The Pharmacokinetics of Fucoidan after Topical Application to Rats
Abstract
:1. Introduction
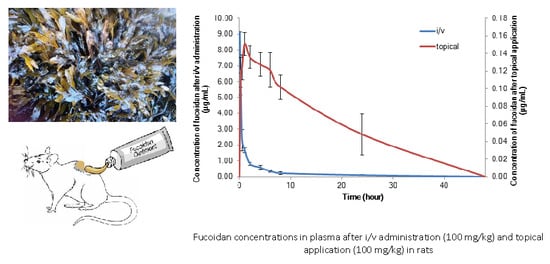
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Animals
3.3. Analysis of Fucoidan in Plasma and Tissue
3.4. Pharmacokinetic and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fitton, H.J.; Stringer, D.S.; Park, A.Y.; Karpiniec, S.N. Therapies from Fucoidan: New Developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuznetsova, T.A. Fucoidan extracted from Fucus evanescens brown algae corrects immunity and hemostasis disorders in experimental endotoxemia. Bull. Exp. Biol. Med. 2009, 147, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohwer, K.; Neupane, S.; Bittkau, K.S.; Galarza Pérez, M.; Dörschmann, P.; Roider, J.; Alban, S.; Klettner, A. Effects of Crude Fucus distichus Subspecies evanescens Fucoidan Extract on Retinal Pigment Epithelium Cells―Implications for Use in Age-Related Macular Degeneration. Mar. Drugs 2019, 17, 538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanina, N. Vaccine Adjuvants Derived from Marine Organisms. Biomolecules 2019, 9, 340. [Google Scholar] [CrossRef] [Green Version]
- Moon, H.J.; Lee, S.H.; Ku, M.J.; Yu, B.C.; Jeon, M.J.; Jeong, S.H.; Stonik, V.A.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.H. Fucoidan inhibits UVB-induced MMP-1 promoter expression and down regulation of type I procollagen synthesis in human skin fibroblasts. Eur. J. Dermatol. 2009, 19, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Moon, H.J.; Park, K.S.; Ku, M.J.; Lee, M.S.; Jeong, S.H.; Imbs, T.I.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.H. Effect of Costaria costata fucoidan on expression of matrix metalloproteinase-1 promoter, mRNA, and protein. J. Nat. Prod. 2009, 72, 1731–1734. [Google Scholar] [CrossRef]
- O’Leary, R.; Rerek, M.; Wood, E.J. Fucoidan modulates the effect of transforming growth factor (TGF)-β1 on fibroblast proliferation and wound repopulation in in vitro models of dermal wound repair. Biol. Pharm. Bull. 2004, 27, 266–270. [Google Scholar] [CrossRef] [Green Version]
- Fitton, J.; Dell’Acqua, G.; Gardiner, V.-A.; Karpiniec, S.; Stringer, D.; Davis, E. Topical benefits of two fucoidan-rich extracts from marine macroalgae. Cosmetics 2015, 2, 66–81. [Google Scholar] [CrossRef] [Green Version]
- Yang, J. Topical Application of Fucoidan Improves Atopic Dermatitis Symptoms in NC/Nga Mice. Phytother. Res. 2012, 26, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Cevher, E.; Hatipoğlu, F.; Oğurtan, Z.; Baş, A.L.; Akbuğa, J. The use of fucosphere in the treatment of dermal burns in rabbits. Eur. J. Pharm. Biopharm. 2008, 69, 189–198. [Google Scholar]
- Senni, K.; Gueniche, F.; Foucault-Bertaud, A.; Igondjo-Tchen, S.; Fioretti, F.; Colliec-Jouault, S.; Durand, P.; Guezennec, J.; Godeau, G.; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006, 445, 56–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwamoto, K.; Hiragun, T.; Takahagi, S.; Yanase, Y.; Morioke, S.; Mihara, S.; Kameyoshi, Y.; Hide, M. Fucoidan suppresses lgE production in peripheral blood mononuclear cells from patients with atopic dermatitis. Arch. Dermatol. Res. 2010, 303, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Tsubura, S.; Suzuki, A. Case report using 4% fucoidan cream for recurrent oral herpes labialis: Patient symptoms markedly improved in terms of time to healing and time to loss of discomfort. Dent. Open J. 2017, 4, 19–23. [Google Scholar] [CrossRef]
- Mourao, P.A.S.; Pereira, M.S. Searching for alternatives to heparin—Sulfated fucans from marine invertebrates. Trends Cardiovas. Med. 1999, 9, 225–232. [Google Scholar] [CrossRef]
