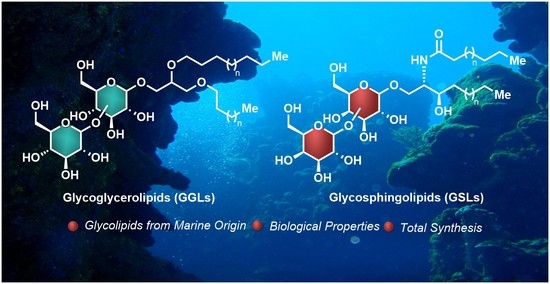
Chemistry and Biology of Bioactive Glycolipids of Marine Origin
Abstract
:1. Introduction
2. Chemistry and Biology of Glycosphingolipids
2.1. Neutral Glycosphingolipids
2.1.1. Cerebrosides
Acanthacerebrosides, Astrocerebrosides, and Asteriacerebrosides
Agelasphins
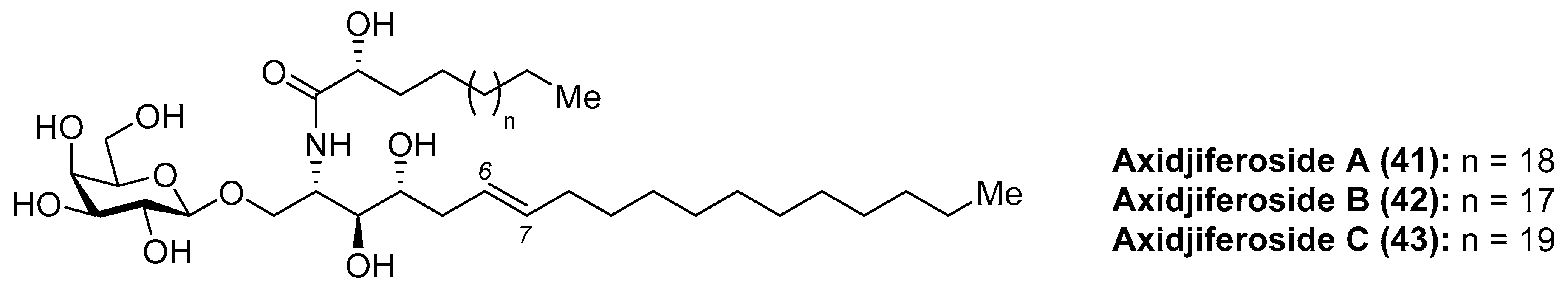
Axidjiferosides
Cerebrosides CE
Halicylindrosides
Phallusides
2.1.2. Diosylceramides
Amphiceramides
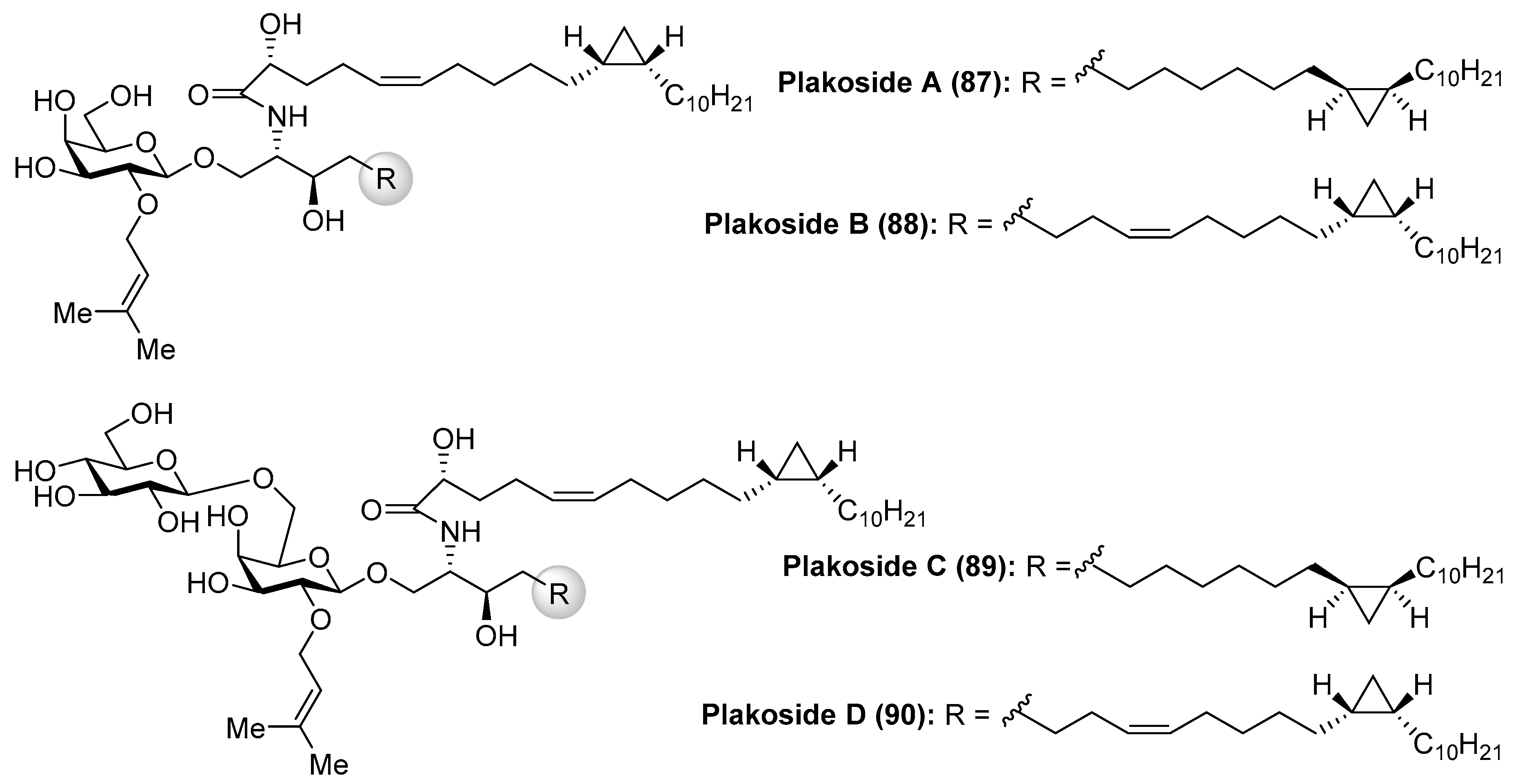
Plakosides
Terpiosides
2.1.3. Neutral Glycosphingolipids with Oligosaccharide Chains
Agelagalastatin
Clarhammosides
Vespariosides
2.2. Acidic Glycosphingolipids
2.2.1. Gangliosides
Ganglioside Hp-s1
Gangliosides HLG
Ganglioside GP-3
Ganglioside CG-1
Gangliosides SJG
Gangliosides CEG
2.2.2. Sulfatides
Axinelloside A
3. Chemistry and Biology of Glycoglycerolipids
3.1. Aminoglycoglycerolipids and Related Glycoglycerolipids
3.2. Crasserides and Isocrasserides
3.3. Myrmekiosides
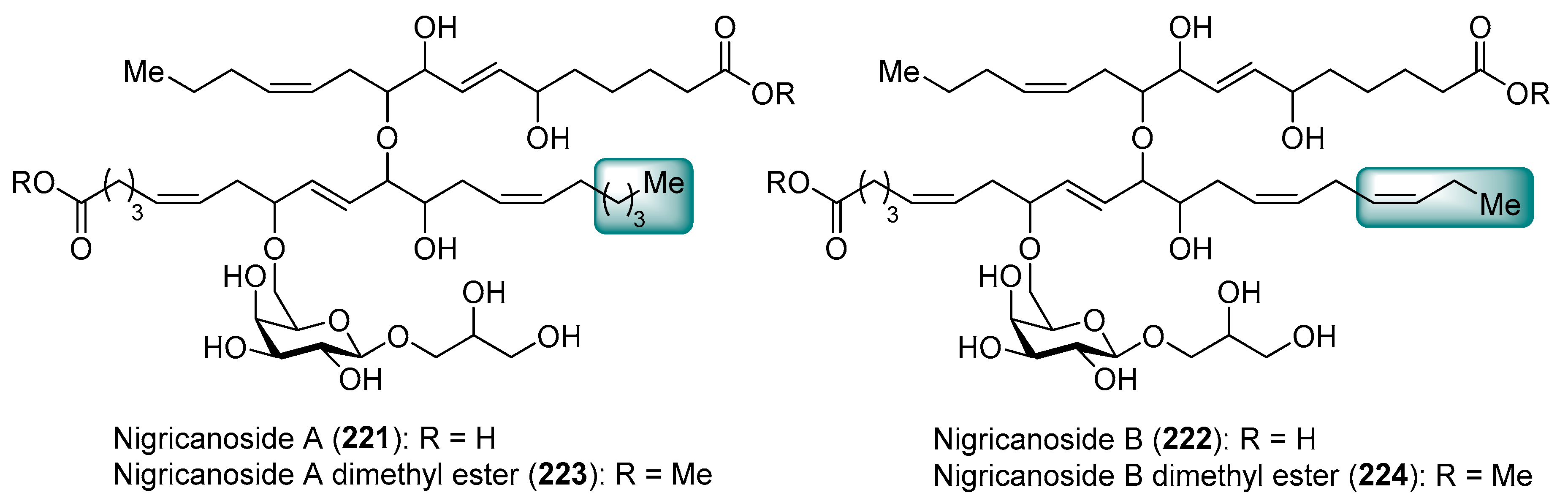
3.4. Nigricanosides
4. Chemistry and Biology of Atypical Glycolipids
4.1. Agminosides
4.2. Ancorinosides
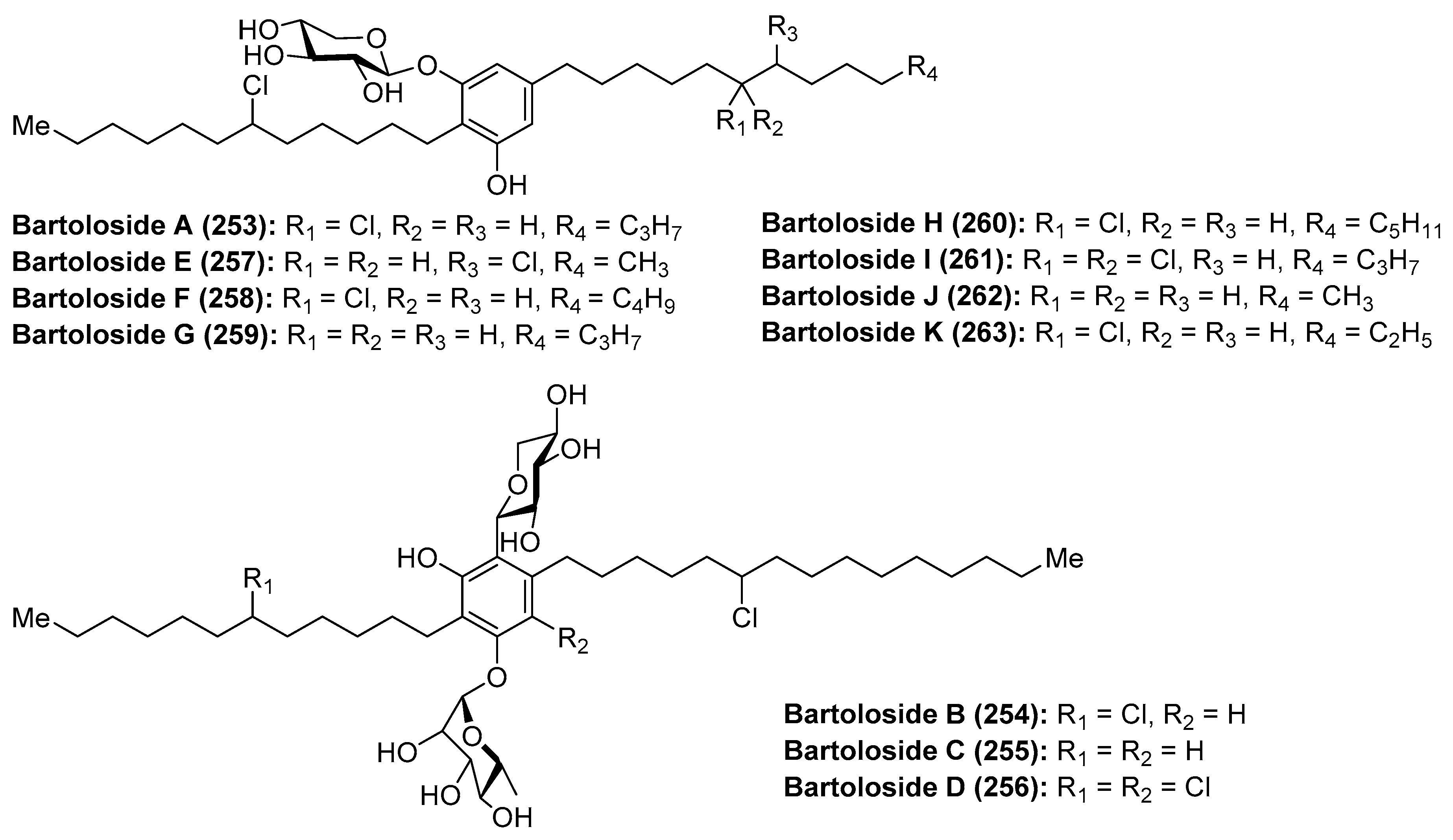
4.3. Bartolosides
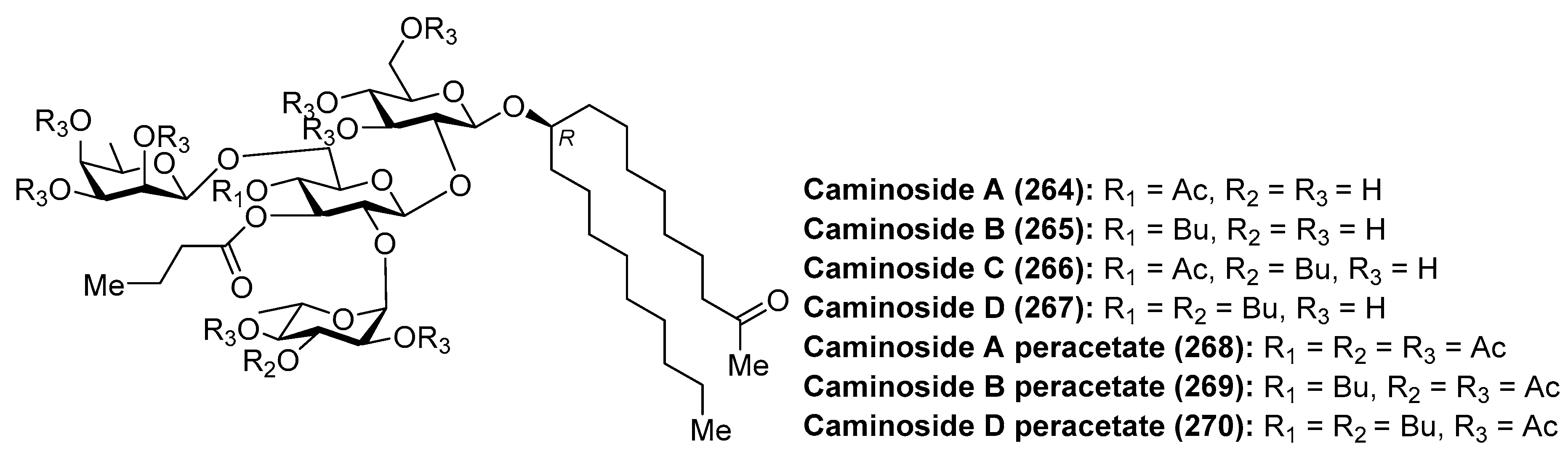
4.4. Caminosides
4.5. Clathrosides and Isoclathrosides
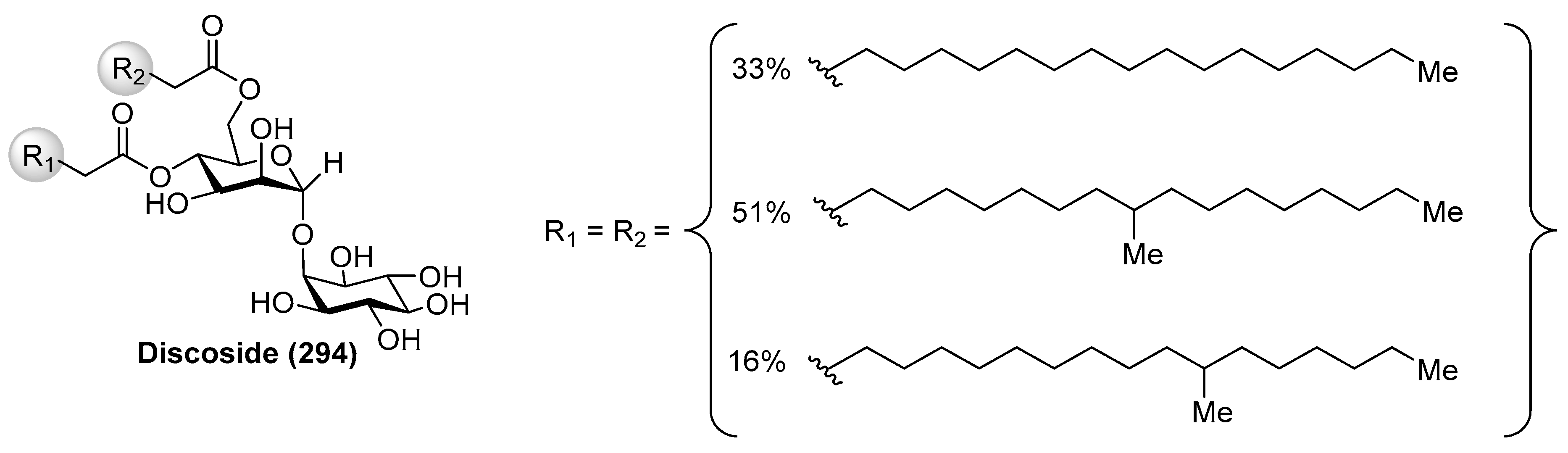
4.6. Discoside
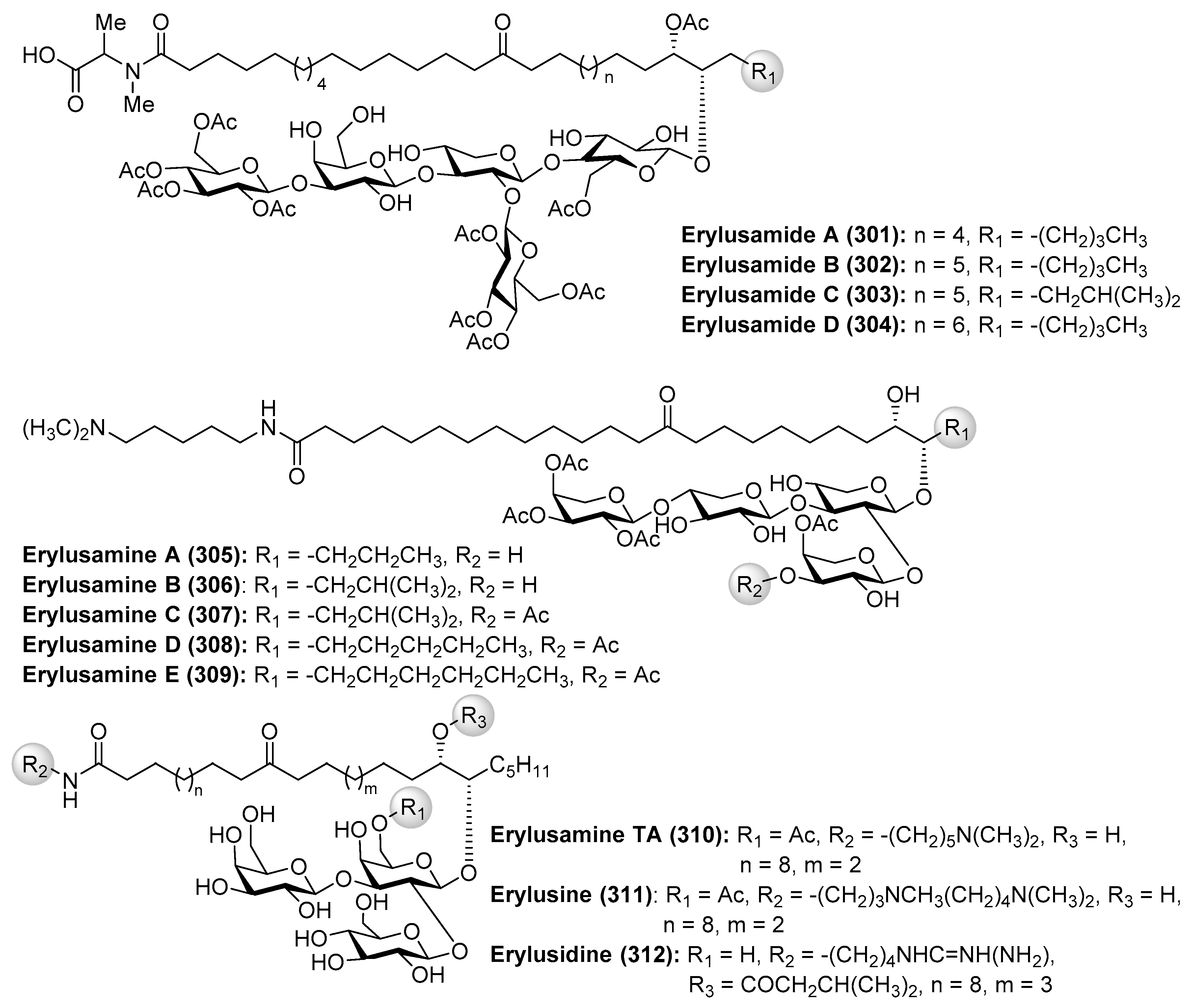
4.7. Erylusamides
4.8. Ieodoglucomides and Ieodoglycolipid
4.9. Pachymoside A
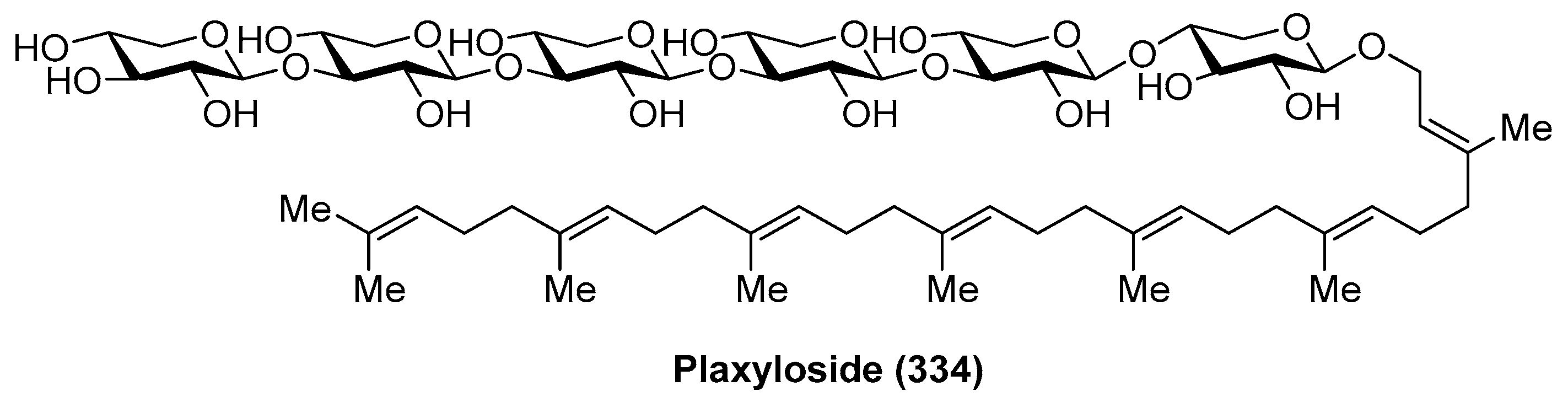
4.10. Plaxyloside
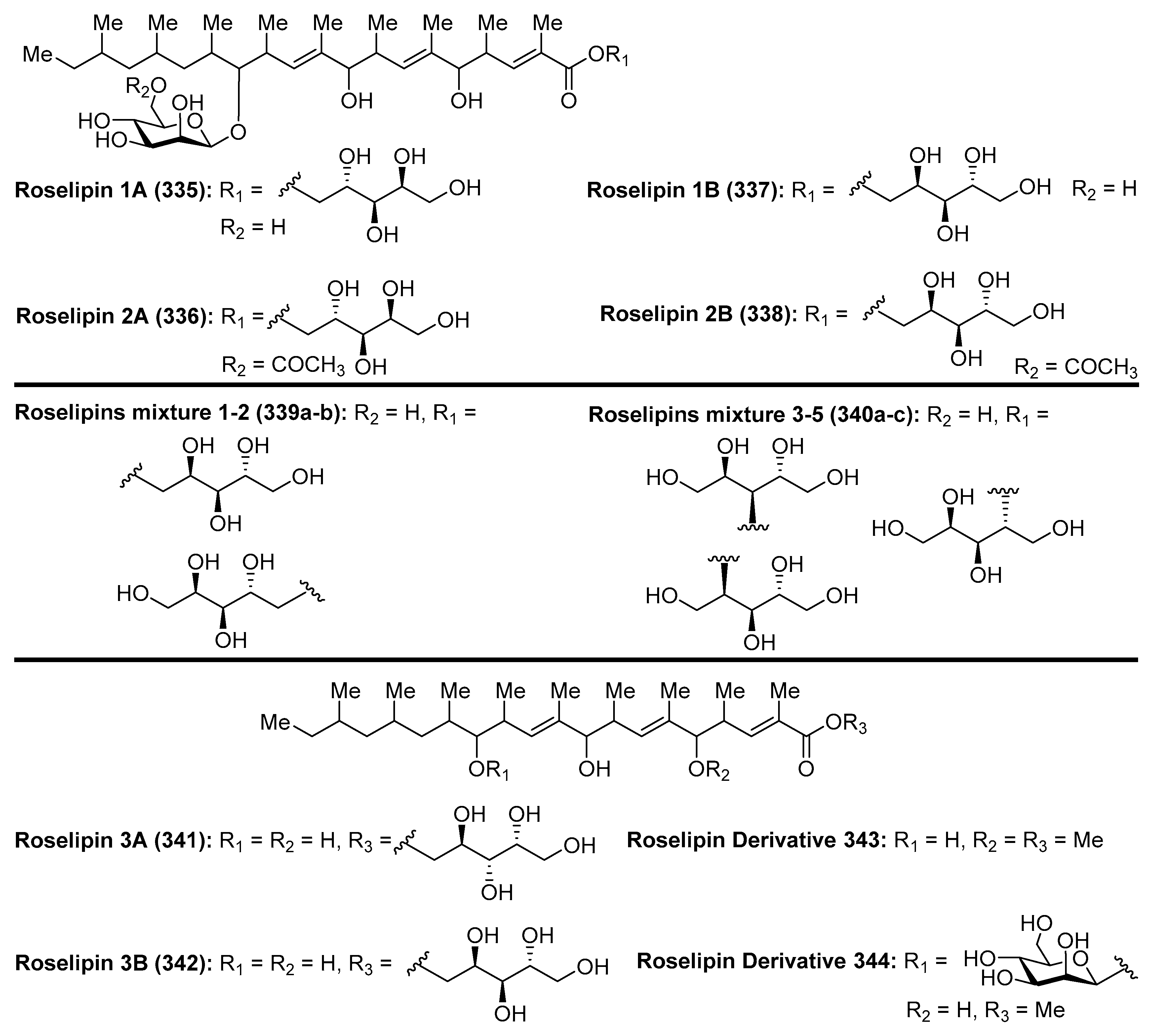
4.11. Roselipins
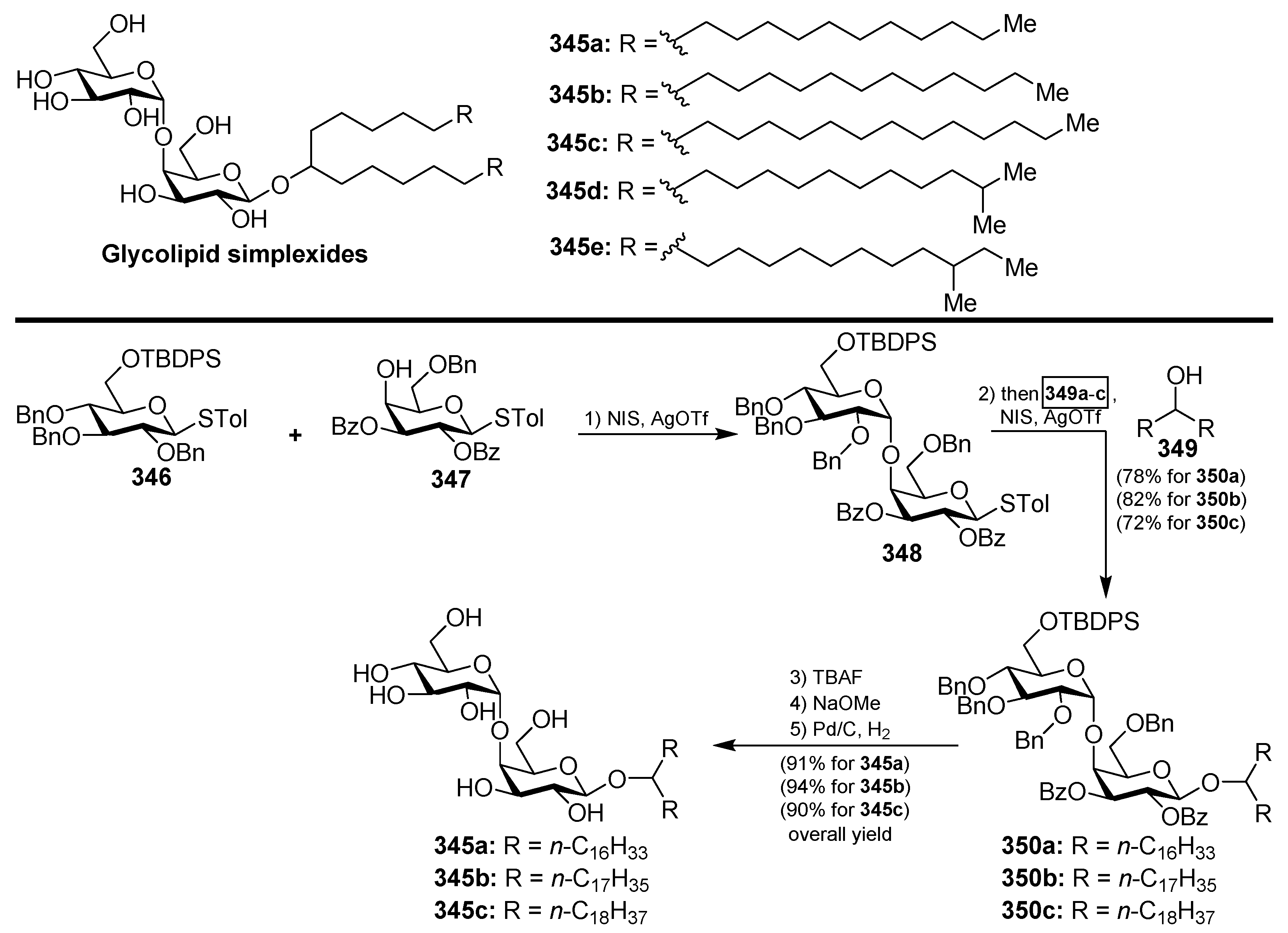
4.12. Simplexides
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Sasaki, D. Glycolipids: New Research; Nova Biomedical: New York, NY, USA, 2007; ISBN 978-1-60456-216-3. [Google Scholar]
- Sweely, C.C. Biochemistry of Lipids, Lipoproteins and Membranes; Benjamin/Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Hakomori, S.-I. Structure and Function of Sphingolipids in Transmembrane Signalling and Cell-Cell Interactions. Biochem. Soc. Trans. 1993, 21, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Biological Roles of Oligosaccharides: All of the Theories are Correct. Glycobiology 1993, 3, 97–130. [Google Scholar] [CrossRef] [PubMed]
- Kolter, T. A View on Sphingolipids and Disease. Chem. Phys. Lipids 2011, 164, 590–606. [Google Scholar] [CrossRef] [PubMed]
- Wennekes, T.; van den Berg, R.J.B.H.N.; Boot, R.G.; van der Marel, G.A.; Overkleeft, H.S.; Aerts, J.M.F.G. Glycosphingolipids—Nature, Function, and Pharmacological Modulation. Angew. Chem. Int. Ed. 2009, 48, 8848–8869. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Tashiro, T. Sphingolipids and Glycosphingolipids—Their Synthesis and Bioactivities. Heterocycles 2011, 83, 951–1003. [Google Scholar] [CrossRef]
- Vankar, Y.D.; Schmidt, R.R. Chemistry of Glycosphingolipids—Carbohydrate Molecules of Biological Significance. Chem. Soc. Rev. 2000, 29, 201–216. [Google Scholar] [CrossRef]
- Farwanah, H.; Kolter, T. Lipidomics of Glycosphingolipids. Metabolites 2012, 2, 134–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, R.X.; Chen, J.H. The Cerebrosides. Nat. Prod. Rep. 2003, 20, 509–534. [Google Scholar] [CrossRef] [PubMed]
- Barreto-Bergter, E.; Sassaki, G.L.; de Souza, L.M. Structural Analysis of Fungal Cerebrosides. Front. Microbiol. 2011, 2, 239. [Google Scholar] [CrossRef] [PubMed]
- Kolter, T. Ganglioside Biochemistry. ISRN Biochem. 2012, 2012, 506160. [Google Scholar] [CrossRef] [PubMed]
- Kates, M. Glycolipids, Phosphoglycolipids and Sulfoglycolipis. Handbook of Lipid Research; Springer: Boston, MA, USA, 1990; Volume 6. [Google Scholar]
- Schnaar, R.L.; Suzuki, A.; Stanley, P. Glycosphingolipids. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009; pp. 129–141. [Google Scholar]
- Kulkarni, S.S. Synthesis of Glycosphingolipids. In Glycochemical Synthesis: Strategies and Applications; Hung, S.-C., Zulueta, M.M.L., Eds.; Wiley: Hoboken, NJ, USA, 2016; pp. 293–326. [Google Scholar]
- Barnathan, G.; Couzinet-Mossion, A.; Wielgosz-Collin, G. Glycolipids from Marine Invertebrates. In Outstanding Marine Molecules: Chemistry, Biology, Analysis, 1st ed.; La Barre, S., Kornprobst, J.-M., Eds.; Wiley-VCH Verlag GmbH and Co., KGaA: Weinheim, Germany, 2014; pp. 99–162. ISBN 9783527681501. [Google Scholar]
- Zhang, J.; Li, C.; Yu, G.; Guan, H. Total Synthesis and Structure–Activity Relationship of Glycoglycerolipids from Marine Organisms. Mar. Drugs 2014, 12, 3634–3659. [Google Scholar] [CrossRef] [PubMed]
