Variation in Lipid Components from 15 Species of Tropical and Temperate Seaweeds
Abstract
:1. Introduction
2. Results
2.1. Total Lipids
2.2. Pigments
2.3. Fatty Acids
2.4. Nutritional Quality Index
2.5. Alpha Tocopherol
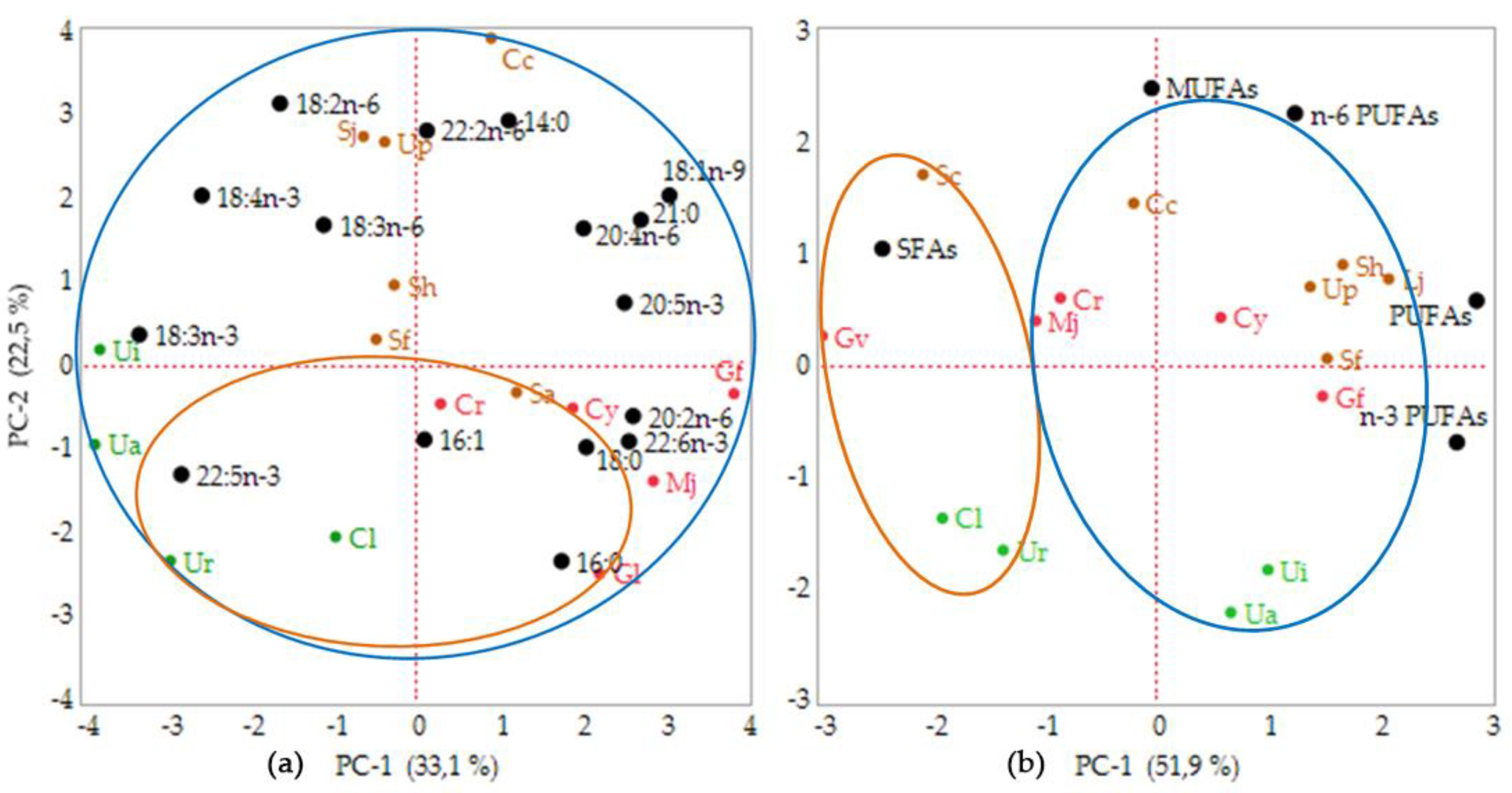
2.6. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Collection and Handling
4.3. Moisture Determination and TL Extraction
4.4. Pigment Analysis
4.5. Fatty Acids Analysis
4.6. α-Toc Analysis
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhargalkar, V.K.; Pereira, N. Seaweed: Promising plant of the millennium. Source 2005, 71, 60–66. [Google Scholar]
- White, W.L.; Wilson, P. World seaweed utilization. In Seaweed Sustainability, 1st ed.; Tiwari, B.K., Troy, D.J., Eds.; Elsevier Inc.: San Diego, CA, USA, 2015; pp. 7–26. ISBN 9780124199583. [Google Scholar]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pareda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Pereira, O.R.; Seca, A.M.L.; Pinto, D.C.G.A.; Silva, A.M.S. Seaweeds as preventive agents for cardiovascular diseases: From nutrients to functional foods. Mar. Drugs 2015, 13, 6838–6865. [Google Scholar] [CrossRef] [PubMed]
- Chater, P.I.; Wilcox, M.D.; Houghton, D.; Pearson, J.P. The role of seaweed bioactives in the control of digestion: Implications for obesity treatments. Food Funct. 2015, 6, 3420–3427. [Google Scholar] [CrossRef] [PubMed]
- Sharifuddin, Y.; Chin, Y.X.; Lim, P.E.; Phang, S.M. Potential bioactive compounds from seaweed for diabetes management. Mar. Drugs 2015, 13, 5447–5491. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Huang, X.; Cheong, K.L. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [PubMed]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and antidiabetic properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef]
- Alves, C.; Silva, J.; Pinteus, S.; Gaspar, H.; Alpoim, M.C.; Botana, L.M.; Pedrosa, R. From marine origin to therapeutics: The antitumor potential of marine algae-derived compounds. Front. Pharmacol. 2018, 9, 1–24. [Google Scholar] [CrossRef]
- Circuncisão, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A.M.S. Minerals from macroalgae origin: Health benefits and risks for consumers. Mar. Drugs 2018, 16, 400. [Google Scholar]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Overview on the antihypertensive and anti-obesity effects of secondary metabolites from seaweeds. Mar. Drugs 2018, 16, 237. [Google Scholar] [CrossRef] [PubMed]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Bioaccessibility of marine carotenoids. Mar. Drugs 2018, 16, 397. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Hirose, A.; Narayan, B.; Baba, Y.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Evaluation of recoverable functional lipid components of several brown seaweeds (Phaeophyta) from Japan with special reference to fucoxanthin and fucosterol contents. J. Phycol. 2009, 45, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Escrig, A.; Gómez-Ordóñez, E.; Rupérez, P. Brown and red seaweeds as potential sources of antioxidant nutraceuticals. J. Appl. Phycol. 2012, 24, 1123–1132. [Google Scholar] [CrossRef]
- Miyashita, K.; Mikami, N.; Hosokawa, M. Chemical and nutritional characteristics of brown seaweed lipids: A review. J. Funct. Foods 2013, 5, 1507–1517. [Google Scholar] [CrossRef]
- Aryee, A.N.; Agyei, D.; Akanbi, T.O. Recovery and utilization of seaweed pigments in food processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Ito, M.; Koba, K.; Hikihara, R.; Ishimaru, M.; Shibata, T.; Hatate, H.; Tanaka, R. Analysis of functional components and radical scavenging activity of 21 algae species collected from the Japanese coast. Food Chem. 2018, 255, 147–156. [Google Scholar] [CrossRef]