- Shanmugam, M.; Mody, K.H. Heparinoid-active sulphated polysaccharides from marine algae as potential blood anticoagulant agents. Curr. Sci. India 2000, 79, 1672–1683. [Google Scholar]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, Q.B.; Zhang, Z.S.; Song, H.F.; Li, P.C. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef]
- Jin, W.H.; Zhang, Q.B.; Wang, J.; Zhang, W.J. A comparative study of the anticoagulant activities of eleven fucoidans. Carbohydr. Polym. 2013, 91, 1–6. [Google Scholar] [CrossRef]
- Lapikova, E.S.; Drozd, N.N.; Tolstenkov, A.S.; Makarov, V.A.; Zvyagintseva, T.N.; Shevchenko, N.M.; Bakunina, I.U.; Besednova, N.N.; Kuznetsova, T.A. Inhibition of thrombin and factor Xa by Fucus evanescens fucoidan and its modified analogs. Bull. Exp. Biol. Med. 2008, 146, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Ushakova, N.A.; Zyuzina, K.A.; Bilan, M.I.; Elizarova, A.L.; Somonova, O.V.; Madzhuga, A.V.; Krylov, V.B.; Preobrazhenskaya, M.E.; Usov, A.I.; et al. Influence of fucoidans on hemostatic system. Mar. Drugs 2013, 11, 2444–2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obluchinsksya, E.D.; Makarova, M.N.; Pozharitskaya, O.N.; Shikov, A.N. Effects of ultrasound treatment on the chemical composition and anticoagulant properties of dry fucus extract. Pharm. Chem. J. 2015, 49, 183–186. [Google Scholar] [CrossRef]
- Irhimeh, M.R.; Fitton, J.H.; Lowenthal, R.M.; Kongtawelert, P. A quantitative method to detect fucoidan in human plasma using a novel antibody. Methods Find. Exp. Clin. Pharmacol. 2005, 27, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Tokita, Y.; Nakajima, K.; Mochida, H.; Iha, M.; Nagamine, T. Development of a fucoidan-specific antibody and measurement of fucoidan in serum and urine by sandwich ELISA. Biosci. Biotechnol. Biochem. 2010, 74, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Pozharitskaya, O.N.; Shikov, A.N.; Faustova, N.M.; Obluchinskaya, E.D.; Kosman, V.M.; Vuorela, H.; Makarov, V.G. Pharmacokinetic and Tissue Distribution of Fucoidan from Fucus vesiculosus after Oral Administration to Rats. Mar. Drugs 2018, 16, 132. [Google Scholar] [CrossRef] [Green Version]
- Lau, W.M.; White, A.W.; Gallagher, S.J.; Donaldson, M.; McNaughton, G.; Heard, C.M. Scope and limitations of the co-drug approach to topical drug delivery. Curr. Pharm. Des. 2008, 14, 794–802. [Google Scholar] [CrossRef]
- Lauterbach, A.; Müller-Goymann, C.C. Applications and limitations of lipid nanoparticles in dermal and transdermal drug delivery via the follicular route. Eur. J. Pharm. Biopharm. 2015, 97, 152–163. [Google Scholar] [CrossRef]
- Rerknimitr, P.; Otsuka, A.; Nakashima, C.; Kabashima, K. Skin Barrier Function and Atopic Dermatitis. Curr. Dermatol. Rep. 2018, 7, 209–220. [Google Scholar] [CrossRef]
- Delgado-Charro, M.; Guy, R.H. Transdermal drug delivery. In Drug Delivery and Targeting for Pharmacists and Pharmaceutical Scientists; Hillery, A.M., Lloyd, A.W., Swarbrick, J., Eds.; Taylor & Francis: London, UK; New York, NY, USA, 2001; pp. 207–236. [Google Scholar]
- Yang, C.; Chung, D.; Shina, I.S.; Lee, H.; Kim, J.; Lee, Y. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008, 43, 433–437. [Google Scholar] [CrossRef]
- Osborne, D.W.; Musakhanian, J. Skin penetration and permeation properties of Transcutol®—neat or diluted mixtures. AAPS PharmSciTech 2018, 19, 3512–3533. [Google Scholar] [CrossRef] [PubMed]
- Athukorala, Y.; Jung, W.K.; Vasanthan, T.; Jeon, Y.J. An anticoagulative polysaccharide from an enzymatic hydrolysate of Ecklonia cava. Carbohydr. Polym. 2006, 66, 184–191. [Google Scholar] [CrossRef]
- Vickers, C.F.H. Reservoir effect of human skin: Pharmacological speculation. In Percutaneous Absorption of Steroids; Mauvais-Jarvis, P., Vickers, C.F.H., Wepierre, J., Eds.; Academic: London, UK, 1980; pp. 19–29. [Google Scholar]