- Banchet-Cadeddu, A.; Hénon, E.; Dauchez, M.; Renault, J.-H.; Monneaux, F.; Haudrechy, A. The Stimulating Adventure of KRN7000. Org. Biomol. Chem. 2011, 9, 3080–3104. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.L.; Teyton, L.; Bendelac, A.; Savage, P.B. Stimulation of Natural Killer T Cells by Glycolipids. Molecules 2013, 18, 15662–15688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, Y.; Higuchi, R.; Isobe, R.; Komori, T. Isolation and Structure of Six New Cerebrosides. Liebigs Ann. Chem. 1988, 1988, 19–24. [Google Scholar] [CrossRef]
- Sugiyama, S.; Honda, M.; Komori, T. Synthesis of Acanthacerebroside A. Liebigs Ann. Chem. 1990, 1990, 1063–1068. [Google Scholar] [CrossRef]
- Higuchi, R.; Kagoshima, M.; Komori, T. Structures of Three New Cerebrosides, Astrocerebroside A, B, and C and of Related Nearly Homogeneous Cerebrosides. Liebigs Ann. Chem. 1990, 1990, 659–663. [Google Scholar] [CrossRef]
- Higuchi, R.; Jhou, J.X.; Inukai, K.; Komori, T. Isolation and Structure of Six New Cerebrosides, Asteriacerebrosides A–F, and Two Known Cerebrosides, Astrocerebroside A and Acanthacerebroside C. Liebigs Ann. Chem. 1991, 1991, 745–752. [Google Scholar] [CrossRef]
- Ishii, T.; Okino, T.; Mino, Y. A Ceramide and Cerebroside from the Starfish Asterias amurensis Lütken and Their Plant-Growth Promotion Activities. J. Nat. Prod. 2006, 69, 1080–1082. [Google Scholar] [CrossRef] [PubMed]
- Park, T.; Park, Y.S.; Rho, J.-R.; Kim, Y.H. Structural Determination of Cerebrosides Isolated from Asterias amurensis Starfish Eggs using High-Energy Collision-Induced Dissociation of Sodium-Adducted Molecules. Rapid Commun. Mass Spectrom. 2011, 25, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Chida, N.; Sakata, N.; Murai, K.; Tobe, T.; Nagase, T.; Ogawa, S. Total Synthesis of Acanthacerebroside A and Astrocerebroside A via a Chiral Epoxide Intermediate Derived from l-Quebrachitol. Bull. Chem. Soc. Jpn. 1998, 71, 259–272. [Google Scholar] [CrossRef]
- Natori, T.; Koezuka, Y.; Higa, T. Agelasphins, Novel α-Galactosylceramides from the Marine Sponge Agelas mauritianus. Tetrahedron Lett. 1993, 34, 5591–5592. [Google Scholar] [CrossRef]
- Natori, T.; Morita, M.; Akimoto, K.; Koezuka, Y. Agelasphins, Novel Antitumor and Immunostimulatory Cerebrosides from the Marine Sponge Agelas mauritianus. Tetrahedron 1994, 50, 2771–2784. [Google Scholar] [CrossRef]
- Morita, M.; Motoki, K.; Akimoto, K.; Natori, T.; Sakai, T.; Sawa, E.; Yamaji, K.; Koezuka, Y.; Kobayashi, E.; Fukushima, H. Structure-Activity Relationship of α-Galactosylceramides against B16-Bearing Mice. J. Med. Chem. 1995, 38, 2176–2187. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G.; Punt, C.J.A.; Ando, Y.; Ruijter, R.; Nishi, N.; Peters, M.; von Blomberg, B.M.E.; Scheper, R.J.; van der Vliet, H.J.J.; van den Eertwegh, A.J.M.; et al. A Phase I Study of the Natural Killer T-Cell Ligand α-Galactosylceramide (KRN7000) in Patients with Solid Tumors. Clin. Cancer Res. 2002, 8, 3702–3709. [Google Scholar] [PubMed]
- Motoki, K.; Kobayashi, E.; Uchida, T.; Fukushima, H.; Koezuka, Y. Antitumor Activities of α-, β-Monogalactosylceramides and Four Diastereomers of an α-Galactosylceramide. Bioorg. Med. Chem. Lett. 1995, 5, 705–710. [Google Scholar] [CrossRef]
- Reddy, B.G.; Silk, J.D.; Salio, M.; Balamurugan, R.; Shepherd, D.; Ritter, G.; Cerundolo, V.; Schmidt, R.R. Nonglycosidic Agonists of Invariant NKT Cells for Use as Vaccine Adjuvants. ChemMedChem 2009, 4, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Costantino, V.; D’Esposito, M.; Fattorusso, E.; Mangoni, A.; Basilico, N.; Parapini, S.; Taramelli, D. Damicoside from Axinella damicornis: The Influence of a Glycosylated Galactose 4-OH Group on the Immunostimulatory Activity of α-Galactoglycosphingolipids. J. Med. Chem. 2005, 48, 7411–7417. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, K.; Natori, T.; Morita, M. Synthesis and Stereochemistry of Agelasphin-9b. Tetrahedron Lett. 1993, 34, 5593–5596. [Google Scholar] [CrossRef]
- Farokhi, F.; Grellier, P.; Clément, M.; Roussakis, C.; Loiseau, P.; Genin-Seward, E.; Kornprobst, J.-M.; Barnathan, G.; Wielgosz-Collin, G. Antimalarial Activity of Axidjiferosides, New β-Galactosylceramides from the African Sponge Axinyssa djiferi. Mar. Drugs 2013, 11, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Hara, E.; Miyamoto, T.; Higuchi, R.; Isobe, R.; Honda, S. Isolation and Structure of Biologically Active Glycosphingolipids from the Sea Cucumber Cucumaria echinata. Eur. J. Org. Chem. 1998, 1998, 371–378. [Google Scholar] [CrossRef]