- Miyashita, K.; Hosokawa, M. Health impact of marine carotenoids. J. Food Bioact. 2018, 1, 31–40. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Chen, K.; Roca, M. In-vitro bioavailability of chlorophyll pigments from edible seaweeds. J. Funct. Foods 2018, 41, 25–33. [Google Scholar] [CrossRef]
- Yoshioka, H.; Kamata, A.; Konishi, T.; Takahashi, J.; Oda, H.; Tamai, T.; Toyohara, H.; Sugahara, T. Inhibitory effect of chlorophyll c2 from brown algae, Sargassum horneri, on degranulation of RBL-2H3 cells. J. Funct. Foods 2013, 5, 204–210. [Google Scholar] [CrossRef]
- Yoshioka, H.; Ishida, M.; Nishi, K.; Oda, H.; Toyohara, H.; Sugahara, T. Studies on anti-allergic activity of Sargassum horneri extract. J. Funct. Foods 2014, 10, 154–160. [Google Scholar] [CrossRef]
- Okai, Y.; Higashi-Okai, K. Potent anti-inflammatory activity of pheophytin a derived from edible green alga, Enteromorpha prolifera (sujiao-nori). Int. J. Immunopharmacol. 1997, 19, 355–358. [Google Scholar] [CrossRef]
- Islam, M.N.; Ishita, I.J.; Jin, S.E.; Choi, R.J.; Lee, C.M.; Kim, Y.S.; Jun, H.A.; Choi, J.S. Anti-inflammatory activity of edible brown alga Saccharina japonica and its constituents pheophorbide a and pheophytin a in LPS-stimulated RAW 264.7 macrophage cells. Food Chem. Toxicol. 2013, 55, 541–548. [Google Scholar] [CrossRef]
- Ina, A.; Hayashi, K.I.; Nozaki, H.; Kamei, Y. Pheophytin a, a low molecular weight compound found in the marine brown alga Sargassum fulvellum, promotes the differentiation of PC12 cells. Int. J. Dev. Neurosci. 2007, 25, 63–68. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Kumar, M.; Kumari, P.; Trivedi, N. Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. J. Appl. Phycol. 2011, 23, 797–810. [Google Scholar] [CrossRef]
- Gebauer, S.K.; Psota, T.L.; Harris, W.S.; Kris-etherton, P.M. n-3 Fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am. J. Clin. Nutr. 2006, 83, 1526–1535. [Google Scholar] [CrossRef]
- Kiso, Y. Pharmacology in health foods: Effects of arachidonic acid and docosahexaenoic acid on the age-related decline in brain and cardiovascular system function. J. Pharmacol. Sci. 2011, 115, 471–475. [Google Scholar] [CrossRef]
- Narayan, B.; Kinami, T.; Miyashita, K.; Park, S.B.; Endo, Y.; Fujimoto, K. Occurrence of conjugated polyenoic fatty acids in seaweeds from the Indian Ocean. Z. Naturforsch. C. 2004, 59, 310–314. [Google Scholar]
- Narayan, B.; Miyashita, K. Comparative evaluation of fatty acid composition of different Sargassum (Fucales, Phaeophyta) species harvested from temperate and tropical waters. J. Aquat. Food Prod. Tech. 2008, 13, 53–70. [Google Scholar] [CrossRef]
- Susanto, E.; Fahmi, A.S.; Abe, M.; Hosokawa, M.; Miyashita, K. Lipids, fatty acids, and fucoxanthin content from temperate and tropical brown seaweeds. Aquat. Procedia 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Gosch, B.J.; Magnusson, M.; Paul, N.A.; de Nys, R. Total lipid and fatty acid composition of seaweeds for the selection of species for oil-based biofuel and bioproducts. GCB Bioenergy 2012, 4, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Thinakaran, T.; Balamurugan, M.; Sivakumar, K. Screening of phycochemical constituents qualitatively and quantitatively certain seaweeds from Gulf of Mannar biosphere reserve. Int. Res. J. Pharm. 2012, 3, 261–265. [Google Scholar]
- McDermid, K.J.; Stuercke, B. Nutritional composition of edible Hawaiian seaweeds. J. Appl. Phycol. 2003, 15, 513–524. [Google Scholar] [CrossRef]
