Polysaccharide from Codium fragile Induces Anti-Cancer Immunity by Activating Natural Killer Cells
Abstract
:1. Introduction
2. Results
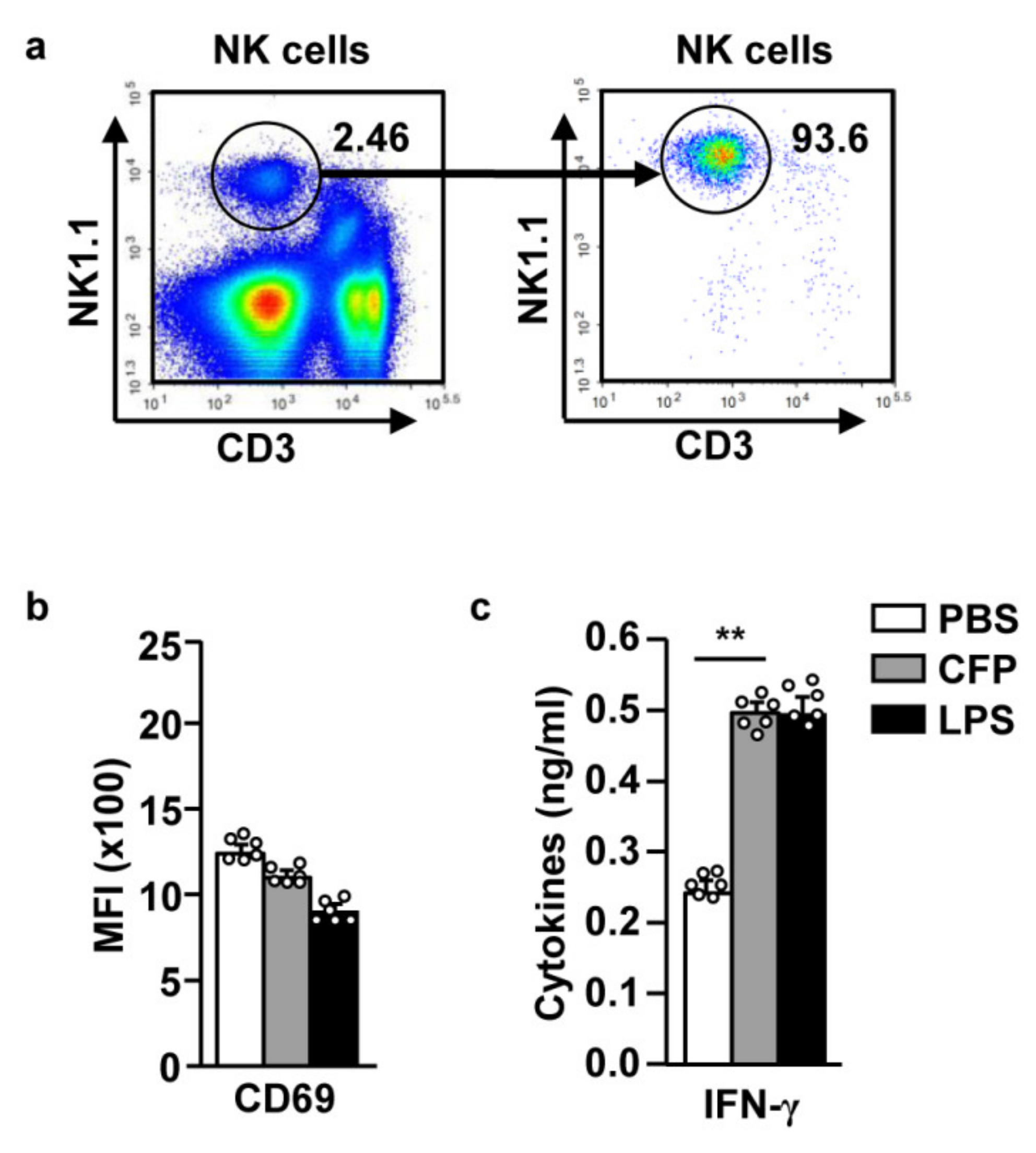
