Dual-Functioning Scaffolds for the Treatment of Spinal Cord Injury: Alginate Nanofibers Loaded with the Sigma 1 Receptor (S1R) Agonist RC-33 in Chitosan Films
Abstract
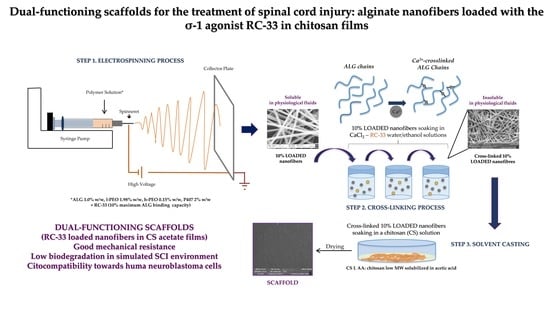
:1. Introduction
2. Results and Discussion
2.1. Synthesis of RC-33 and Assessment of ALG/RC-33 Interaction
2.2. Preparation of Nanofibers Loaded with RC-33 by Electrospinning
2.3. Preparation and Characterization of CS Films
3. Materials and Methods
3.1. Materials
3.2. RC-33 HCl Synthesis
3.3. Binding and Drug Release Study by Dialysis Equilibrium
3.4. Preparation of the Polymer Solutions for Electrospinning
3.4.1. Blank ALG/PEOs Solution
3.4.2. RC-33 Loaded ALG/PEOs Solutions
3.5. Characterization of the Polymer Solutions for Electrospinning
3.5.1. Rheological Analysis
3.5.2. Dynamic Surface Tension Measurements
3.5.3. Conductivity Measurements
3.6. Fiber Preparation and Morphological Characterization
3.7. Fiber Loading Capacity
3.8. Assessment of Fiber Mechanical Properties
3.9. Fiber Cross-Linking and Morphological Characterization
3.10. Film Preparation
3.11. Dual-Functioning Scaffold Preparation
3.12. Film and Dual-Functioning Scaffold Characterization
3.12.1. Mechanical Properties
3.12.2. Hydration Properties
3.12.3. Wettability
3.12.4. Biodegradation
3.12.5. In Vitro Cell Biocompatibility Assay
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Silva, N.A.; Sousa, N.; Reis, R.L.; Salgado, A.J. From basics to clinical: A comprehensive review on spinal cord injury. Prog. Neurobiol. 2014, 114, 25–57. [Google Scholar] [CrossRef]
- WHO—World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury (accessed on 6 November 2019).
- Oyinbo, C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta. Neurobiol. Exp. 2011, 71, 281–299. [Google Scholar]
- Kabu, S.; Gao, Y.; Kwon, B.K.; Labhasetwar, V. Drug delivery, cell-based therapies, and tissue engineering approaches for spinal cord injury. J. Control. Release 2015, 219, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, C.S.; Fehlings, M. Concise review: Bridging the gap: Novel neuroregenerative and neuroprotective strategies inspinal cord injury. Stem Cells Transl. Med. 2016, 5, 914–924. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.; Lucke-Wold, B.P.; Mookerjee, S.A.; Cavendish, J.Z.; Robson, M.J.; Scandinaro, A.L.; Matsumoto, R.R. Role of sigma-1 receptors in neurodegenerative diseases. J. Pharmacol. Sci. 2015, 127, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.W.; Lin, T.Y.; Wang, C.C.; Wang, S.J. σ-1 Receptor agonist SKF10047 inhibits glutamate release in rat cerebral cortex nerve endings. J. Pharmacol. Exp. Ther. 2012, 341, 532–542. [Google Scholar] [CrossRef] [Green Version]
- Hirata, Y.; Yamamoto, H.; Atta, M.S.; Mahmoud, S.; Oh-hashi, K.; Kiuchi, K. Chloroquine inhibits glutamate-induced death of a neuronal cell line by reducing reactive oxygen species through sigma-1 receptor. J. Neurochem. 2011, 119, 839–847. [Google Scholar] [CrossRef]
- Su, T.P.; Su, T.C.; Nakamura, Y.; Tsai, S.Y. The Sigma-1 Receptor as a Pluripotent Modulator in Living Systems. Trends Pharmacol. Sci. 2016, 37, 262–278. [Google Scholar] [CrossRef] [Green Version]
- Collina, S.; Rui, M.; Stotani, S.; Bignardi, E.; Rossi, D.; Curti, D.; Giordanetto, F.; Malacrida, A.; Scuteri, A.; Cavaletti, G. Are sigma receptor modulators a weapon against multiple sclerosis disease? Future Med. Chem. 2017, 9, 2029–2051. [Google Scholar] [CrossRef]
- Rui, M.; Rossino, G.; Rossi, D.; Collina, S. Sigma receptors as new target for multiple sclerosis. In Emerging Drugs and Targets for Multiple Sclerosis, 1st ed.; Martinez, A., Ed.; RSC: Croydon, UK, 2019; Volume 70, pp. 264–284. [Google Scholar]
- Rossi, D.; Urbano, M.; Pedrali, A.; Serra, M.; Zampieri, D.; Mamolo, M.G.; Laggner, C.; Zanette, C.; Florio, C.; Schepmann, B.; et al. Design, synthesis and SAR analysis of novel selective σ1 ligands (Part2). Bioorg. Med. Chem. 2010, 18, 1204–1212. [Google Scholar] [CrossRef]