- Nomura, M.; Kamogawa, H.; Susanto, E.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Seasonal variations of total lipids, fatty acid composition, and fucoxanthin contents of Sargassum horneri (Turner) and Cystoseira hakodatensis (Yendo) from the northern seashore of Japan. J. Appl. Phycol. 2013, 25, 1159–1169. [Google Scholar] [CrossRef]
- Khotimchenko, S.V.; Yakovleva, I.M. Lipid composition of the red alga Tichocarpus crinitus exposed to different levels of photon irradiance. Phytochemistry 2005, 66, 73–79. [Google Scholar] [CrossRef]
- Ganesan, M.; Mairh, O.P.; Eswaran, K.; Subba Rao, P.V. Effect of salinity, light intensity and nitrogen source on growth and composition of Ulva fasciata Delile (Chlorophyta, Ulvales). Indian J. Mar. Sci. 1999, 28, 70–73. [Google Scholar]
- Zhao, Z.; Zhao, F.; Yao, J. Early development of germlings of Sargassum thunbergii (Fucales, Phaeophyta) under laboratory conditions. J. Appl. Phycol. 2008, 925–931. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Algal lipids, fatty acids and sterols. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 119–166. ISBN 978-0-85709-868-9. [Google Scholar]
- Chen, K.; Ríos, J.J.; Pérez-Gálvez, A.; Roca, M. Comprehensive chlorophyll composition in the main edible seaweeds. Food Chem. 2017, 228, 625–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, K.H.; Cheung, P.C.K. Nutritional evaluation of some subtropical red and green seaweeds. Food Chem. 2000, 71, 475–482. [Google Scholar] [CrossRef]
- Burtin, P. Nutritional value of seaweeds. J. Environ. Agric. Food Chem. 2003, 2, 498–503. [Google Scholar]
- Sánchez-Machado, D.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Fonseca, P.C.; Carneiro, M.A.A.; Moreira, W.S.C. Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour. Technol. 2006, 97, 2402–2406. [Google Scholar] [CrossRef]
- Van Ginneken, V.J.T.; Helsper, J.P.F.G.; De Visser, W.; Van Keulen, H.; Brandenburg, W.A. Polyunsaturated fatty acids in various macroalgal species from north Atlantic and tropical seas. Lipids Health Dis. 2011, 10, 4–11. [Google Scholar] [CrossRef]
- Kumari, P.; Bijo, A.J.; Mantri, V.A.; Reddy, C.R.K.; Jha, B. Fatty acid profiling of tropical marine macroalgae: An analysis from chemotaxonomic and nutritional perspectives. Phytochemistry 2013, 86, 44–56. [Google Scholar] [CrossRef]
- Boulom, S.; Robertson, J.; Hamid, N.; Ma, Q.; Lu, J. Seasonal changes in lipid, fatty acid, α-tocopherol and phytosterol contents of seaweed, Undaria pinnatifida, in the Marlborough Sounds, New Zealand. Food Chem. 2014, 161, 261–269. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Sho, H. History and characteristics of Okinawan longevity food. Asia Pac. J. Clin. Nutr. 2001, 10, 159–164. [Google Scholar] [CrossRef]
- McHugh, D.J. A Guide to the Seaweed Industry; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; pp. 1–105. ISBN 92-5-104958-0. [Google Scholar]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Moussavou, G.; Kwak, D.H.; Obiang-Obonou, B.W.; Maranguy, C.A.O.; Dinzouna-Boutamba, S.D.; Lee, D.H.; Pissibangnga, O.G.M.; Ko, K.; Seo, J.I.; Choo, J.K. Anticancer effects of different seaweeds on human colon and breast cancers. Mar. Drugs 2014, 12, 4898–4911. [Google Scholar] [CrossRef] [PubMed]
- Pádua, D.; Rocha, E.; Gargiulo, D.; Ramos, A.A. Bioactive compounds from brown seaweeds: Phloroglucinol, fucoxanthin and fucoidan as promising therapeutic agents against breast cancer. Phytochem. Lett. 2015, 14, 91–98. [Google Scholar] [CrossRef]
- Macartain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Special article nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef]
