Immunostimulatory Effects of Polysaccharides from Spirulina platensis In Vivo and Vitro and Their Activation Mechanism on RAW246.7 Macrophages
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of PSP on Spleen and Thymus Indices in Cy-Treated Mice
2.2. Effect of PSP on Hemopoietic Function in Cy-Treated Mice
2.3. Effect of PSP on Cytokine Levels in Sera from Cy-Treated Mice
2.4. Effect of PSPs on Cell Proliferation
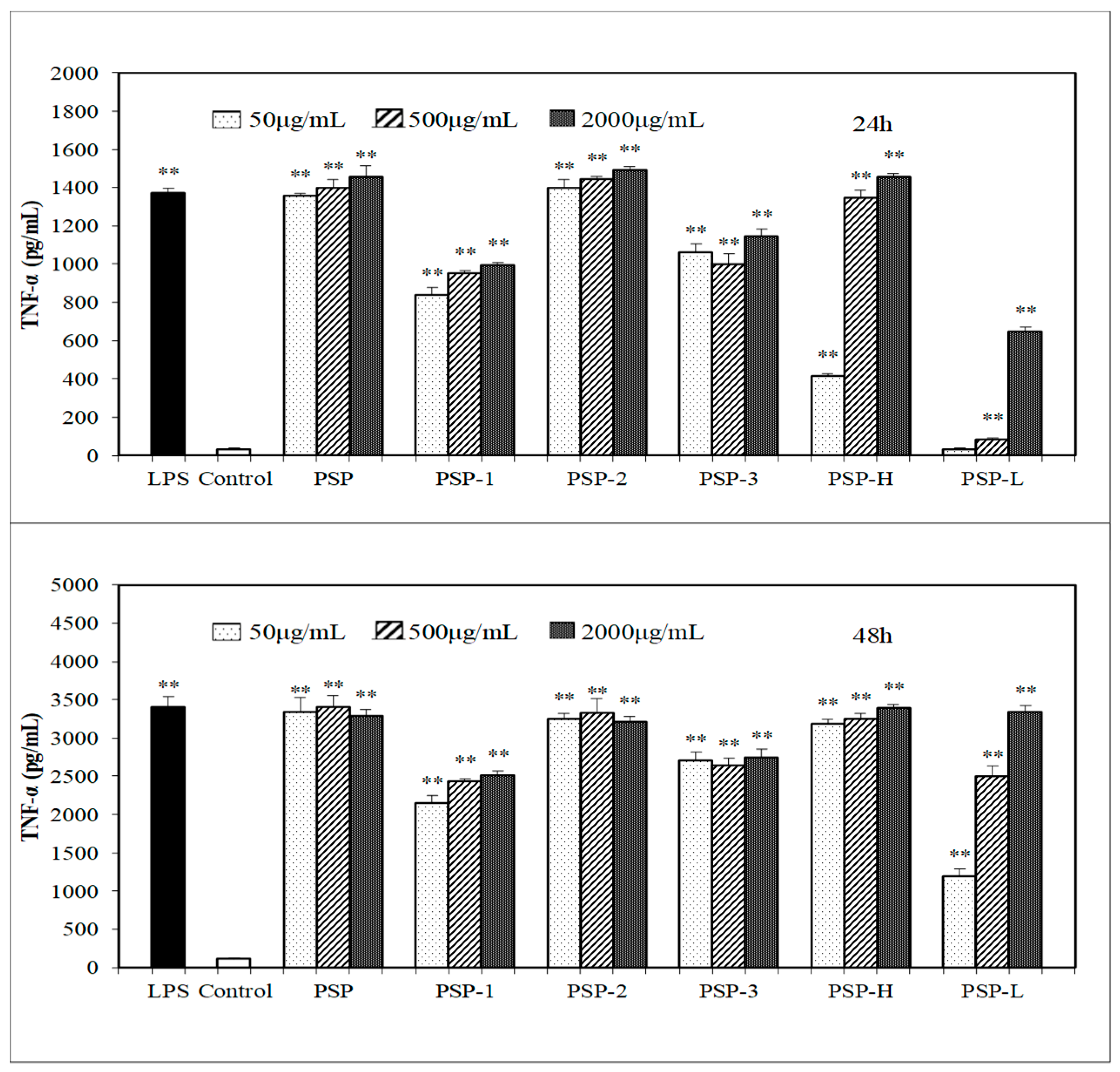
2.5. Effect of PSPs on the Production of NO, IL-6, and TNF-α by RAW 264.7 Cells
2.6. Effects of PSPs on Phagocytic Activity of RAW264.7 Cells
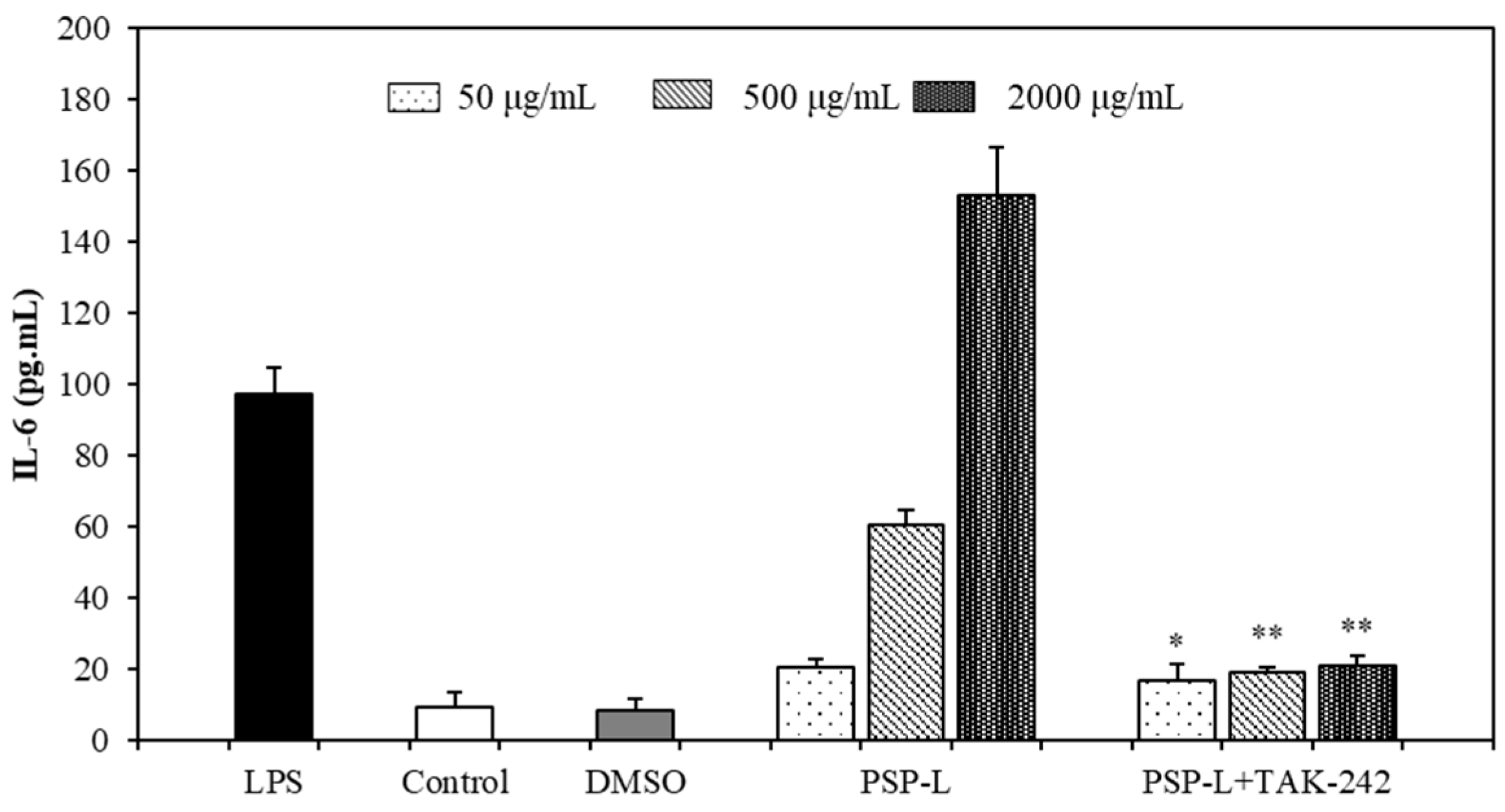
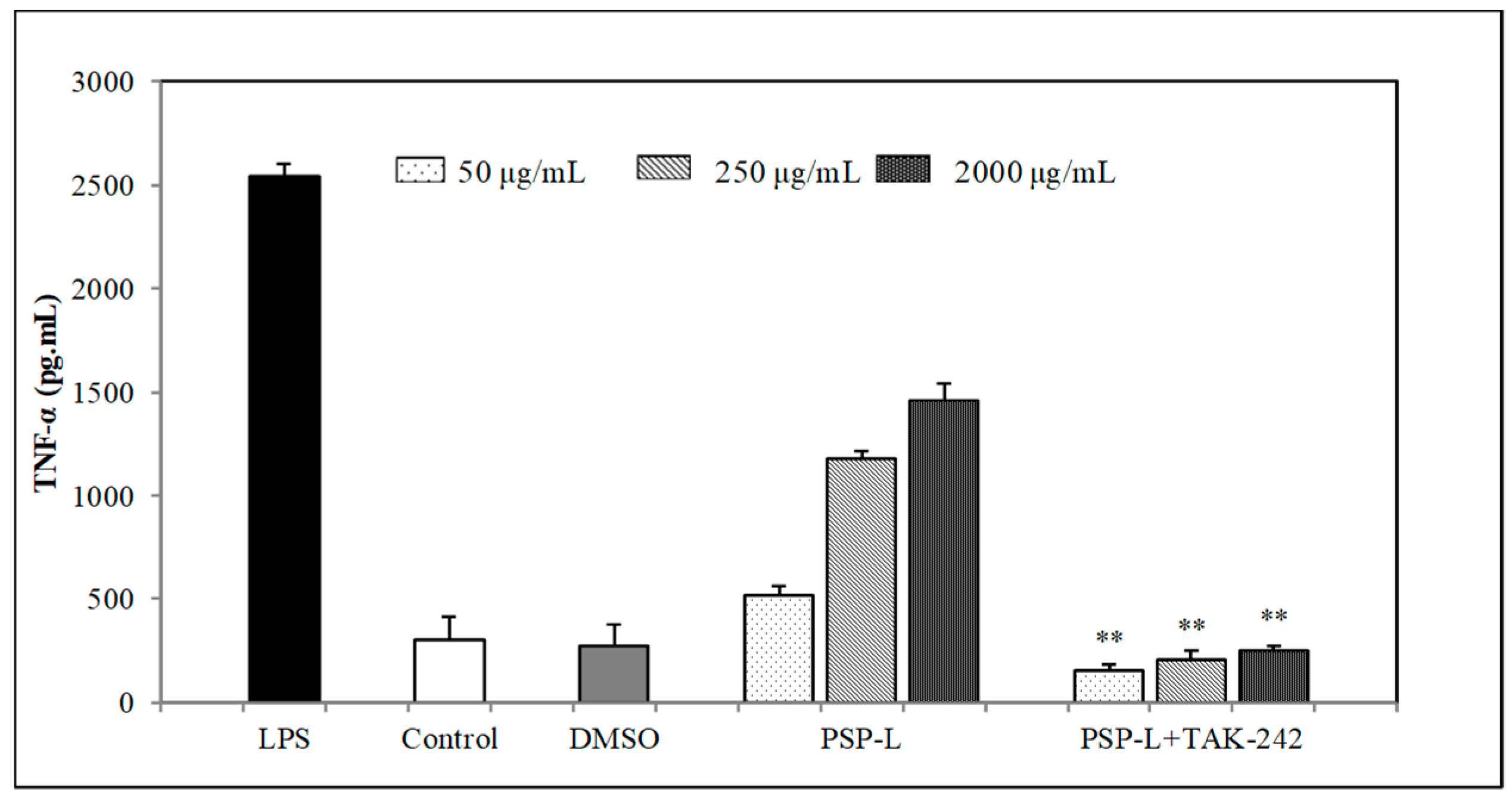
2.7. Effects of TLR4 Inhibitor on the Cytokines Secretion by RAW264.7 Cells
2.8. Western Blot Analysis
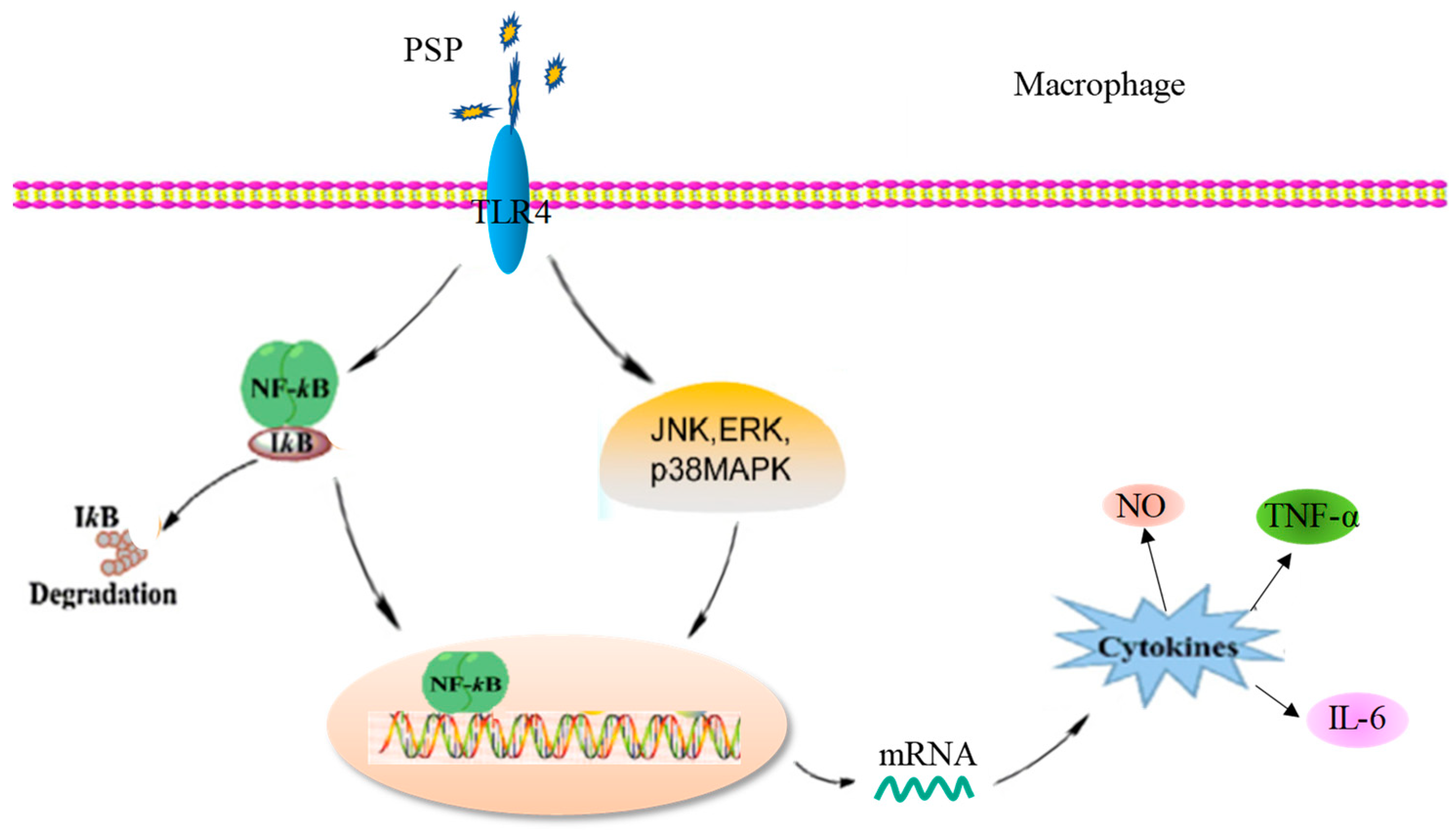
2.9. Effects of PSP-L on the Activation of NF-κB Signaling Pathway
3. Discussion
4. Materials and methods
4.1. Reagent
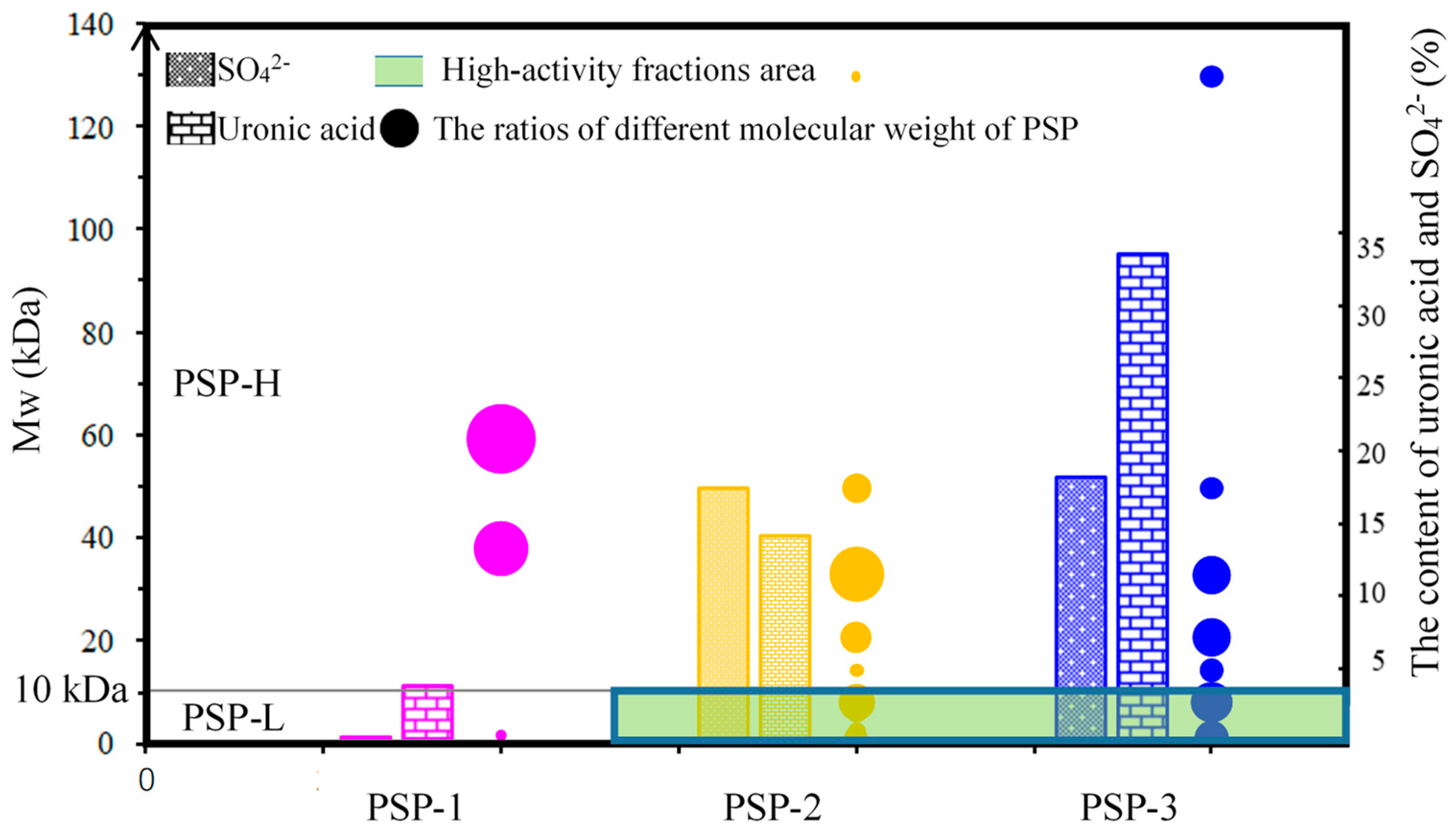
4.2. Preparation and Quantitative Analysis of PSP
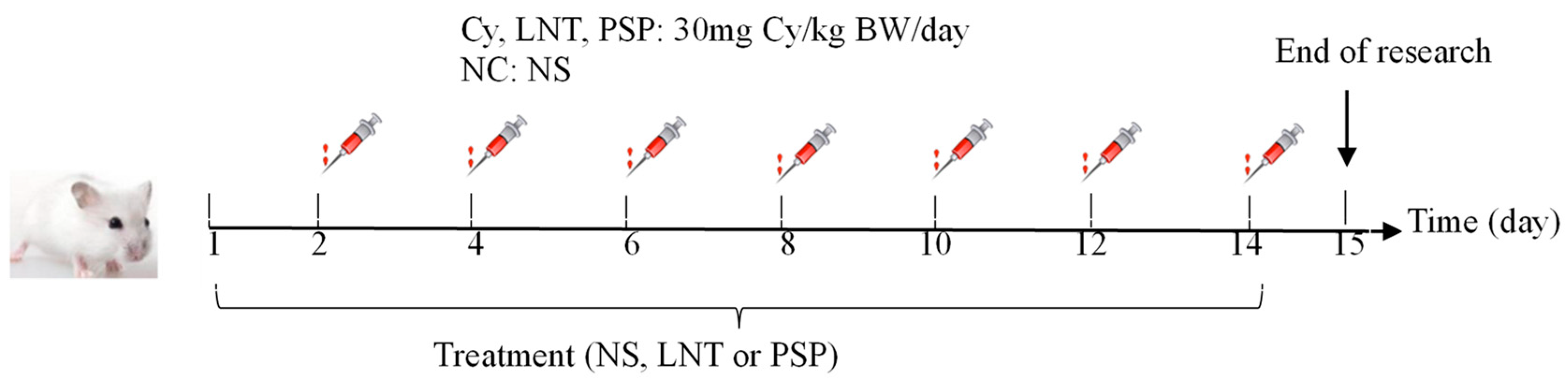
4.3. Animals and Experimental Design
4.4. Calculation of Splenic and Thymic Indices
4.5. Peripheral White Blood Cell and Peripheral Blood Lymphocytes Cells Counts
4.6. Determination of IL-10, INF-γ, and TNF-α in Serum
4.7. Cell Culture
4.8. Assay for Cell Viability
4.9. Spleen Lymphocyte Proliferation Assay
4.10. Measurement of NO, TNF-α, IL-6 Cytokine Production