2.1. CFP Upregulates the Expression of CD69 in Splenic NK Cells
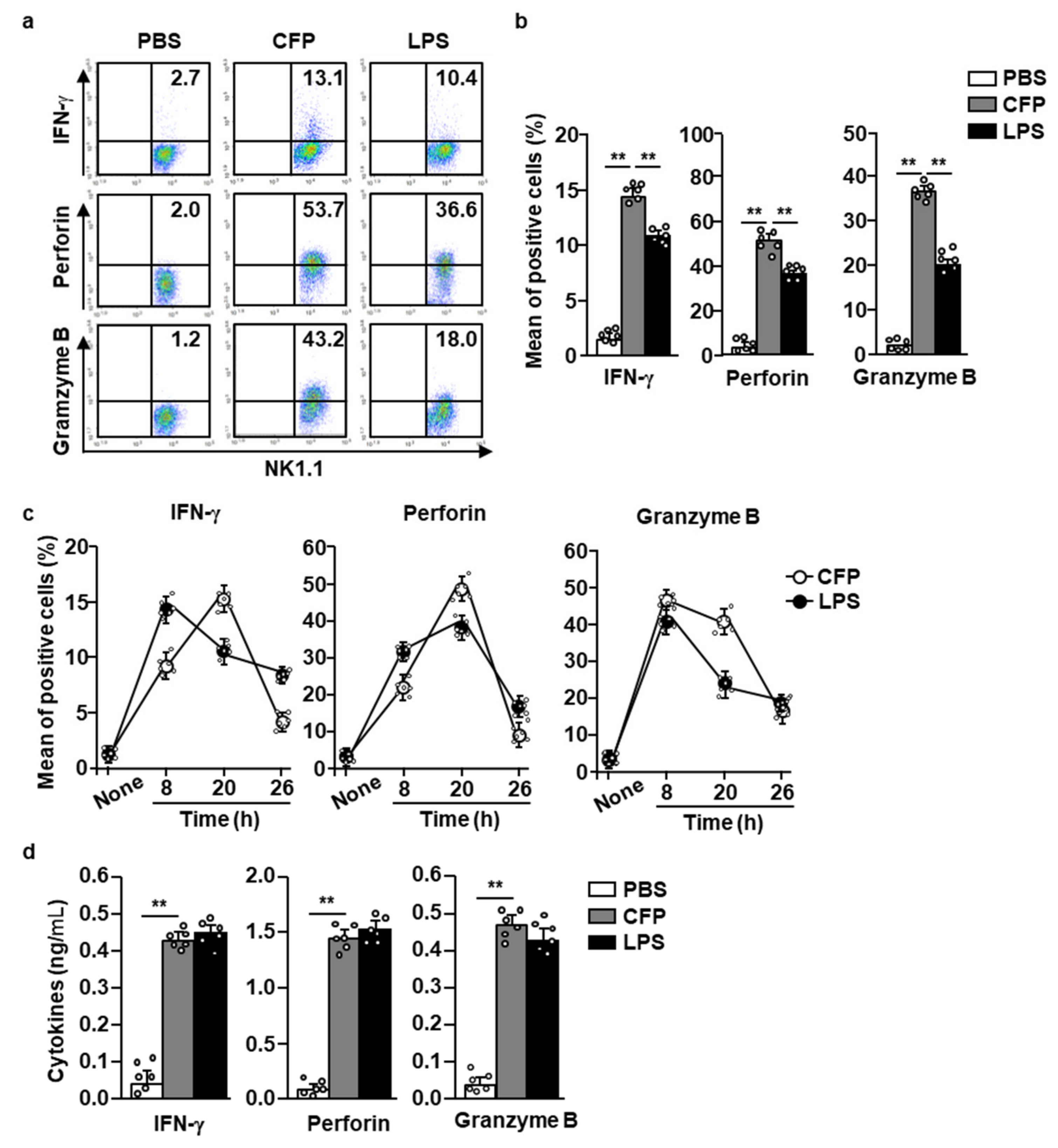
2.2. CFP Induces the Splenic NK Cell-Mediated Production of Cytotoxic Mediators