- Li, H.; Matsunaga, S.; Fusetani, N. Halicylindrosides, Antifungal and Cytotoxic Cerebrosides from the Marine Sponge Halichondria cylindrata. Tetrahedron 1995, 51, 2273–2280. [Google Scholar] [CrossRef]
- Murakami, T.; Taguchi, K. Stereocontrolled Synthesis of Novel Phytosphingosine-type Glucosaminocerebrosides. Tetrahedron 1999, 55, 989–1004. [Google Scholar] [CrossRef]
- Durin, R.; Zubia, E.; Ortega, M.J.; Naranjo, S.; Salv, J. Phallusides, New Glucosphingolipids from the Ascidian Phallusia fumigata. Tetrahedron 1998, 54, 14597–14602. [Google Scholar] [CrossRef]
- Karlsson, K.-A.; Leffler, H.; Samuelsson, B.E. Characterization of Cerebroside (Monoglycosylceramide) from the Sea Anemone, Metridium senile. Biochim. Biophys. Acta 1979, 574, 79–93. [Google Scholar] [CrossRef]
- Kawai, G.; Ikeda, Y. Fruiting-Inducing Activity of Cerebrosides Observed with Schizophyllum commune. Biochim. Biophys. Acta 1982, 719, 612–618. [Google Scholar] [CrossRef]
- Hammami, S.; Bergaoui, A.; Boughalleb, N.; Romdhane, A.; Khoja, I.; Ben Halima Kamel, M.; Mighri, Z. Antifungal Effects of Secondary Metabolites Isolated from Marine Organisms Collected from the Tunisian Coast. C. R. Chim. 2010, 13, 1397–1400. [Google Scholar] [CrossRef]
- Black, F.J.; Kocienski, P.J. Synthesis of Phalluside-1 and Sch II using 1,2-Metallate Rearrangements. Org. Biomol. Chem. 2010, 8, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Costantino, V.; Fattorusso, E.; Imperatore, C.; Mangoni, A.; Teta, R. Amphiceramide A and B, Novel Glycosphingolipids from the Marine Sponge Amphimedon compressa. Eur. J. Org. Chem. 2009, 2009, 2112–2119. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Mangoni, A.; Di Rosa, M.; Ianaro, A. Glycolipids from Sponges. 6. Plakoside A and B, Two Unique Prenylated Glycosphingolipids with Immunosuppressive Activity from the Marine Sponge Plakortis simplex. J. Am. Chem. Soc. 1997, 119, 12465–12470. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Mangoni, A. Glycolipids from Sponges. Part 9: Plakoside C and D, Two Further Prenylated Glycosphingolipids from the Marine Sponge Ectyoplasia ferox. Tetrahedron 2000, 56, 5953–5957. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Li, J.; Zenke, G. Total Synthesis and Biological Evaluation of Glycolipids Plakosides A, B and Their Analogs. Helv. Chim. Acta 2000, 83, 1977–2006. [Google Scholar] [CrossRef]
- Seki, M.; Kayo, A.; Mori, K. Synthesis of (2S,3R,11S,12R,2′′′R,11′′′S,12′′′R)-Plakoside A, a Prenylated and Immunosuppressive Marine Galactosphingolipid with Cyclopropane-Containing Alkyl Chains. Tetrahedron Lett. 2001, 42, 2357–2360. [Google Scholar] [CrossRef]
- Seki, M.; Mori, K. Synthesis of a Prenylated and Immunosuppressive Marine Galactosphingolipid with Cyclopropane-Containing Alkyl Chains: (2S,3R,11S,12R,2′′′R, 5′′′Z,11′′′S,12′′′R)-Plakoside A and Its (2S,3R,11R,12S,2′′′R,5′′′Z,11′′′R,12′′′S) Isomer. Eur. J. Org. Chem. 2001, 2001, 3797–3809. [Google Scholar] [CrossRef]
- Mori, K.; Tashiro, T.; Akasaka, K.; Ohrui, H.; Fattorusso, E. Determination of the Absolute Configuration at the Two Cyclopropane Moieties of Plakoside A, an Immunosuppressive Marine Galactosphingolipid. Tetrahedron Lett. 2002, 43, 3719–3722. [Google Scholar] [CrossRef]
- Tashiro, T.; Akasaka, K.; Ohrui, H.; Fattorusso, E.; Mori, K. Determination of the Absolute Configuration at the Two Cyclopropane Moieties of Plakoside A, an Immunosuppressive Marine Galactosphingolipid. Eur. J. Org. Chem. 2002, 2002, 3659–3665. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Imperatore, C.; Mangoni, A.; Teta, R. Terpioside from the Marine Sponge Terpios sp., the First Glycosphingolipid Having an l-Fucofuranose Unit. Eur. J. Org. Chem. 2008, 2008, 2130–2134. [Google Scholar] [CrossRef]
- Cutignano, A.; De Palma, R.; Fontana, A. A Chemical Investigation of the Antarctic Sponge Lyssodendoryx flabellata. Nat. Prod. Res. 2012, 26, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Costantino, V.; Fattorusso, E.; Mangoni, A.; Teta, R.; Panza, E.; Ianaro, A. Terpioside B, a Difucosyl GSL from the Marine Sponge Terpios sp. is a Potent Inhibitor of NO Release. Bioorg. Med. Chem. 2010, 18, 5310–5315. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Xu, J.; Gingrich, D.E.; Williams, M.D.; Doubek, D.L.; Chapuis, J.-C.; Schmidt, J.M. Antineoplastic agents. Part 395. Isolation and Structure of Agelagalastatin from the Papua New Guinea Marine Sponge Agelas sp. Chem. Commun. 1999, 915–916. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, B.-Y.; Jeon, H.B.; Kim, K.S. Total Synthesis of Agelagalastatin. Org. Lett. 2006, 8, 3971–3974. [Google Scholar] [CrossRef] [PubMed]
