge-derived Penicillium chrysogenum strain. Tetrahedron 2005, 61, 7252–7265. [Google Scholar] [CrossRef]
- Cabrera, G.M.; Butler, M.; Rodriguez, M.A.; Godeas, A.; Haddad, R.; Eberlin, M.N. A sorbicillinoid urea from an intertidal Paecilomyces marquandii. J. Nat. Prod. 2006, 69, 1806–1808. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Peng, J.; Zhu, T.; Gu, Q.; Keyzers, R.A.; Li, D. Sorbicillamines A-E, nitrogen-containing sorbicillinoids from the deep-sea-derived fungus Penicillium sp. F23-2. J. Nat. Prod. 2013, 76, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Trifonov, L.S.; Dreiding, A.S.; Hoesch, L.; Rast, D.M. Isolation of four hexaketides from Verticillium intertextum. Helv. Chim. Acta 1981, 64, 1843–1846. [Google Scholar] [CrossRef]
- Miklos, F.; Bozo, K.; Galla, Z.; Haukka, M.; Fulop, F. Traceless chirality transfer from a norbornene β-amino acid to pyrimido[2,1-a]isoindole enantiomers. Tetrahedron Asymmetr. 2017, 28, 1401–1406. [Google Scholar] [CrossRef]
- Houlihan, W.J.; Kelly, L.; Pankuch, J.; Koletar, J.; Brand, L.; Janowsky, A.; Kopajtic, T.A. Mazindol analogues as potential inhibitors of the cocaine binding site at the dopamine transporter. J. Med. Chem. 2002, 45, 4097–4109. [Google Scholar] [CrossRef] [PubMed]
- Miklos, F.; Toth, Z.; Haenninen, M.M.; Sillanpaeae, R.; Forro, E.; Fueloep, F. Retro-Diels-Alder protocol for the synthesis of pyrrolo[1,2-a]pyrimidine and pyrimido[2,1-a] isoindole enantiomers. Eur. J. Org. Chem. 2013, 2013, 4887–4894. [Google Scholar] [CrossRef]
- Yu, G.; Wang, S.; Wang, L.; Che, Q.; Zhu, T.; Zhang, G.; Gu, Q.; Guo, P.; Li, D. Lipid-lowering polyketides from the fungus Penicillium Steckii HDN13-279. Mar. Drugs 2018, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, X.; Zhang, G.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Inducing secondary metabolite production by combined culture of Talaromyces aculeatus and Penicillium variabile. J. Nat. Prod. 2017, 80, 3167–3171. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Kong, X.; Zhu, T.; Gu, Q.; Li, D. Penipyrols A-B and peniamidones A-D from the mangrove derived Penicillium solitum GWQ-143. Arch. Pharm. Res. 2015, 38, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Spartan’ 14; Wavefunction Inc.: Irvine, CA, USA, 2013.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bruhn, T.; Hemberger, Y.; Schaumlöffel, A.; Bringmann, G. SpecDis, Version 1.53; University of Wuerzburg: Wurzburg, Germany, 2011. [Google Scholar]






| No. | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH, Mult. (J in Hz) | δC | δH, Mult. (J in Hz) | |
| 1 | 125.6 | 125.6 | ||
| 2 | 115.6 | 115.8 | ||
| 3 | 155.7 | 155.8 | ||
| 4 | 118.9 | 119.2 | ||
| 5 | 148.4 | 148.4 | ||
| 6 | 124.8 | 130.7 | ||
| 7 | 75.2 | 75.2 | ||
| 9 | 167.1 | 168.0 | ||
| 10 | 31.0 | 2.48 dd (17.1, 6.0), H-10a 2.82 dd (17.1, 6.0), H-10b | 34.0 | 2.35 m, H-10a 2.61 m, H-10b |
| 11 | 48.2 | 4.94 t (7.55) | 50.2 | 4.31 s |
| 13 | 169.0 | 169.5 | ||
| 14 | 10.1 | 2.38 s | 10.2 | 2.33 s |
| 15 | 10.7 | 2.11 s | 10.7 | 2.10 s |
| 16 | 36.9 | 2.41 dt (11.8, 4.0) 1.90 dt (11.8, 4.0) | 35.9 | 2.45 m 2.13 m |
| 17 | 27.1 | 1.80 dt (11.8, 5.8) 1.12 dt (11.8, 5.8) | 27.3 | 1.66 m 1.32 m |
| 18 | 130.4 | 5.19 m | 130.5 | 5.24 m |
| 19 | 124.8 | 5.17m | 125.1 | 5.19 m |
| 20 | 18.2 | 1.48 d (4.2) | 18.3 | 1.48 d (5.7) |
| 21 | 172.6 | 171.4 | ||
| 3-OH | 8.62 s | 8.60 s | ||
| 5-OH | 8.77 s | 8.76 s | ||
| 8-NH | 8.04 s | 8.03 s | ||
| 21-OH | 13.12 brs | 12.74 brs | ||
| No. | 3 a | |
|---|---|---|
| δC | δH, Mult. (J in Hz) | |
| 2 | 165.3 | |
| 3 | 97.3 | |
| 4 | 165.2 | |
| 5 | 99.9 | 5.98 s |
| 6 | 161.2 | |
| 7 | 28.4 | 2.67 t (6.95) |
| 8 | 30.2 | 2.59 t (6.95) |
| 9 | 172.4 | |
| 10 | 52.0 | 3.58 s |
| 11 | 8.8 | 1.72 s |
| 4-OH | 11.15 brs | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; He, X.; Che, Q.; Zhang, G.; Zhu, T.; Gu, Q.; Li, D. Sorbicillasins A–B and Scirpyrone K from a Deep-Sea-Derived Fungus, Phialocephala sp. FL30r. Mar. Drugs 2018, 16, 245. https://doi.org/10.3390/md16070245
Zhang Z, He X, Che Q, Zhang G, Zhu T, Gu Q, Li D. Sorbicillasins A–B and Scirpyrone K from a Deep-Sea-Derived Fungus, Phialocephala sp. FL30r. Marine Drugs. 2018; 16(7):245. https://doi.org/10.3390/md16070245
Chicago/Turabian StyleZhang, Zhenzhen, Xueqian He, Qian Che, Guojian Zhang, Tianjiao Zhu, Qianqun Gu, and Dehai Li. 2018. "Sorbicillasins A–B and Scirpyrone K from a Deep-Sea-Derived Fungus, Phialocephala sp. FL30r" Marine Drugs 16, no. 7: 245. https://doi.org/10.3390/md16070245
APA StyleZhang, Z., He, X., Che, Q., Zhang, G., Zhu, T., Gu, Q., & Li, D. (2018). Sorbicillasins A–B and Scirpyrone K from a Deep-Sea-Derived Fungus, Phialocephala sp. FL30r. Marine Drugs, 16(7), 245. https://doi.org/10.3390/md16070245