- Barry, B.W. Dermatological Formulations: Percutaneous Absorption; Dekker: New York, NY, USA, 1983; pp. 95–126. [Google Scholar]
- Roberts, M.S.; Cross, S.E.; Anissimov, Y.G. Factors affecting the formation of a skin reservoir for topically applied solutes. Skin Pharmacol. Physiol. 2004, 17, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Hatanaka, T.; Sugibayashi, K.; Omiya, H. Prediction of skin permeability of drugs: Comparison of human and hairless rat skin. J. Pharm. Pharmacol. 1992, 44, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Van Ravenzwaay, B.; Leibold, E. A comparison between in vitro rat and human and in vivo rat skin absorption studies. Hum. Exp. Toxicol. 2004, 23, 421–430. [Google Scholar] [CrossRef] [PubMed]
- International Federation of Pharmaceutical Manufacturers & Associations (IFPMA). ICH, Q2A, Harmonized Tripartite Guideline, Text on Validation of Analytical Procedures. In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, 1–5 March 1994. [Google Scholar]
- International Federation of Pharmaceutical Manufacturers & Associations (IFPMA). ICH, Q2B, Harmonized Tripartite Guideline, Validation of Analytical Procedure: Methodology, IFPMA. In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, 1–8 March 1996. [Google Scholar]



| Parameter | Range |
|---|---|
| Accuracy, % | |
| ULOQ (1.13 μg/g) | 0.6–3.4 |
| Middle-quality control (0.56 μg/g) | 1.7–9.7 |
| Low-quality control (0.14 μg/g) | 9.2–14.8 |
| LLOQ (0.014 μg/g) | 2.9–12.3 |
| Intraday/Interday precision (RSD), % | |
| ULOQ (1.13 μg/g) | 0.9–1.9/3.5 |
| Middle-quality control (0.56 μg/g) | 3.4–6.1/7.6 |
| Low-quality control (0.14 μg/g) | 2.3–5.0/6.8 |
| LLOQ (0.014 μg/g) | 3.7–4.7/10.9 |
| LOD, μg/g | 0.0035 |
| Sample | Dose | Parameter | ||||
|---|---|---|---|---|---|---|
| (mg/kg) | AUC0–48 (µg·h/mL) | MRT (h) | T1/2 (h) | Cmax (μg/mL) | Tmax (h) | |
| Single dose | ||||||
| Plasma, i/v | 100 | 10.83 ± 0.32 | 8.08 ± 1.92 | 9.47 ± 2.34 | 9.15 ± 0.60 | - |
| Plasma, topical | 50 | 0.74 ± 0.11 | 20.99 ± 10.74 | 14.41 ± 7.52 | 0.12 ± 0.02 | 1.20 ± 0.44 |
| Plasma, topical | 100 | 1.92 ± 0.94 | 30.20 ± 13.70 | 20.75 ± 9.43 | 0.15 ± 0.01 | 1.00 ± 0.00 |
| Plasma, topical | 150 | 3.08 ± 0.21 | 29.94 ± 4.51 | 20.60 ± 3.07 | 0.18 ± 0.01 | 1.20 ± 0.01 |
| Skin*, topical | 100 | 0.94 ± 0.11 | 9.00 ± 4.36 | 6.28 ± 3.46 | 0.27 ± 0.01 | 0.25 ± 0.00 |
| Striated muscle*, dermal | 100 | 2.22 ± 1.18 | 16.15 ± 4.40 | 10.64 ± 9.94 | 0.31 ± 0.12 | 1.80 ± 1.30 |
| Repeated doses (5 days) | ||||||
| Plasma, topical | 100 | 2.10 ± 0.69 | 41.12 ± 10.70 | 28.06 ± 7.92 | 0.18 ± 0.05 | 1.10 ± 0.55 |
| Tissues | 15 min | 30 min | 1 h | 2 h | 4 h | 6 h | 8 h |
|---|---|---|---|---|---|---|---|
| Skin | 0.274 ± 0.013 | 0.231 ± 0.028 | 0.224 ± 0.029 | 0.138 ± 0.032 | 0.105 ± 0.010 | 0.092 ± 0.008 | 0.050 ± 0.046 |
| Muscle | 0.141 ± 0.033 | 0.162 ± 0.049 | 0.268 ± 0.156 | 0.238 ± 0.067 | 0.196 ± 0.053 | 0.150 ± 0.037 | 0.115 ± 0.043 |
| Plasma | 0.119 ± 0.017 | 0.124 ± 0.014 | 0.151 ± 0.013 | 0.136 ± 0.013 | 0.127 ± 0.012 | 0.121 ± 0.019 | 0.102 ± 0.014 |
| M-P ratio | 1.18 | 1.31 | 1.77 | 1.75 | 1.54 | 1.24 | 1.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozharitskaya, O.N.; Shikov, A.N.; Obluchinskaya, E.D.; Vuorela, H. The Pharmacokinetics of Fucoidan after Topical Application to Rats. Mar. Drugs 2019, 17, 687. https://doi.org/10.3390/md17120687
Pozharitskaya ON, Shikov AN, Obluchinskaya ED, Vuorela H. The Pharmacokinetics of Fucoidan after Topical Application to Rats. Marine Drugs. 2019; 17(12):687. https://doi.org/10.3390/md17120687
Chicago/Turabian StylePozharitskaya, Olga N., Alexander N. Shikov, Ekaterina D. Obluchinskaya, and Heikki Vuorela. 2019. "The Pharmacokinetics of Fucoidan after Topical Application to Rats" Marine Drugs 17, no. 12: 687. https://doi.org/10.3390/md17120687