- Costantino, V.; Fatturusso, E.; Imperatore, C.; Mangoni, A. Glycolipids from Sponges. 13. Clarhamnoside, the First Rhamnosylated α-Galactosylceramide from Agelas clathrodes. Improving Spectral Strategies for Glycoconjugate Structure Determination. J. Org. Chem. 2004, 69, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Li, C.; Liu, Y.; Zhang, Z.; Li, Y. Concise Synthesis of Clarhamnoside, a Novel Glycosphingolipid Isolated from the Marine Sponge Agela clathrodes. Carbohydr. Res. 2007, 342, 2003–2013. [Google Scholar] [CrossRef] [PubMed]
- Costantino, V.; Fattorusso, E.; Imperatore, C.; Mangoni, A. Vesparioside from the Marine Sponge Spheciospongia vesparia, the First Diglycosylceramide with a Pentose Sugar Residue. Eur. J. Org. Chem. 2005, 2005, 368–373. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Imperatore, C.; Mangoni, A. Glycolipids from Sponges. 20. J-Coupling Analysis for Stereochemical Assignments in Furanosides: Structure Elucidation of Vesparioside B, a Glycosphingolipid from the Marine Sponge Spheciospongia vesparia. J. Org. Chem. 2008, 73, 6158–6165. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.-C.; Zhu, S.-Y.; Cao, H.; Yang, J.-S. Total Synthesis of Marine Glycosphingolipid Vesparioside B. J. Am. Chem. Soc. 2016, 138, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Ijuin, T.; Kitajima, K.; Song, Y.; Kitazume, S.; Inoue, S.; Haslam, S.M.; Morris, H.R.; Dell, A.; Inoue, Y. Isolation and identification of novel sulfated and nonsulfated oligosialyl glycosphingolipids from sea urchin sperm. Glycoconj. J. 1996, 13, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Tanabe, K.; Miyamoto, T.; Kusumoto, T.; Inagaki, M.; Higuchi, R. Isolation and Structure of a Monomethylated Ganglioside Possessing Neuritogenic Activity from the Ovary of the Sea Urchin Diadema setosum. Chem. Pharm. Bull. 2008, 56, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-S.; Sawant, R.C.; Yang, S.-A.; Liao, Y.-J.; Liao, J.-W.; Badsara, S.S.; Luo, S.-Y. Synthesis of ganglioside Hp-s1. RSC Adv. 2014, 4, 47752–47761. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.-F.; Shih, C.-H.; Su, Y.-T.; Yao, C.-H.; Lian, J.-F.; Liao, C.-C.; Hsia, C.-W.; Shui, H.-A.; Rani, R. The Total Synthesis of a Ganglioside Hp-s1 Analogue Possessing Neuritogenic Activity by Chemoselective Activation Glycosylation. Org. Biomol. Chem. 2012, 10, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.-T.; Yeh, C.-H.; Yang, S.-A.; Lin, C.-Y.; Tai, H.-J.; Shelke, G.B.; Reddy, D.M.; Yu, A.L.; Luo, S.-Y. Design, Synthesis, and Biological Evaluation of Ganglioside Hp-s1 Analogues Varying at Glucosyl Moiety. ACS Chem. Neurosci. 2016, 7, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Shelke, G.B.; Chen, B.-R.; Yang, S.-A.; Kuo, T.-M.; Syu, Y.-L.; Ko, Y.-C.; Luo, S.-Y. Mild and Highly α-Selective O-Sialylation Method Based on Pre-Activation: Access to Gangliosides Hp-s1, DSG-A, and Their Analogues. Asian J. Org. Chem. 2017, 6, 1556–1560. [Google Scholar] [CrossRef]
- Wu, Y.-F.; Tsai, Y.-F.; Guo, J.-R.; Yu, C.-P.; Yu, H.-M.; Liao, C.-C. First Total Synthesis of Ganglioside DSG-A Possessing Neuritogenic Activity. Org. Biomol. Chem. 2014, 12, 9345–9349. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Matsubara, R.; Kaneko, M.; Miyamoto, T.; Higuchi, R. Isolation and Structure of a Biologically Active Ganglioside Molecular Species from the Sea Cucumber Holothuria leucospilota. Chem. Pharm. Bull. 2001, 49, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Iwayama, Y.; Ando, H.; Ishida, H.; Kiso, M. A First Total Synthesis of Ganglioside HLG-2. Chem. Eur. J. 2009, 15, 4637–4648. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.-F.; Wang, Y.; Xiong, D.-C.; Ye, X.-S. Stereoselective Synthesis of the Trisaccharide Moiety of Ganglioside HLG-2. J. Org. Chem. 2014, 79, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, R.; Inoue, S.; Inagaki, K.; Sakai, M.; Miyamoto, T.; Komori, T.; Inagaki, M.; Isobe, R. Isolation and Structure of a New Biologically Active Ganglioside Molecular Species from the Starfish Asterina pectinifera. Chem. Pharm. Bull. 2006, 54, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Sawa, M.; Tamai, H.; Imamura, A.; Ando, H.; Ishida, H.; Kiso, M. The Total Synthesis of Starfish Ganglioside GP3 Bearing a Unique Sialyl Glycan Architecture. Chem. Eur. J. 2016, 22, 8323–8331. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, R.; Mori, T.; Sugata, T.; Yamada, K.; Miyamoto, T. Partial Synthesis of a Sea Cucumber Ganglioside Analogue from a Starfish Cerebroside. Eur. J. Org. Chem. 1999, 1999, 145–147. [Google Scholar] [CrossRef]