- Rossi, D.; Marra, A.; Picconi, P.; Serra, M.; Catenacci, L.; Sorrenti, M.; Laurini, E.; Fermeglia, M.; Pricl, S.; Brambilla, S.; et al. Identification of RC-33 as a potent and selective σ1 receptor agonist potentiating NGF-induced neurite outgrowth in PC12 cells. Part 2: G-scale synthesis, physicochemical characterization and in vitro metabolic stability. Bioorg. Med. Chem. 2013, 21, 2577–2586. [Google Scholar] [CrossRef]
- Rossi, D.; Pedrali, A.; Gaggeri, R.; Marra, A.; Pignataro, L.; Laurini, E.; Dal Col, V.; Fermeglia, M.; Pricl, S.; Schepmann, D.; et al. Chemical, pharmacological, and in vitro metabolic stability studies on enantiomerically pure RC-33 compounds: Promising neuroprotective agents acting as σ₁ receptor agonists. Chem. Med. Chem. 2013, 8, 1514–1527. [Google Scholar] [CrossRef]
- Rossi, D.; Pedrali, A.; Marra, A.; Pignataro, L.; Schepmann, D.; Wünsch, B.; Ye, L.; Leuner, K.; Peviani, M.; Curti, D.; et al. Studies on the enantiomers of RC-33 as neuroprotective agents: Isolation, configurational assignment, and preliminary biological profile. Chirality 2013, 25, 814–822. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Muzzarelli, R.A.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Rossi, S.; Ferrari, F.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M. Wound healing: Hemoderivatives and biopolymers. In Concise Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; Mishra, M.K., Ed.; CRC Press: Boca Raton, FL, USA, 2017; Volume 2, pp. 1642–1660. [Google Scholar]
- Rossi, S.; Vigani, B.; Puccio, A.; Bonferoni, M.C.; Sandri, G.; Ferrari, F. Chitosan ascorbate nanoparticles for the vaginal delivery of antibiotic drugs in atrophic vaginitis. Mar. Drugs 2017, 15, 319. [Google Scholar] [CrossRef] [Green Version]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Hyaluronic acid and chitosan-based nanosystems: A new dressing generation for wound care. Expert Opin. Drug Deliv. 2019, 16, 715–740. [Google Scholar] [CrossRef]
- Puccio, A.; Ferrari, F.; Rossi, S.; Bonferoni, M.C.; Sandri, G.; Dacarro, C.; Grisoli, P.; Caramella, C. Comparison of functional and biological properties of chitosan and hyaluronic acid, to be used for the treatment of mucositis in cancer patients. J. Drug Del. Sci. Tech. 2011, 21, 241–247. [Google Scholar] [CrossRef]
- Xu, W.; Chi, L.; Xu, R.; Ke, Y.; Luo, C.; Cai, J.; Qiu, M.; Gozal, D.; Liu, R. Increased production of reactive oxygen species contributes to motor neuron death in a compression mouse model of spinal cord injury. Spinal Cord 2005, 43, 204–213. [Google Scholar] [CrossRef]
- Jia, Z.; Zhu, H.; Li, J.; Wang, X.; Misra, H.; Li, Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord 2012, 50, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Tator, C.H.; Shoichet, M.S. Chitosan implants in the rat spinal cord: Biocompatibility and biodegradation. J. Biomed. Mater. Res. A 2011, 97, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Z.; Zhang, A.; Wang, T.; Chen, W. Repair of thoracic spinal cord injury by chitosan tube implantation in adult rats. Biomaterials 2009, 30, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Z.; Yang, Y. Morphological and electrophysiological evidence for regeneration of transected spinal cord fibers and restoration of motor functions in adult rats. Chin. Sci. Bull. 2006, 51, 918–926. [Google Scholar] [CrossRef]