4.11. Determination of Pinocytic Capability of RAW264.7 Cells
4.12. Investigation of Membrane Receptors
4.13. Western Blot Analysis
4.14. Immunofluorescence Staining Analysis of Nuclear Translocation of NF-κB
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgia, J.P. Role of hepatic cytochrome p450s in the pharmacokinetics and toxicity of cyclophosphamide: Studies with the hepatic cytochrome p450 reductase null mouse. Cancer Res. 2005, 10, 65. [Google Scholar]
- Ehrke, M.J. Immunomodulation in cancer therapeutics. Int. Immunopharmacol. 2003, 3, 1105–1119. [Google Scholar] [CrossRef]
- Chen, X.; Nie, W.; Fan, S.; Zhang, J.; Wang, Y.; Lu, J.; Jin, L. A polysaccharide from Sargassum fusiforme protects against immunosuppression in cyclophosphamide-treated mice. Carbohydr. Polym. 2012, 90, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, S.Z.; Ying, H.Z.; Dai, X.Y.; Li, X.X.; Yu, C.H.; Ye, H.C. Chemical characterization and immunostimulatory effects of a polysaccharide from Polygoni Multiflori Radix Praeparata in cyclophosphamide-induced anemic mice. Carbohydr. Polym. 2012, 88, 1476–1482. [Google Scholar] [CrossRef]
- Nie, S.-P.; Xie, M.-Y. A review on the isolation and structure of tea polysaccharides and their bioactivities. Food Hydrocoll. 2011, 25, 144–149. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Shokri, H.; Khosravi, A.R.; Taghavi, M. Efficacy of Spirulina platensis on immune functions in cancer mice with systemic candidiasis. J. Mycol. Res. 2014, 1, 7–13. [Google Scholar]