- Kaneko, M.; Kisa, F.; Yamada, K.; Miyamoto, T.; Higuchi, R. Structure of Neuritogenic Active Ganglioside from the Sea Cucumber Stichopus japonicus. Eur. J. Org. Chem. 1999, 1999, 3171–3174. [Google Scholar] [CrossRef]
- Kaneko, M.; Kisa, F.; Yamada, K.; Miyamoto, T.; Higuchi, R. Structure of a New Neuritogenic-Active Ganglioside from the Sea Cucumber Stichopus japonicus. Eur. J. Org. Chem. 2003, 2003, 1004–1008. [Google Scholar] [CrossRef]
- Tamai, H.; Ando, H.; Tanaka, H.-N.; Hosoda-Yabe, R.; Yabe, T.; Ishida, H.; Kiso, M. The Total Synthesis of the Neurogenic Ganglioside LLG-3 Isolated from the Starfish Linckia laevigata. Angew. Chem. Int. Ed. 2011, 50, 2330–2333. [Google Scholar] [CrossRef] [PubMed]
- Tamai, H.; Imamura, A.; Ogawa, J.; Ando, H.; Ishida, H.; Kiso, M. First Total Synthesis of Ganglioside GAA-7 from Starfish Asterias amurensis versicolor. Eur. J. Org. Chem. 2015, 2015, 5199–5211. [Google Scholar] [CrossRef]
- Kaneko, M.; Yamada, K.; Miyamoto, T.; Inagaki, M.; Higuchi, R. Neuritogenic Activity of Gangliosides from Echinoderms and Their Structure-Activity Relationship. Chem. Pharm. Bull. 2007, 55, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Kisa, F.; Yamada, K.; Miyamoto, T.; Inagaki, M.; Higuchi, R. Isolation and Structure of Biologically Active Monosialo-Gangliosides from the Sea Cucumber Cucumaria echinata. Chem. Pharm. Bull. 2006, 54, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Kisa, F.; Yamada, K.; Miyamoto, T.; Inagaki, M.; Higuchi, R. Isolation and Structure of Biologically Active Disialo- and Trisialo-Gangliosides from the Sea Cucumber Cucumaria echinata. Chem. Pharm. Bull. 2006, 54, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Warabi, K.; Hamada, T.; Nakao, Y.; Matsunaga, S.; Hirota, H.; van Soest, R.W.M.; Fusetani, N. Axinelloside A, an Unprecedented Highly Sulfated Lipopolysaccharide Inhibiting Telomerase, from the Marine Sponge, Axinella infundibula. J. Am. Chem. Soc. 2005, 127, 13262–13270. [Google Scholar] [CrossRef] [PubMed]
- Warabi, K.; Matsunaga, S.; van Soest, R.W.M.; Fusetani, N. Dictyodendrins A-E, the First Telomerase-Inhibitory Marine Natural Products from the Sponge Dictyodendrilla verongiformis. J. Org. Chem. 2003, 68, 2765–2770. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Walczak, M.A. Synthesis of Asymmetrically Substituted scyllo-Inositol. Tetrahedron Lett. 2016, 57, 3281–3283. [Google Scholar] [CrossRef]
- Guang, J.; Rumlow, Z.A.; Wiles, L.M.; O’Neill, S.; Walczak, M.A. Sulfated Liposaccharides Inspired by Telomerase Inhibitor Axinelloside A. Tetrahedron Lett. 2017, 58, 4867–4871. [Google Scholar] [CrossRef]
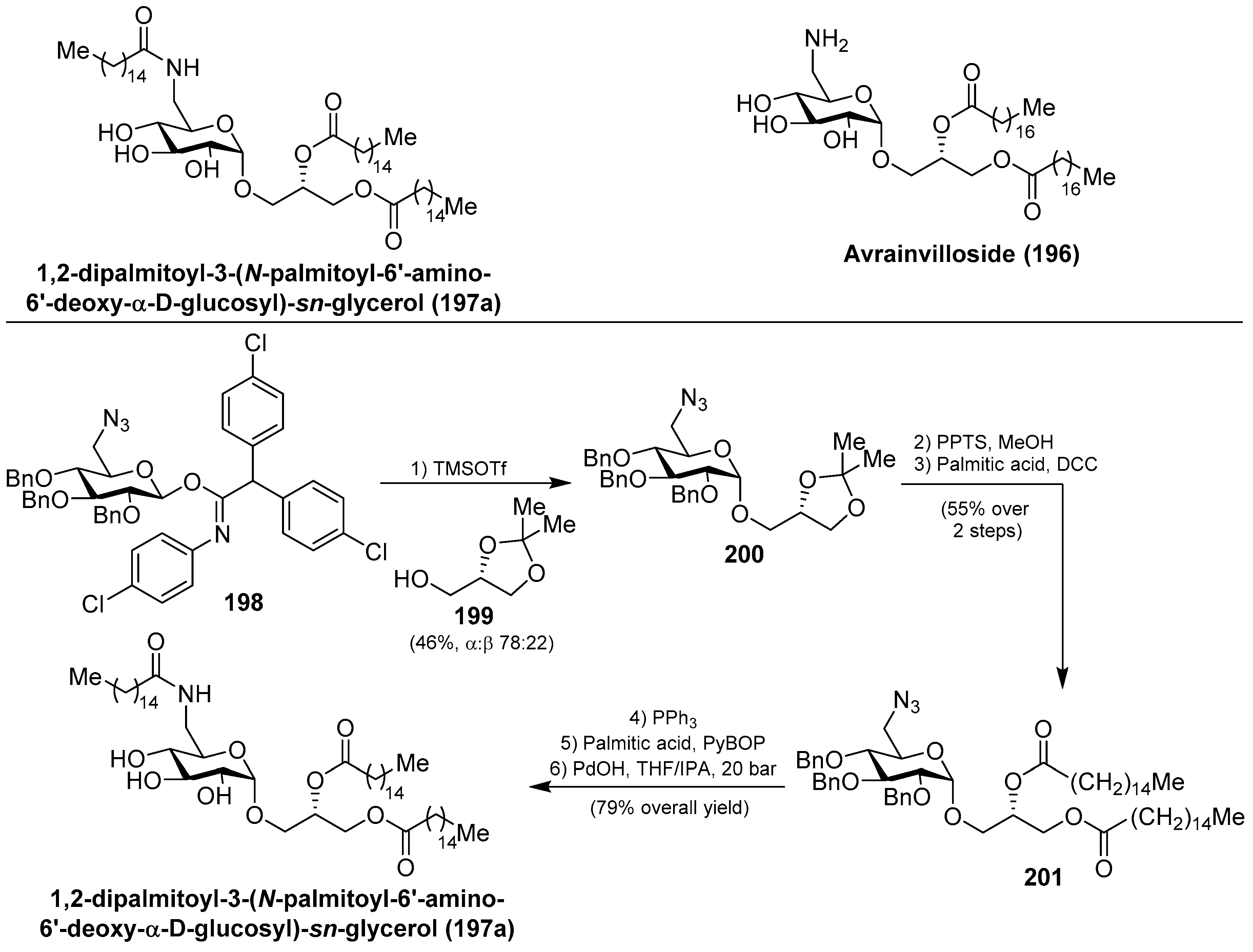
- Andersen, R.J.; Taglialatela-Scafati, O. Avrainvilloside, a 6-Deoxy-6-aminoglucoglycerolipid from the Green Alga Avrainvillea nigricans. J. Nat. Prod. 2005, 68, 1428–1430. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.-N.; Tang, S.; Johnson, R.K.; Mattern, M.P.; Lazo, J.S.; Sharlow, E.R.; Harich, K.; Kingston, D.G.I. New Glycolipid Inhibitors of Myt1 Kinase. Tetrahedron 2005, 61, 883–887. [Google Scholar] [CrossRef]
- Göllner, C.; Philipp, C.; Dobner, B.; Sippl, W.; Schmidt, M. First Total Synthesis of 1,2-dipalmitoyl-3-(N-palmitoyl-6′-amino-6′-deoxy-α-d-glucosyl)-sn-glycerol—A Glycoglycerolipid of a Marine Alga with a High Inhibitor Activity against Human Myt1-Kinase. Carbohydr. Res. 2009, 344, 1628–1631. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, J.; Li, C.; Guan, H.; Yu, G. Synthesis of Glycoglycerolipid of 1,2-dipalmitoyl-3-(N-palmitoyl-6′-amino-6′-deoxy-α-d-glucosyl)-sn-glycerol and its Analogues, Inhibitors of Human Myt1-Kinase. Carbohydr. Res. 2012, 355, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Y.; Wang, W.; Zhang, X.; Li, C.; Guan, H. Synthesis and Antiviral Evaluation of 6′-acylamido-6′-deoxy-α-d-mannoglycerolipids. Carbohydr. Res. 2013, 381, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, Y.; Zhang, J.; Zhao, Z.; Yu, G.; Guan, H. Synthesis of 6′-acylamido-6′-deoxy-α-d-galactoglycerolipids. Carbohydr. Res. 2013, 376, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, J.; Ma, H.; Sun, L.; Zhang, X.; Yu, G.; Guan, H.; Wang, W.; Li, C. Synthesis and Anti-Influenza A Virus Activity of 6′-amino-6′-deoxy-glucoglycerolipids Analogs. Mar. Drugs 2016, 14, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Costantino, V.; Fattorusso, E.; Mangoni, A. Isolation of Five-Membered Cyclitol Glycolipids, Crasserides: Unique Glycerides from the Sponge Pseudoceratina crassa. J. Org. Chem. 1993, 58, 186–191. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Imperatore, C.; Mangoni, A. Glycolipids from Sponge. 11. Isocrasserides, Novel Glycolipids with a Five-Membered Cyclitol Widely Distributed in Marine Sponges. J. Nat. Prod. 2002, 65, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Higuchi, K.; Kato, A.; Murakami, N.; Kobayashi, M. Myrmekiosides A and B, Novel Mono-O-alkyl-diglycosylglycerols Reversing Tumor Cell Morphology of ras-Transformed Cells from a Marine Sponge of Myrmekioderma sp. Tetrahedron 1999, 55, 14865–14870. [Google Scholar] [CrossRef]
- Farokhi, F.; Wielgosz-Collin, G.; Robic, A.; Debitus, C.; Malleter, M.; Roussakis, C.; Kornprobst, J.-M.; Barnathan, G. Antiproliferative Activity against Human non-Small Cell Lung Cancer of Two O-alkyl-diglycosylglycerols from the Marine Sponges Myrmekioderma dendyi and Trikentrion laeve. Eur. J. Med. Chem. 2012, 49, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, C.; Sun, L.; Yu, G.; Guan, H. Total Synthesis of Myrmekioside A, a Mono-O-alkyl-diglycosylglycerol from Marine Sponge Myrmekioderma sp.: Total Synthesis of Myrmekioside A. Eur. J. Org. Chem. 2015, 2015, 4246–4253. [Google Scholar] [CrossRef]
- Williams, D.E.; Sturgeon, C.M.; Roberge, M.; Andersen, R.J. Nigricanosides A and B, Antimitotic Glycolipids Isolated from the Green Alga Avrainvillea nigricans Collected in Dominica. J. Am. Chem. Soc. 2007, 129, 5822–5823. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, T.; Fujiwara, K.; Okamoto, S.; Kondo, Y.; Akiba, U.; Ishigaki, Y.; Katoono, R.; Suzuki, T. Double Bond Formation Based on Nitroaldol Reaction and Radical Elimination: A Prototype Segment Connection Method for the Total Synthesis of Nigricanoside A Dimethyl Ester. Tetrahedron Lett. 2018, 59, 1846–1850. [Google Scholar] [CrossRef]
- Kinashi, N.; Fujiwara, K.; Tsunoda, T.; Katoono, R.; Kawai, H.; Suzuki, T. A stereoselective Method for the Construction of the C8′–O–C6′′ Ether of Nigricanoside-A: Synthesis of Simple Models for the C20 Lipid Chain/Galactosyl Glycerol Segment. Tetrahedron Lett. 2013, 54, 4564–4567. [Google Scholar] [CrossRef]
- Kurashina, Y.; Kuwahara, S. Stereoselective Synthesis of a Protected Form of (6R,7E,9S,10R,12Z)-6,9,10-trihydroxy-7,12-hexadecadienoic Acid. Biosci. Biotechnol. Biochem. 2012, 76, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Espindola, A.P.D.M.; Crouch, R.; DeBergh, J.R.; Ready, J.M.; MacMillan, J.B. Deconvolution of Complex NMR Spectra in Small Molecules by Multi Frequency Homonuclear Decoupling (MDEC). J. Am. Chem. Soc. 2009, 131, 15994–15995. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Koswatta, P.; DeBergh, J.R.; Fu, P.; Pan, E.; MacMillan, J.B.; Ready, J.M. Structure Elucidation of Nigricanoside A through Enantioselective Total Synthesis. Chem. Sci. 2015, 6, 2932–2937. [Google Scholar] [CrossRef] [PubMed]
- Wojnar, J.M.; Northcote, P.T. The Agminosides: Naturally Acetylated Glycolipids from the New Zealand Marine Sponge Raspailia agminata. J. Nat. Prod. 2011, 74, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Ohta, E.; Ikegami, S. Ancorinoside A: A Novel Tetramic Acid Glycoside from the Marine Sponge, Ancorina sp. which Specifically Inhibits Blastulation of Starfish Embryos. J. Org. Chem. 1997, 62, 6452–6453. [Google Scholar] [CrossRef]
- Fujita, M.; Nakao, Y.; Matsunaga, S.; Seiki, M.; Itoh, Y.; van Soest, R.W.M.; Fusetani, N. Ancorinosides B–D, Inhibitors of Membrane Type 1 Matrix Metalloproteinase (MT1-MMP), from the Marine Sponge Penares sollasi Thiele. Tetrahedron 2001, 57, 1229–1234. [Google Scholar] [CrossRef]
- Ohta, E.; Ohta, S.; Ikegami, S. Ancorinoside A Mg Salt from the Marine Sponge, Ancorina sp., which Specifically Inhibits Blastulation of Starfish Embryos. Tetrahedron 2001, 57, 4699–4703. [Google Scholar] [CrossRef]