- Takaichi, S. Distributions, biosyntheses and functions of carotenoids in algae. Agro Food Ind. Hi Tech 2013, 24, 55–58. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-A y mice. J. Agric. Food Chem. 2007, 55, 7701–7706. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Dougherty, R.C.; Strain, H.H.; Svec, W.A.; Uphaus, R.A.; Katz, J.J. The structure, properties, and distribution of chlorophyll c. J. Am. Chem. Soc. 1970, 92, 2826–2833. [Google Scholar] [CrossRef]
- Katz, J.J.; Norris, J.R.; Shipman, L.L.; Thurnauer, M.C.; Wasielewski, M.R. Chlorophyll function in the photosynthetic reaction center. Annu. Rev. Biophys. Bioeng. 1978, 7, 393–434. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Voloshin, R.A.; Korol’kova, D.V.; Tomo, T.; Shen, J.R. Chlorophylls d and f and their role in primary photosynthetic processes of cyanobacteria. Biochemistry 2016, 81, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Nishida, N.; Nota, J.; Kitani, T.; Aoishi, K.; Takahashi, H.; Sugahara, T.; Hato, N. Efficacy of chlorophyll c2 for seasonal allergic rhinitis: Single-center double-blind randomized control trial. Eur. Arch. Otorhinolaryngol. 2016, 273, 4289–4294. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Roca, M. Cooking effects on chlorophyll profile of the main edible seaweeds. Food Chem. 2018, 266, 368–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffrey, S.W. Preparation and some properties of crystalline chlorophyll c1 and c2 from marine algae. Biochim. Biophys. Acta 1972, 279, 15–33. [Google Scholar] [CrossRef]
- Wilhelm, C. Purification and identification of chlorophyll c1 from the green alga Mantoniella squamata. Biochim. Biophys. Acta 1987, 892, 23–29. [Google Scholar] [CrossRef]
- Gelzinis, A.; Butkus, V.; Songaila, E.; Augulis, R.; Gall, A.; Büchel, C.; Robert, B.; Abramavicius, D.; Zigmantas, D.; Valkunas, L. Mapping energy transfer channels in fucoxanthin-chlorophyll protein complex. Biochim. Biophys. Acta - Bioenerg. 2015, 1847, 241–247. [Google Scholar] [CrossRef]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Strzalka, K. Photosynthetic pigments in diatoms. Mar. Drugs 2015, 13, 5847–5881. [Google Scholar] [CrossRef]
- Apt, K.E.; Clendennen, S.K.; Powers, D.A.; Grossman, A.R. The gene family encoding the fucoxanthin chlorophyll proteins from the brown alga Macrocystis pyrifera. Mol. Gen. Genet. 1995, 246, 455–464. [Google Scholar] [CrossRef]
- Mikami, K.; Hosokawa, M. Biosynthetic pathway and health benefits of fucoxanthin, an algae-specific xanthophyll in brown seaweeds. Int. J. Mol. Sci. 2013, 14, 13763–13781. [Google Scholar] [CrossRef]
- Schubert, N.; García-Mendoza, E.; Pacheco-Ruiz, I. Carotenoid composition of marine red algae. J. Phycol. 2006, 42, 1208–1216. [Google Scholar] [CrossRef]
- Cho, M.; Lee, H.S.; Kang, I.J.; Won, M.H.; You, S.G. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chem. 2011, 127, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Ki, J.S. A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Takagi, T. Tocopherol content of Japanese algae and its seasonal variation. Agric. Biol. Chem. 1987, 51, 3115–3118. [Google Scholar]
- Santos, S.A.O.; Vilela, C.; Freire, C.S.R.; Abreu, M.H.; Rocha, S.M.; Silvestre, A.J.D. Chlorophyta and Rhodophyta macroalgae: A source of health promoting phytochemicals. Food Chem. 2015, 183, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Praiboon, J.; Palakas, S.; Noiraksa, T.; Miyashita, K. Seasonal variation in nutritional composition and anti-proliferative activity of brown seaweed, Sargassum oligocystum. J. Appl. Phycol. 2018, 30, 101–111. [Google Scholar] [CrossRef]