- Qureshi, M.A. Spirulina platensis exposure enhances macrophage phagocytic function in cats. Immunopharmacol. Immunotoxicol. 1996, 3, 457–463. [Google Scholar] [CrossRef]
- Khan, M.; Varadharaj, S.; Ganesan, L.P.; Shobha, J.C.; Naidu, M.U.; Parinandi, N.L.; Tridandapani, S.; Kutala, V.K.; Kuppusamy, P. C-phycocyanin protects against ischemia-reperfusion injury of heart through involvement of p38 MAPK and ERK signaling. J. Physiol. Heart Circ. Physiol. 2006, 290, 2136–2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Sheikh, S.M.; Shalaby, M.A.M.; Hafez, R.A.; Metwally, W.S.A.; El-Ayoty, Y.M. The Immunomodulatory Effects of Probiotic Bacteria on Peripheral Blood Mononuclear Cells (Pbmcs) of Allergic Patients. Am. J. Immunol. 2014, 10, 116–130. [Google Scholar] [CrossRef]
- Grzanna, R.; Polotsky, A.; Phan, P.V.; Pugh, N.; Pasco, D.; Frondoza, C.G. Immolina, a High–Molecular-Weight Polysaccharide Fraction of Spirulina, Enhances Chemokine Expression in Human Monocytic THP-1 Cells. J. Altern. Complementary Med. 2006, 12, 429–435. [Google Scholar] [CrossRef]
- Pugh, N.; Ross, S.A.; ElSohly, H.N.; ElSohly, M.A.; Pasco, D.S. Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, aphanizomenon flos-aquae and Chlorella pyrenoidosa. Planta Med. 2001, 67, 737–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Shen, M.; Wang, Z.; Wang, Y.; Xie, M.; Xie, J. Sulfated polysaccharide from Cyclocarya paliurus enhances the immunomodulatory activity of macrophages. Carbohydr. Polym. 