- Petermichl, M.; Schobert, R. Total Synthesis of the Diglycosidic Tetramic Acid Ancorinoside A. Chem. Eur. J. 2017, 23, 14743–14746. [Google Scholar] [CrossRef] [PubMed]
- Leão, P.N.; Nakamura, H.; Costa, M.; Pereira, A.R.; Martins, R.; Vasconcelos, V.; Gerwick, W.H.; Balskus, E.P. Biosynthesis-Assisted Structural Elucidation of the Bartolosides, Chlorinated Aromatic Glycolipids from Cyanobacteria. Angew. Chem. Int. Ed. 2015, 54, 11063–11067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afonso, T.B.; Costa, M.S.; Rezende de Castro, R.; Freitas, S.; Silva, A.; Schneider, M.P.C.; Martins, R.; Leão, P.N. Bartolosides E–K from a Marine Coccoid Cyanobacterium. J. Nat. Prod. 2016, 79, 2504–2513. [Google Scholar] [CrossRef] [PubMed]
- Linington, R.G.; Robertson, M.; Gauthier, A.; Finlay, B.B.; van Soest, R.; Andersen, R.J. Caminoside A, an Antimicrobial Glycolipid Isolated from the Marine Sponge Caminus sphaeroconia. Org. Lett. 2002, 4, 4089–4092. [Google Scholar] [CrossRef] [PubMed]
- Linington, R.G.; Robertson, M.; Gauthier, A.; Finlay, B.B.; MacMillan, J.B.; Molinski, T.F.; van Soest, R.; Andersen, R.J. Caminosides B−D, Antimicrobial Glycolipids Isolated from the Marine Sponge Caminus sphaeroconia. J. Nat. Prod. 2006, 69, 173–177. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, J.B.; Linington, R.G.; Andersen, R.J.; Molinski, T.F. Stereochemical Assignment in Acyclic Lipids Across Long Distance by Circular Dichroism: Absolute Stereochemistry of the Aglycone of Caminoside A. Angew. Chem. Int. Ed. 2004, 43, 5946–5951. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Han, X.; Yu, B. First Total Synthesis of Caminoside A, an Antimicrobial Glycolipid from Sponge. Synlett 2005, 3, 437–440. [Google Scholar] [CrossRef]
- Zhang, Z.; Zong, C.; Song, G.; Lv, G.; Chun, Y.; Wang, P.; Ding, N.; Li, Y. Total Synthesis of Caminoside B, a Novel Antimicrobial Glycolipid Isolated from the Marine Sponge Caminus sphaeroconia. Carbohydr. Res. 2010, 345, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Costantino, V.; Fattorusso, E.; Imperatore, C.; Mangoni, A. Glycolipids from Sponges. Part 17. Clathrosides and Isoclathrosides, Unique Glycolipids from the Caribbean Sponge Agelas clathrodes. J. Nat. Prod. 2006, 69, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Costantino, V.; Fattorusso, E.; Mangoni, A. Glycolipids from Sponges. Part 16. Discoside, a Rare myo-Inositol-Containing Glycolipid from the Caribbean Sponge Discodermia dissoluta. J. Nat. Prod. 2005, 68, 1527–1530. [Google Scholar] [CrossRef] [PubMed]
- Florence, G.J.; Aslam, T.; Miller, G.J.; Milne, G.D.S.; Conway, S.J. Synthesis of the Marine Glycolipid Dioctadecanoyl Discoside. Synlett 2009, 19, 3099–3102. [Google Scholar] [CrossRef]
- Gaspar, H.; Cutignano, A.; Grauso, L.; Neng, N.; Cachatra, V.; Fontana, A.; Xavier, J.; Cerejo, M.; Vieira, H.; Santos, S. Erylusamides: Novel Atypical Glycolipids from Erylus cf. deficiens. Mar. Drugs 2016, 14, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Sata, N.; Asai, N.; Matsunaga, S.; Fusetani, N. Erylusamines, IL-6 Receptor Antagonists, from the Marine Sponge, Erylus placenta. Tetrahedron 1994, 50, 1105–1110. [Google Scholar] [CrossRef]
- Fusetani, N.; Sata, N.; Matsunaga, S. Isolation and Structure Elucidation of Erylusamine B, a New Class of Marine Natural Products, which Blocked an IL-6 Receptor, from the Marine Sponge Erylus placenta Thiele. Tetrahedron Lett. 1993, 34, 4067–4070. [Google Scholar] [CrossRef]
- Goobes, R.; Rudi, A.; Kashman, Y.; Ilan, M.; Loya, Y. Three New Glycolipids from a Red Sea Sponge of the Genus Erylus. Tetrahedron 1996, 52, 7921–7928. [Google Scholar] [CrossRef]
- Tareq, F.S.; Kim, J.H.; Lee, M.A.; Lee, H.-S.; Lee, Y.-J.; Lee, J.S.; Shin, H.J. Ieodoglucomides A and B from a Marine-Derived Bacterium Bacillus licheniformis. Org. Lett. 2012, 14, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Tareq, F.S.; Lee, H.-S.; Lee, Y.-J.; Lee, J.S.; Shin, H.J. Ieodoglucomide C and Ieodoglycolipid, New Glycolipids from a Marine-Derived Bacterium Bacillus licheniformis 09IDYM23. Lipids 2015, 50, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.R.; Jithender, E.; Prasad, K.R. Total Syntheses of the Proposed Structure for Ieodoglucomides A and B. J. Org. Chem. 2013, 78, 4251–4260. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.R.; Jithender, E.; Singh, A.; Ummanni, R. Stereoisomers of Ieodoglucomides A and B: Synthesis and Evaluation of Anticancer Activity. Synthesis 2014, 46, 822–827. [Google Scholar] [CrossRef]