- Gerasimenko, N.; Logvinov, S. Seasonal composition of lipids, fatty acids pigments in the brown alga Sargassum pallidum: The potential for health. Open J. Mar. Sci. 2016, 06, 498–523. [Google Scholar] [CrossRef]
- Nelson, M.M.; Phleger, C.F.; Nichols, P.D. Seasonal lipid composition in macroalgae of the northeastern Pacific Ocean. Bot. Mar. 2002, 45, 58–65. [Google Scholar] [CrossRef]
- Honya, M.; Kinoshita, T.; Ishikawa, M.; Mori, H.; Nisizawa, K. Seasonal variation in the lipid content of cultured Laminaria japonica: Fatty acids, sterols, β-carotene and tocopherol. J. Appl. Phycol. 1994, 6, 25–29. [Google Scholar] [CrossRef]
- Sanina, N.M.; Goncharova, S.N.; Kostetsky, E.Y. Seasonal changes of fatty acid composition and thermotropic behavior of polar lipids from marine macrophytes. Phytochemistry 2008, 69, 1517–1527. [Google Scholar] [CrossRef]
- Gerasimenko, N.I.; Busarova, N.G.; Moiseenko, O.P. Seasonal changes in the content of lipids, fatty acids, and pigments in brown alga Costaria costata. Russ. J. Plant Physiol. 2010, 57, 205–211. [Google Scholar] [CrossRef]
- Gerasimenko, N.I.; Skriptsova, A.V.; Busarova, N.G.; Moiseenko, O.P. Effects of the season and growth stage on the contents of lipids and photosynthetic pigments in brown alga Undaria pinnatifida. Russ. J. Plant Physiol. 2011, 58, 885–891. [Google Scholar] [CrossRef]
- Altamirano, M.; Flores-Moya, A.; Conde, F.; Figueroa, F.L. Growth seasonality, photosynthetic pigments, and carbon and nitrogen content in relation to environmental factors: A field study of Ulva olivascens (Ulvales, Chlorophyta). Phycologia 2000, 39, 50–58. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Giannenasa, I.; Florou-Paneria, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; Osman, M.E.H. Seasonal fluctuation of photosynthetic pigments of most common red seaweeds species collected from Abu Qir, Alexandria, Egypt. Rev. Biol. Mar. Oceanogr. 2016, 51, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Sampath-Wiley, P.; Neefus, C.D.; Jahnke, L.S. Seasonal effects of sun exposure and emersion on intertidal seaweed physiology: Fluctuations in antioxidant contents, photosynthetic pigments and photosynthetic efficiency in the red alga Porphyra umbilicalis Kützing (Rhodophyta, Bangiales). J. Exp. Mar. Biol. Ecol. 2008, 361, 83–91. [Google Scholar] [CrossRef]
- Pereira, D.C.; Trigueiro, T.G.; Colepicolo, P.; Marinho-Soriano, E. Seasonal changes in the pigment composition of natural population of Gracilaria domingensis (Gracilariales, Rhodophyta). Braz. J. Pharmacogn. 2012, 22, 874–880. [Google Scholar] [CrossRef]
- Zavodnik, N. Seasonal variations in rate of photosynthetic activity and chemical composition of the littoral seaweeds common to North Adriatic part II. Wrangelia penicillata C. AG. Bot. Mar. 1973, 16, 166–170. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N.; Hirose, A.; Stephen, N.; Gowda, L.R.; Hosokawa, M.; Miyashita, K. Edible Japanese seaweed, wakame (Undaria pinnatifida) as an ingredient in pasta: Chemical, functional and structural evaluation. Food Chem. 2009, 115, 501–508. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Res. Int. 2010, 43, 2289–2294. [Google Scholar] [CrossRef]
- Garrido, J.L.; Zapata, M. High performance liquid chromatography of chlorophylls c3, c1, c2 and a and of carotenoids of chromophyte algae on a polymeric octadecyl silica column. Chromatographia 1993, 35, 543–547. [Google Scholar] [CrossRef]