2017, 174, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.M.; Wang, J.F.; Zha, X.Q.; Pan, L.H.; Zhang, H.L.; Luo, J.P. Structural characterization and immunomodulatory activity of a new polysaccharide from jellyfish. Carbohydr. Polym. 2017, 159, 188–194. [Google Scholar] [CrossRef]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-R.; Yang, Z.-Q.; Hu, T.J.; Yan, Z.T.; Niu, T.X.; Wang, L.; Cui, D.A.; Wang, M. Immunomodulatory activity in vitro and in vivo of polysaccharide from Potentilla anserina. Fitoterapia 2010, 81, 1117–1124. [Google Scholar] [CrossRef]
- Hou, F.X.; Yang, H.F.; Yu, T.; Chen, W. The immunosuppressive effects of 10 mg/kg cyclophosphamide in Wistar rats. Environ. Toxicol. Pharmacol. 2007, 24, 30–36. [Google Scholar] [CrossRef]
- Huang, G.C.; Wu, L.S.; Chen, L.G.; Yang, L.L.; Wang, C.C. Immuno-enhancement effects of Huang Qi Liu Yi Tang in a murine model of cyclophosphamide-induced leucopenia. J. Ethnopharmacol. 2007, 109, 229–235. [Google Scholar] [CrossRef]
- Wei, X.J.; Hu, T.J.; Chen, J.R.; Wei, Y.Y. Inhibitory effect of carboxymethylpachymaran on cyclophosphamide-induced oxidative stress in mice. Int. J. Biol. Macromol. 2011, 49, 801–805. [Google Scholar] [CrossRef]