- Bozkurt, G.; Mothe, A.J.; Zahir, T.; Kim, H.; Shoichet, M.S.; Tator, C.H. Chitosan channels containing spinal cord-derived stem/progenitor cells for repair of subacute spinal cord injury in the rat. Neurosurgery 2010, 67, 1733–1744. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lu, X.; Feng, G.; Gu, Z.; Sun, Y.; Bao, G.; Xu, G.; Lu, Y.; Chen, J.; Xu, L.; et al. Chitosan scaffolds induce human dental pulp stem cells to neural differentiation: Potential roles for spinal cord injury therapy. Cell Tissue Res. 2016, 366, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.K. Neuroprotective properties of chitosan and its derivatives. Mar. Drugs 2010, 8, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Chedly, J.; Soares, S.; Montembault, A.; von Boxberg, Y.; Veron-Ravaille, M.; Mouffle, C.; Benassy, M.N.; Taxi, J.; David, L.; Nothias, F. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials 2017, 138, 91–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawrotek, K.; Marqueste, T.; Modrzejewska, Z.; Zarzycki, R.; Rusak, A.; Decherchi, P. Thermogelling chitosan lactate hydrogel improves functional recovery after a C2 spinal cord hemisection in rat. J. Biomed. Mater. Res. A 2017, 105, 2004–2019. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, X.; Li, Y.; Jin, Q.; Tang, W.; Chen, Q.; Aili, D.; Qian, H. Application of stem cells and chitosan in the repair of spinal cord injury. Int. J. Dev. Neurosci. 2019, 76, 80–85. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of alginate microspheres in therapeutics delivery and cell culture: Past, present and future. Int. J. Pharm. 2019, 569, 118627. [Google Scholar] [CrossRef] [PubMed]
- Prang, P.; Müller, R.; Eljaouhari, A.; Heckmann, K.; Kunz, W.; Weber, T.; Faber, C.; Vroemen, M.; Bogdahn, U.; Weidner, N. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials 2006, 27, 3560–3569. [Google Scholar] [CrossRef] [PubMed]
- Pawar, K.; Mueller, R.; Caioni, M.; Prang, P.; Bogdahn, U.; Kunz, W.; Weidner, N. Increasing capillary diameter and the incorporation of gelatin enhance axon outgrowth in alginate-based anisotropic hydrogels. Acta Biomater. 2011, 7, 2826–2834. [Google Scholar] [CrossRef] [PubMed]
- Pawar, K.; Prang, P.; Müller, R.; Caioni, M.; Bogdahn, U.; Kunz, W.; Weidner, N. Intrinsic and extrinsic determinants of central nervous system axon outgrowth into alginate-based anisotropic hydrogels. Acta Biomater. 2015, 27, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Günther, M.I.; Weidner, N.; Müller, R.; Blesch, A. Cell-seeded alginate hydrogel scaffolds promote directed linear axonal regeneration in the injured rat spinal cord. Acta Biomater. 2015, 27, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Raspa, A.; Pugliese, R.; Maleki, M.; Gelain, F. Recent therapeutic approaches for spinal cord injury. Biotechnol. Bioeng. 2016, 113, 253–259. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Ferrari, F. Design and criteria of electrospun fibrous scaffolds for the treatment of spinal cord injury. Neural Regen. Res. 2017, 12, 1786–1790. [Google Scholar]
- Faccendini, A.; Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Nanofiber Scaffolds as Drug Delivery Systems to Bridge Spinal Cord Injury. Pharmaceuticals 2017, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Colello, R.J.; Chow, W.N.; Bigbee, J.W.; Lin, C.; Dalton, D.; Brown, D.; Jha, B.S.; Mathern, B.E.; Lee, K.D.; Simpson, D.G. The incorporation of growth factor and chondroitinase ABC into an electrospun scaffold to promote axon regrowth following spinal cord injury. J. Tissue Eng. Regen. Med. 2016, 10, 656–668. [Google Scholar] [CrossRef]
- Cao, H.; Liu, T.; Chew, S.Y. The application of nanofibrous scaffolds in neural tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1055–1064. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Milanesi, G.; Bonferoni, M.C.; Sandri, G.; Bruni, G.; Ferrari, F. Electrospun Alginate Fibers: Mixing of Two Different Poly(ethylene oxide) Grades to Improve Fiber Functional Properties. Nanomaterials 2018, 12, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Milanesi, G.; Bruni, G.; Ferrari, F. Coated electrospun alginate-containing fibers as novel delivery systems for regenerative purposes. Int. J. Nanomed. 2018, 13, 6531–6550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Shao, H.; Hu, X. Morphology and structure of electrospun mats from regenerated silk fibroin aqueous solutions with adjusting pH. Int. J. Biol. Macromol. 2007, 41, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.M.; Zhang, Y.Z.; Ramakrishna, S.; Lim, C.T. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer 2004, 45, 5361–5368. [Google Scholar] [CrossRef]