- Prevot, A.F.; Mordret, F.X. Utilisation des colonnes capillaires de verre pour l’analyse des corps gras par chromotographie en phase gazeuse. Rev. Fr. Corps Gras. 1979, 23, 409–423. [Google Scholar]
- Matos, Â.P.; Feller, R.; Moecke, E.H.S.; de Oliveira, J.V.; Junior, A.F.; Derner, R.B.; Sant`Anna, E.S. Chemical characterization of six microalgae with potential utility for food application. J. Am. Oil Chem. Soc. 2016, 93, 963–972. [Google Scholar] [CrossRef]
- Poerschmann, J.; Spijkerman, E.; Langer, U. Fatty acid patterns in Chlamydomonas sp. as a marker for nutritional regimes and temperature under extremely acidic conditions. Microb. Ecol. 2004, 48, 78–89. [Google Scholar] [CrossRef] [PubMed]
| Harvesting Location | Phylum | Family | Seaweeds | Local Name | Collection Date | Total Lipids (mg∙g−1 DW) |
|---|---|---|---|---|---|---|
| Tual, Indonesia a,d | Chlorophyta | Caulerpaceae | Caulerpa lentillifera | Lat | Feb-17 | 15.75 ± 0.82 |
| Hakodate, Japan b,f | Chlorophyta | Ulvaceae | Ulva australis | Anaaosa | Jun-17 | 62.48 ± 3.05 |
| Hakodate, Japan b,f | Chlorophyta | Ulvaceae | Ulva intestinalis | Bouaonori | Jun-17 | 37.46 ± 7.56 |
| Tual, Indonesia a,d | Chlorophyta | Ulvaceae | Ulva reticulata | Lumut daun | May-17 | 22.70 ± 3.01 |
| Hakodate, Japan b,f | Ochrophyta | Agaraceae | Costaria costata | Sujime | May-17 | 33.71 ± 1.86 |
| Hakodate, Japan b,f | Ochrophyta | Alariaceae | Undaria pinnatifida | Wakame | May-17 | 58.10 ± 4.56 |
| Hakodate, Japan b,f | Ochrophyta | Laminariaceae | Saccharina japonica | Konbu | May-17 | 37.42 ± 6.23 |
| Tual, Indonesia a,c,d | Ochrophyta | Sargassaceae | Sargassum aquifoliuma | Pama | Feb-17 | 20.87 ± 0.70 |
| Hakodate, Japan b,f | Ochrophyta | Sargassaceae | Sargassum fusiforme | Hijiki | May-17 | 48.54 ± 1.61 |
| Iwate, Japan b,g | Ochrophyta | Sargassaceae | Sargassum horneri | Akamoku | May-15 | 55.97 ± 2.51 |
| Hakodate, Japan b,f | Rhodophyta | Endocladiaceae | Gloiopeltis furcata | Fukurofunori | May-17 | 8.94 ± 1.45 |
| Hakodate, Japan b,f | Rhodophyta | Gigartinaceae | Chondrus yendoi | Kurohaginnansou | Jun-17 | 6.91 ± 0.21 |
| Hakodate, Japan b,f | Rhodophyta | Gigartinaceae | Mazzaella japonica | Akabaginnansou | Jun-17 | 14.25 ± 0.30 |
| Jepara, Indonesia a,e | Rhodophyta | Gracilariaceae | Gracilariopsis longissima | agar-agar | May-17 | 8.86 ± 0.15 |
| Hakodate, Japan b,f | Rhodophyta | Rhodomelaceae | Chondria crassicaulis | Yuna | Jun-17 | 49.77 ± 2.40 |
| Seaweeds | Chl a | Phy a | Chl b | Phy b | Chl c (c1 + c2) b | Total Chlorophylls |
|---|---|---|---|---|---|---|
| Caulerpa lentillifera | n.d. | 118.68 ± 44.41 | 16.83 ± 4.60 | 90.28 ± 8.97 | n.d. | 225.79 |
| Ulva australis | 81.90 ± 4.94 | 26.17 ± 3.27 | 64.45 ± 9.36 | n.d. | n.d. | 172.52 |
| Ulva intestinalis | 115.57 ± 51.28 | 165.98 ± 14.70 | 101.50 ± 29.21 | 64.38 ± 11.30 | n.d. | 447.43 |
| Ulva reticulata | n.d. | 115.85 ± 21.17 | 18.04 ± 0.61 | 267.69 ± 158.86 | n.d. | 401.58 |
| Costaria costata | 16.28 ± 3.80 | 291.92 ± 67.84 | n.d. | n.d. | 21.78 ± 0.84 | 329.97 |
| Undaria pinnatifida | 54.67 ± 14.60 | 423.42 ± 57.72 | n.d. | n.d. | 38.58 ± 5.03 | 518.67 |
| Saccharina japonica | 26.13 ± 16.79 | 425.42 ± 19.17 | n.d. | n.d. | 17.11 ± 2.91 | 469.24 |
| Sargassum aquifoliuma | 12.17 ± 4.02 | 149.64 ± 56.00 | n.d. | n.d. | 2.12 ± 0.21 | 163.93 |
| Sargassum fusiforme | 210.72 ± 43.63 | 107.59 ± 30.30 | n.d. | n.d. | 18.20 ± 0.34 | 336.51 |
| Sargassum horneri | 268.82 ± 59.93 | 220.86 ± 22.84 | n.d. | n.d. | 23.29 ± 4.9 | 512.97 |
| Gloiopeltis furcata | 2.98 ± 1.96 | 28.21 ± 4.92 | n.d. | n.d. | n.d. | 31.19 |
| Chondrus yendoi | 4.77 ± 2.10 | 29.29 ± 2.21 | n.d. | n.d. | n.d. | 34.06 |