- Fu, Y.F.; Jiang, L.H.; Zhao, W.D.; Xi-Nan, M.; Huang, S.Q.; Yang, J.; Hu, T.J.; Chen, H.L. Immunomodulatory and antioxidant effects of total flavonoids of Spatholobus suberectus Dunn on PCV2 infected mice. Sci. Rep. 2017, 7, 1–9. [Google Scholar]
- Yu, Q.; Nie, S.P.; Wang, J.Q.; Liu, X.Z.; Yin, P.F.; Huang, D.F.; Li, W.J.; Gong, D.M.; Xie, M.Y. Chemoprotective effects of Ganoderma atrum polysaccharide in cyclophosphamide-induced mice. Int. J. Biol. Macromol. 2014, 64, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Coffman, R.L. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [PubMed]
- Wang, J.; Tong, X.; Li, P.; Cao, H.; Su, W. Immuno-enhancement effects of Shenqi Fuzheng Injection on cyclophosphamide-induced immunosuppression in Balb/c mice. J. Ethnopharmacol. 2012, 139, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Cui, S.H.; Zha, X.Q.; Bansal, V.; Jiang, Y.L.; Asghar, M.N.; Wang, J.H.; Pan, L.H.; Xu, B.F.; Luo, J.P. Structure and bioactivity of a polysaccharide extracted from protocorm-like bodies of Dendrobium huoshanense. Int. J. Biol. Macromol. 2015, 72, 664–672. [Google Scholar] [PubMed]
- Bogdan, C.; Röllinghoff, M.; Diefenbach, A. The role of nitric oxide in innate immunity. Immunol. Rev. 2000, 173, 17–26. [Google Scholar] [CrossRef]
- Yoon, S.-B.; Lee, Y.J.; Park, S.K.; Kim, H.C.; Bae, H.; Kim, H.M.; Ko, S.G.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J. Ethnopharmacol. 2009, 125, 286–290. [Google Scholar] [CrossRef]