2.3. CFP Directly Enhances Splenic NK Cell-Mediated IFN-γ Secretion
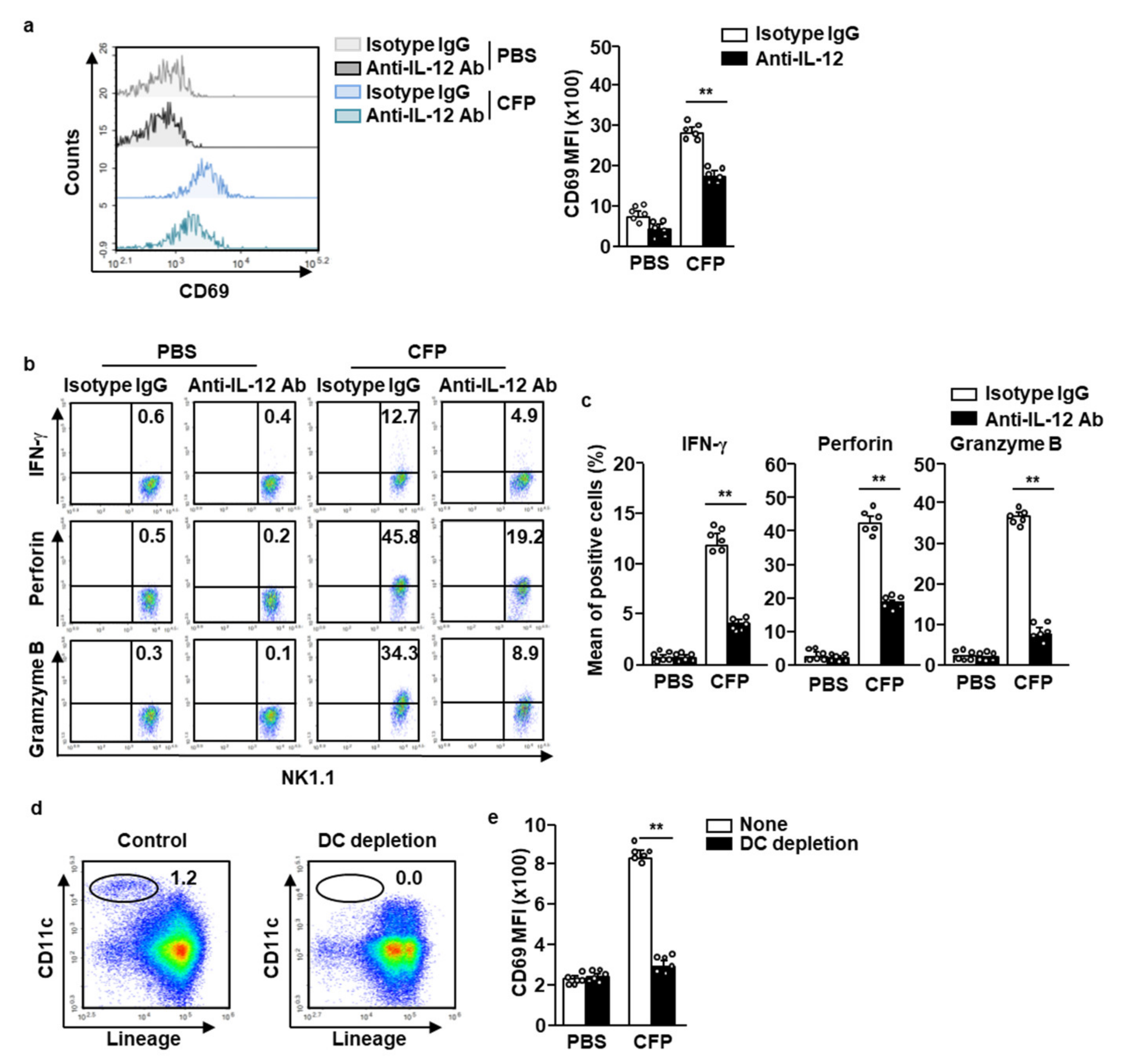
2.4. CFP-Induced Activation of NK Cells Is Partially Dependent on IL-12