| Mazzaella japonica | 6.70 ± 1.28 | 16.25 ± 1.85 | n.d. | n.d. | 7.63 ± 1.25 | 30.58 |
| Gracilariopsis longissima | n.d. | 14.66 ± 2.24 | n.d. | n.d. | n.d. | 14.66 |
| Chondria crassicaulis | 1.74 ± 1.19 | 322.72 ± 56.48 | n.d. | n.d. | 10.18 ± 1.29 | 334.64 |
| Seaweeds | β-Car | α-Car | Zx | Lut | Vx | Nx | Fx | Total Carotenoids |
|---|---|---|---|---|---|---|---|---|
| Caulerpa lentillifera | 1.84 ± 0.70 | 17.15 ± 2.96 | n.d. | 1.02 ± 0.53 | n.d. | n.d. | n.d. | 20.01 |
| Ulva australis | 58.24 ± 6.52 | 63.01 ± 0.04 | n.d. | 124.82 ± 13.14 | 23.30 ± 0.74 | 185.56 ± 59.65 | n.d. | 454.93 |
| Ulva intestinalis | 39.91 ± 9.54 | 17.83 ± 6.66 | n.d. | 77.78 ± 18.14 | 13.15 ± 4.19 | 153.70 ± 84.20 | n.d. | 302.37 |
| Ulva reticulata | 3.89 ± 0.39 | 4.83 ± 0.35 | n.d. | 14.12 ± 2.85 | n.d. | 30.63 ± 7.84 | n.d. | 53.47 |
| Costaria costata | 7.31 ± 0.86 | n.d. | n.d. | n.d. | 1.45 ± 0.19 | n.d. | 97.60 ± 12.87 | 106.36 |
| Undaria pinnatifida | 25.58 ± 3.71 | n.d. | 12.03 ± 3.99 | n.d. | 3.51 ± 0.61 | n.d. | 169.48 ± 12.98 | 210.61 |
| Saccharina japonica | 18.10 ± 0.61 | n.d. | 3.04 ± 0.13 | n.d. | 1.47 ± 0.25 | n.d. | 154.71 ± 11.29 | 177.32 |
| Sargassum aquifoliuma | 12.51 ± 1.24 | n.d. | 2.45 ± 0.32 | n.d. | n.d. | n.d. | 108.44 ± 9.17 | 123.40 |
| Sargassum fusiforme | 44.70 ± 7.52 | n.d. | 7.06 ± 1.65 | n.d. | 12.15 ± 2.45 | n.d. | 140.93 ± 12.98 | 204.84 |
| Sargassum horneri | 42.70 ± 6.70 | n.d. | 29.21 ± 2.72 | n.d. | 4.74 ± 0.98 | n.d. | 216.50 ± 31.97 | 293.15 |
| Gloiopeltis furcata | 8.99 ± 2.53 | 3.73 ± 0.70 | 0.47 ± 0.30 | +(8.76 ± 2.31) c | n.d. | n.d. | 3.43 ± 0.48 | 25.38 |
| Chondrus yendoi | 2.07 ± 0.19 | 4.60 ± 0.47 | n.d. | 1.78 ± 0.81 | n.d. | n.d. | 2.57 ± 0.07 | 11.02 |
| Mazzaella japonica | 2.23 ± 0.18 | 1.95 ± 0.27 | n.d. | 0.62 ± 0.12 | n.d. | n.d. | 7.26 ± 0.42 | 12.06 |
| Gracilariopsis longissima | 1.12 ± 0.25 | n.d. | +(0.75 ± 0.16) b | n.d. | n.d. | n.d. | n.d. | 1.87 |
| Chondria crassicaulis | 13.78 ± 2.40 | 8.19 ± 0.82 | +(4.94 ± 1.66) b | n.d. | n.d. | n.d. | 67.76 ± 9.63 | 94.67 |
| Seaweeds | Σ C16 | Σ C18 | Σ C20 | Σ SFAs | Σ MUFAs | Σ PUFAs | Σ n-3 PUFAs | Σ n-6 PUFAs | Σ n-3 PUFAs b |
|---|---|---|---|---|---|---|---|---|---|
| Caulerpa lentillifera | 33.69 ± 0.64 | 13.57 ± 0.91 | 2.84 ± 0.53 | 29.80 ± 1.65 | 9.08 ± 2.75 | 13.06 ± 0.32 | 7.31 ± 0.41 | 5.75 ± 0.10 | 0.01 |
| Ulva australis | 24.37 ± 0.35 | 35.41 ± 0.44 | 4.23 ± 0.08 | 25.67 ± 0.47 | 3.45 ± 0.24 | 36.23 ± 0.82 | 29.00 ± 0.66 | 7.22 ± 0.22 | 6.83 |
| Ulva intestinalis | 23.26 ± 1.27 | 36.61 ± 0.87 | 7.72 ± 2.31 | 24.37 ± 1.21 | 4.24 ± 0.87 | 39.16 ± 1.54 | 29.03 ± 2.02 | 10.14 ± 0.65 | 9.84 |
| Ulva reticulata | 44.66 ± 0.38 | 23.96 ± 0.38 | 6.01 ± 0.63 | 43.01 ± 0.46 | 6.88 ± 0.42 | 25.75 ± 0.52 | 23.25 ± 0.36 | 2.50 ± 0.43 | 0.02 |
| Costaria costata | 23.88 ± 1.12 | 30.84 ± 1.13 | 22.00 ± 1.92 | 37.46 ± 2.02 | 15.92 ± 1.46 | 36.59 ± 2.62 | 15.38 ± 2.15 | 21.21 ± 0.79 | 0.53 |
| Undaria pinnatifida | 25.73 ± 1.22 | 38.31 ± 4.44 | 23.01 ± 0.39 | 31.12 ± 1.75 | 14.07 ± 0.90 | 48.19 ± 5.72 | 28.94 ± 5.71 | 19.25 ± 0.30 | 4.66 |
| Saccharina japonica | 16.79 ± 0.75 | 38.83 ± 0.79 | 26.93 ± 0.18 | 24.35 ± 1.18 | 15.76 ± 0.64 | 51.28 ± 0.61 | 31.24 ± 1.91 | 20.04 ± 1.57 | 2.26 |
| Sargassum aquifoliuma | 48.45 ± 2.70 | 21.83 ± 0.75 | 13.46 ± 0.30 | 46.80 ± 5.31 | 18.88 ± 1.40 | 22.90 ± 0.89 | 6.26 ± 0.37 | 16.64 ± 0.52 | 0.10 |
| Sargassum fusiforme | 24.80 ± 0.52 | 27.01 ± 0.43 | 30.38 ± 0.45 | 27.62 ± 0.58 | 11.95 ± 0.22 | 47.32 ± 1.08 | 30.22 ± 0.78 | 17.11 ± 0.27 | 2.66 |