- Borazjani, N.J.; Tabarsa, M.; You, S.; Rezaei, M. Purification, molecular properties, structural characterization, and immunomodulatory activities of water soluble polysaccharides from Sargassum angustifolium. Int. J. Biol. Macromol. 2018, 109, 793–802. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Xie, G.; Kirpotina, L.N.; Klein, R.A.; Jutila, M.A.; Quinn, M.T. Macrophage immunomodulatory activity of polysaccharides isolated from Opuntia polyacantha. Int. Immunopharmacol. 2008, 8, 1455–1466. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Zheng, G.; You, L.; Wen, L.; Li, C.; Fu, X.; Zhou, L. Structural characterization and macrophage immunomodulatory activity of a polysaccharide isolated from Gracilaria lemaneiformis. J. Funct. Foods 2017, 33, 286–296. [Google Scholar] [CrossRef]
- Pan, G.; Xie, Z.; Huang, S.; Tai, Y.; Cai, Q.; Jiang, W.; Sun, J.; Yuan, Y. Immune-enhancing effects of polysaccharides extracted from Lilium lancifolium Thunb. Int. Immunopharmacol. 2017, 52, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Xie, J.H.; Shen, M.Y.; Nie, S.P.; Xie, M.Y. Sulfated modification of polysaccharides: Synthesis, characterization and bioactivities. Trends Food Sci. Technol. 2018, 74, 147–157. [Google Scholar] [CrossRef]
- Shi, F.; Yan, X.; Cheong, K.L.; Liu, Y. Extraction, purification, and characterization of polysaccharides from marine algae Gracilaria lemaneiformis with anti-tumor activity. Process Biochem. 2018, 73, 197–203. [Google Scholar] [CrossRef]