- Warabi, K.; Zimmerman, W.T.; Shen, J.; Gauthier, A.; Robertson, M.; Finlay, B.B.; van Soest, R.; Andersen, R.J. Pachymoside A—A Novel Glycolipid Isolated from the Marine Sponge Pachymatisma johnstonia. Can. J. Chem. 2004, 82, 102–112. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Imperatore, C.; Mangoni, A. Plaxyloside from the Marine Sponge Plakortis simplex: An Improved Strategy for NMR Structural Studies of Carbohydrate Chains. Eur. J. Org. Chem. 2001, 2001, 4457–4462. [Google Scholar] [CrossRef]
- Ōmura, S.; Tomoda, H.; Tabata, N.; Ohyama, Y.; Abe, T.; Namikoshi, M. Roselipins, Novel Fungal Metabolites Having a Highly Methylated Fatty Acid Modified with a Mannose and an Arabinitol. J. Antibiot. 1999, 52, 586–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomoda, H.; Ohyama, Y.; Abe, T.; Tabata, N.; Namikoshi, M.; Yamaguchi, Y.; Masuma, R.; Ōmura, S. Roselipins, Inhibitors of Diacylglycerol Acyltransferase, Produced by Gliocladium roseum KF-1040. J. Antibiot. 1999, 52, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Tabata, N.; Ohyama, Y.; Tomoda, H.; Abe, T.; Namikoshi, M.; Ōmura, S. Structure Elucidation of Roselipins, Inhibitors of Diacylglycerol Acyltransferase Produced by Gliocladium roseum KF-1040. J. Antibiot. 1999, 52, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Ayers, S.; Zink, D.L.; Mohn, K.; Powell, J.S.; Brown, C.M.; Bills, G.; Grund, A.; Thompson, D.; Singh, S.B. Anthelmintic Constituents of Clonostachys candelabrum. J. Antibiot. 2010, 63, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Inokoshi, J.; Kawamoto, K.; Takagi, Y.; Matsuhama, M.; Ōmura, S.; Tomoda, H. Expression of Two Human Acyl-CoA: Diacylglycerol Acyltransferase Isozymes in Yeast and Selectivity of Microbial Inhibitors toward the Isozymes. J. Antibiot. 2009, 62, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, H.; Tabata, N.; Ohyama, Y.; Ōmura, S. Core Structure in Roselipins Essential for Eliciting Inhibitory Activity against Diacylglycerol Acyltransferase. J. Antibiot. 2003, 56, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Collado, J.; Singh, S.B.; Jayasuriya, H.; Dewey, R.; Polishook, J.D.; Dombrowski, A.W.; Zink, D.L.; Platas, G.; Pelaez, F.; et al. Isolation, Structure, and HIV-1-Integrase Inhibitory Activity of Structurally Diverse Fungal Metabolites. J. Ind. Microbiol. Biotechnol. 2003, 30, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Ondeyka, J.G.; Herath, K.B.; Jayasuriya, H.; Polishook, J.D.; Bills, G.F.; Dombrowski, A.W.; Mojena, M.; Koch, G.; DiSalvo, J.; DeMartino, J.; et al. Discovery of Structurally Diverse Natural Product Antagonists of Chemokine Receptor CXCR3. Mol. Divers. 2005, 9, 123–129. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Mangoni, A.; Di Rosa, M.; Ianaro, A. Glycolipids from Sponges. VII. Simplexides, Novel Immunosuppressive Glycolipids from the Caribbean Sponge Plakortis simplex. Bioorg. Med. Chem. Lett. 1999, 9, 271–276. [Google Scholar] [CrossRef]
- Loffredo, S.; Staiano, R.I.; Granata, F.; Costantino, V.; Borriello, F.; Frattini, A.; Lepore, M.T.; Mangoni, A.; Marone, G.; Triggiani, M. Simplexide Induces CD1d-Dependent Cytokine and Chemokine Production from Human Monocytes. PLoS ONE 2014, 9, 111326. [Google Scholar] [CrossRef] [PubMed]
- Lü, G.; Wang, P.; Liu, Q.; Zhang, Z.; Zhang, W.; Li, Y. Reactivity-based One-pot Synthesis of Immunosuppressive Glycolipids from the Caribbean Sponge Plakortis simplex. Chin. J. Chem. 2009, 27, 2217–2222. [Google Scholar]
- Li, J.; Li, W.; Yu, B. A Divergent Approach to the Synthesis of Simplexides and Congeners via a Late-Stage Olefin Cross-Metathesis Reaction. Org. Biomol. Chem. 2013, 11, 4971–4974. [Google Scholar] [CrossRef] [PubMed]
















































© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng-Sánchez, I.; Sarabia, F. Chemistry and Biology of Bioactive Glycolipids of Marine Origin. Mar. Drugs 2018, 16, 294. https://doi.org/10.3390/md16090294
Cheng-Sánchez I, Sarabia F. Chemistry and Biology of Bioactive Glycolipids of Marine Origin. Marine Drugs. 2018; 16(9):294. https://doi.org/10.3390/md16090294
Chicago/Turabian StyleCheng-Sánchez, Iván, and Francisco Sarabia. 2018. "Chemistry and Biology of Bioactive Glycolipids of Marine Origin" Marine Drugs 16, no. 9: 294. https://doi.org/10.3390/md16090294