- Rogina, A. Electrospinning process: Versatile preparation method for biodegradable and natural polymers and biocomposite systems applied in tissue engineering and drug delivery. Appl. Surf. Sci. 2014, 296, 221–230. [Google Scholar] [CrossRef]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.J. Electrospun nanofibers: New generation materials for advanced applications. Mater. Sci. Eng. B 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Rossi, S.; Vigani, B.; Bonferoni, M.C.; Sandri, G.; Caramella, C.; Ferrari, F. Rheological analysis and mucoadhesion: A 30 year-old and still active combination. J. Pharm. Biomed. Anal. 2018, 156, 232–238. [Google Scholar] [CrossRef]
- Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Caramella, C. Characterization of chitosan hydrochloride-mucin interaction by means of viscosimetric and turbidimetric measurements. Eur. J. Pharm. Sci. 2000, 10, 251–257. [Google Scholar] [CrossRef]
- Gadgey, K.K.; Sharma, G.S. Investigation of mechanical properties of chitosan based films: A review. Int. J. Adv. Res. Eng. Technol. 2018, 8, 93–102. [Google Scholar]
- Quetzeri-Santiago, M.A.; Castrejón-Pita, A.A.; Castreon-Pita, R. The Effect of Surface Roughness on the Contact Line and Splashing Dynamics of Impacting Droplets. Sci. Rep. 2019, 9, 15030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junfang, W.; Lipinski, M.M. Autophagy in Neurotrauma: Good, Bad, or Dysregulated. Cells 2019, 8, 693. [Google Scholar]
- Abraham, J.; Balasubramanian, A.S.; Theodore, D.R.; Nagarajan, S.; Apte, C.A.; Chandi, S. Spinal cord edema, 5-hydroxytriptamine, lipid peroxidation, and lysosomal enzyme release after acute contusion and compression injury in primates. Cent. Nerv. Trauma 1985, 2, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.A.; Chen, F.J.; Cui, H.L.; Lin, T.; Guo, N.; Wu, H.G. Efficacy of chitosan and sodium alginate scaffolds for repair of spinal cord injury in rats. Neural Regen. Res. 2018, 13, 502–509. [Google Scholar] [PubMed]
- Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Bettinetti, G.P.; Caramella, C. Characterization of a diltiazem-lambda carrageenan complex. Int. J. Pharm. 2000, 200, 207–216. [Google Scholar] [CrossRef]
- Rezvanian, M.; Ahmad, N.; Mohd Amin, M.C.; Ng, S.F. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef]














| CS | Acid | Glycerol | |
|---|---|---|---|
| CSL AA | 2 | 1 | 1 |
| CSL GA | 1 | 0.7 | 0.7 |
| CSM AA | 2 | 1 | 1 |
| CSM GA | 1.4 | 0.7 | 0.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Rui, M.; Collina, S.; Fagiani, F.; Lanni, C.; Ferrari, F. Dual-Functioning Scaffolds for the Treatment of Spinal Cord Injury: Alginate Nanofibers Loaded with the Sigma 1 Receptor (S1R) Agonist RC-33 in Chitosan Films. Mar. Drugs 2020, 18, 21. https://doi.org/10.3390/md18010021
Vigani B, Rossi S, Sandri G, Bonferoni MC, Rui M, Collina S, Fagiani F, Lanni C, Ferrari F. Dual-Functioning Scaffolds for the Treatment of Spinal Cord Injury: Alginate Nanofibers Loaded with the Sigma 1 Receptor (S1R) Agonist RC-33 in Chitosan Films. Marine Drugs. 2020; 18(1):21. https://doi.org/10.3390/md18010021
Chicago/Turabian StyleVigani, Barbara, Silvia Rossi, Giuseppina Sandri, Maria Cristina Bonferoni, Marta Rui, Simona Collina, Francesca Fagiani, Cristina Lanni, and Franca Ferrari. 2020. "Dual-Functioning Scaffolds for the Treatment of Spinal Cord Injury: Alginate Nanofibers Loaded with the Sigma 1 Receptor (S1R) Agonist RC-33 in Chitosan Films" Marine Drugs 18, no. 1: 21. https://doi.org/10.3390/md18010021