- Wu, X.; Li, R.; Zhao, Y.; Liu, Y. Separation of polysaccharides from Spirulina platensis by HSCCC with ethanol-ammonium sulfate ATPS and their antioxidant activities. Carbohydr. Polym. 2017, 173, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.P.; Li, X.L.; Wu, Y.; Zhou, L.; Wang, Z.Z.; Xiao, W. Effect of Lentinan on Peyer’s patch structure and function in an immunosuppressed mouse model. Int. J. Biol. Macromol. 2019, 137, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, H.Y.; Yu, J.D.; Qu, C.H.; Wang, Q.; Yang, S.; Ma, S.C.; Ni, J. Quality control and immunological activity of lentinan samples produced in China. Int. J. Biol. Macromol. 2020, 159, 129–136. [Google Scholar] [CrossRef]
- Wang, M.; Yang, X.B.; Zhao, J.W.; Lu, C.J.; Zhu, W. Structural characterization and macrophage immunomodulatory activity of a novel polysaccharide from Smilax glabra Roxb. Carbohydr. Polym. 2017, 156, 390–402. [Google Scholar] [CrossRef]








| Group | Spleen Index | Thymus Index | PWBC (×109/L) | PBL (×109/L) |
|---|---|---|---|---|
| NC | 2.97 ± 0.23 | 2.54 ± 0.2 | 4.04 ± 0.75 | 3.25 ± 0.38 |
| Cy | 1.86 ± 0.15 ** | 1.11 ± 0.14 ** | 1.47 ± 0.42 ** | 1.05 ± 0.17 ** |
| LNT | 2.46 ± 0.3 *,# | 1.87 ± 0.27 **,# | 2.38 ± 0.54 **,# | 1.84 ± 0.32 **,## |
| PSPL | 2.02 ± 0.08 ** | 1.18 ± 0.34 ** | 1.96 ± 0.69 ** | 1.51 ± 0.34 **,# |