| Sargassum horneri | 26.35 ± 1.30 | 30.25 ± 0.96 | 30.49 ± 1.14 | 26.98 ± 1.49 | 14.24 ± 0.25 | 49.00 ± 2.36 | 26.97 ± 1.64 | 22.03 ± 1.16 | 4.03 |
| Gloiopeltis furcata | 23.34 ± 1.31 | 19.36 ± 0.11 | 44.89 ± 2.24 | 26.97 ± 1.45 | 18.05 ± 0.23 | 45.33 ± 2.36 | 38.11 ± 2.12 | 7.22 ± 0.24 | 0.46 |
| Chondrus yendoi | 33.12 ± 0.48 | 12.60 ± 0.23 | 42.35 ± 0.89 | 37.28 ± 0.62 | 9.89 ± 0.19 | 43.91 ± 0.85 | 24.23 ± 0.81 | 19.68 ± 0.21 | 0.68 |
| Mazzaella japonica | 40.50 ± 0.38 | 16.42 ± 0.05 | 30.12 ± 0.29 | 45.29 ± 0.51 | 14.49 ± 0.61 | 31.21 ± 0.72 | 20.05 ± 0.50 | 11.15 ± 0.24 | 0.10 |
| Gracilariopsis longissima | 46.44 ± 0.91 | 10.16 ± 0.50 | 15.21 ± 0.69 | 47.78 ± 2.36 | 11.18 ± 1.73 | 13.90 ± 0.64 | 1.63 ± 0.26 | 12.28 ± 0.71 | 0.01 |
| Chondria crassicaulis | 38.41 ± 1.54 | 19.93 ± 1.20 | 18.50 ± 0.69 | 36.88 ± 1.23 | 20.49 ± 0.88 | 28.15 ± 1.37 | 19.82 ± 0.71 | 8.33 ± 0.82 | 2.73 |
| Seaweeds | n-6/n-3 PUFAs | AI | TI | h/H | UI |
|---|---|---|---|---|---|
| Caulerpa lentillifera | 0.79 ± 0.05 | 1.53 ± 0.28 | 0.96 ± 0.13 | 0.44 ± 0.04 | 3.00 ± 0.07 |
| Ulva australis | 0.25 ± 0.01 | 0.64 ± 0.01 | 0.26 ± 0.01 | 1.04 ± 0.02 | 3.27 ± 0.01 |
| Ulva intestinalis | 0.35 ± 0.05 | 0.57 ± 0.04 | 0.23 ± 0.01 | 1.39 ± 0.13 | 3.19 ± 0.03 |
| Ulva reticulata | 0.11 ± 0.02 | 1.31 ± 0.05 | 0.35 ± 0.27 | 0.33 ± 0.02 | 3.67 ± 0.01 |
| Costaria costata | 1.40 ± 0.18 | 1.37 ± 0.16 | 0.54 ± 0.08 | 1.28 ± 0.12 | 3.63 ± 0.06 |
| Undaria pinnatifida | 0.68 ± 0.13 | 0.69 ± 0.08 | 0.28 ± 0.06 | 1.66 ± 0.23 | 3.71 ± 0.04 |
| Saccharina japonica | 0.65 ± 0.09 | 0.67 ± 0.04 | 0.20 ± 0.02 | 2.23 ± 0.08 | 3.88 ± 0.04 |
| Sargassum aquifoliuma | 2.66 ± 0.07 | 1.42 ± 0.25 | 1.23 ± 0.20 | 0.67 ± 0.07 | 3.40 ± 0.01 |
| Sargassum fusiforme | 0.57 ± 0.01 | 0.62 ± 0.02 | 0.24 ± 0.01 | 1.76 ± 0.06 | 3.93 ± 0.00 |
| Sargassum horneri | 0.82 ± 0.05 | 0.53 ± 0.05 | 0.26 ± 0.03 | 1.82 ± 0.16 | 3.86 ± 0.02 |
| Gloiopeltis furcata | 0.19 ± 0.00 | 0.50 ± 0.05 | 0.19 ± 0.02 | 2.46 ± 0.26 | 4.75 ± 0.01 |
| Chondrus yendoi | 0.81 ± 0.03 | 0.79 ± 0.03 | 0.41 ± 0.02 | 1.48 ± 0.05 | 4.46 ± 0.01 |
| Mazzaella japonica | 0.56 ± 0.01 | 1.14 ± 0.03 | 0.58 ± 0.03 | 0.97 ± 0.03 | 4.49 ± 0.00 |
| Gracilariopsis longissima | 7.69 ± 1.58 | 2.03 ± 0.24 | 2.80 ± 0.25 | 0.44 ± 0.03 | 3.87 ± 0.02 |
| Chondria crassicaulis | 0.42 ± 0.03 | 1.19 ± 0.09 | 0.47 ± 0.03 | 0.86 ± 0.05 | 4.13 ± 0.03 |
| Seaweeds | α-Toc |
|---|---|
| Caulerpa lentillifera | 0.87 ± 0.21 |
| Ulva australis | 0.44 ± 0.01 |
| Ulva intestinalis | 0.83 ± 0.06 |
| Ulva reticulata | 1.13 ± 0.08 |
| Costaria costata | 1.37 ± 0.10 |
| Undaria pinnatifida | 1.09 ± 0.05 |
| Saccharina japonica | 1.82 ± 0.02 |
| Sargassum aquifoliuma | 2.40 ± 0.02 |
| Sargassum fusiforme | 3.56 ± 0.08 |
| Sargassum horneri | 3.65 ± 0.031 |
| Gloiopeltis furcata | 2.71 ± 0.34 |
| Chondrus yendoi | 9.34 ± 0.19 |
| Mazzaella japonica | 1.72 ± 0.009 |
| Gracilariopsis longissima | 2.58 ± 0.015 |
| Chondria crassicaulis | 0.54 ± 0.009 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susanto, E.; Fahmi, A.S.; Hosokawa, M.; Miyashita, K. Variation in Lipid Components from 15 Species of Tropical and Temperate Seaweeds. Mar. Drugs 2019, 17, 630. https://doi.org/10.3390/md17110630
Susanto E, Fahmi AS, Hosokawa M, Miyashita K. Variation in Lipid Components from 15 Species of Tropical and Temperate Seaweeds. Marine Drugs. 2019; 17(11):630. https://doi.org/10.3390/md17110630
Chicago/Turabian StyleSusanto, Eko, A. Suhaeli Fahmi, Masashi Hosokawa, and Kazuo Miyashita. 2019. "Variation in Lipid Components from 15 Species of Tropical and Temperate Seaweeds" Marine Drugs 17, no. 11: 630. https://doi.org/10.3390/md17110630