| PSPM | 2.16 ± 0.17 *,# | 1.38 ± 0.27 ** | 2.06 ± 0.641 **,# | 1.65 ± 0.13 **,# |
| PSPH | 2.26 ± 0.06 *,# | 1.55 ± 0.22 *,# | 2.27 ± 0.47 **,## | 1.79 ± 0.48 **,# |
| Group | Cy-Induced (mg/kg/day) (Day 2, 4, 6……14) | Drug and Dosage (mg/kg) Once a Day |
|---|---|---|
| NC | normal saline | NS |
| Cy | 30 mg Cy/kg BW/day | NS |
| LNT | 30 mg Cy/kg BW/day | 500 mg LNT/kg BW/day |
| PSPL | 30 mg Cy/kg BW/day | 500 mg PSP/kg BW/day |
| PSPM | 30 mg Cy/kg BW/day | 1000 mg PSP/kg BW/day |
| PSPH | 30 mg Cy/kg BW/day | 1500 mg PSP/kg BW/day |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Liu, Z.; Liu, Y.; Yang, Y.; Shi, F.; Cheong, K.-L.; Teng, B. Immunostimulatory Effects of Polysaccharides from Spirulina platensis In Vivo and Vitro and Their Activation Mechanism on RAW246.7 Macrophages. Mar. Drugs 2020, 18, 538. https://doi.org/10.3390/md18110538
Wu X, Liu Z, Liu Y, Yang Y, Shi F, Cheong K-L, Teng B. Immunostimulatory Effects of Polysaccharides from Spirulina platensis In Vivo and Vitro and Their Activation Mechanism on RAW246.7 Macrophages. Marine Drugs. 2020; 18(11):538. https://doi.org/10.3390/md18110538
Chicago/Turabian StyleWu, Xueyan, Zhicong Liu, Yang Liu, Yu Yang, Fulin Shi, Kit-Leong Cheong, and Bo Teng. 2020. "Immunostimulatory Effects of Polysaccharides from Spirulina platensis In Vivo and Vitro and Their Activation Mechanism on RAW246.7 Macrophages" Marine Drugs 18, no. 11: 538. https://doi.org/10.3390/md18110538