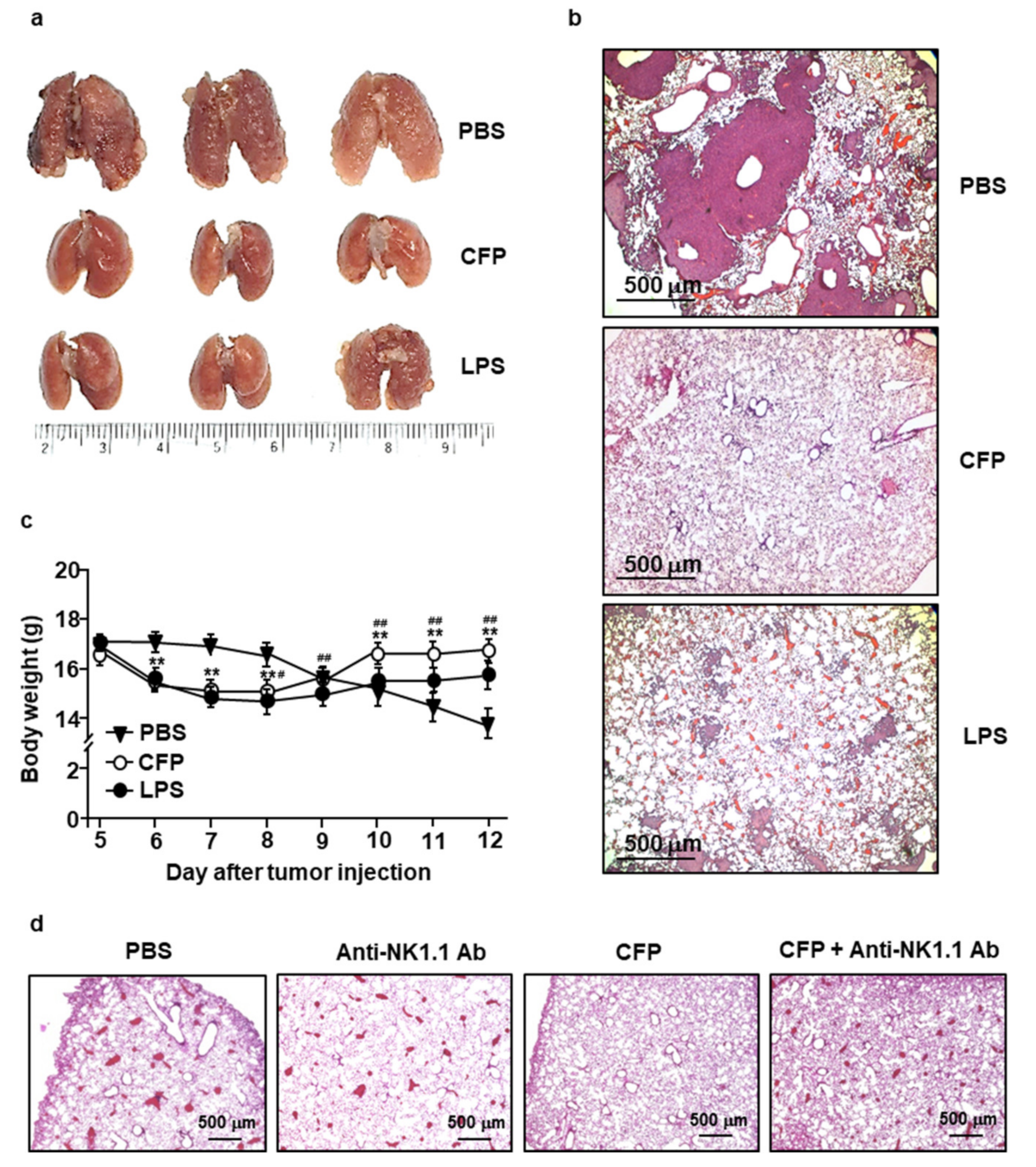
2.5. CFP Prevents Tumor Cell Infiltration in Lung Tissues
3. Discussions
4. Materials and Methods
4.1. Ethics Statement
4.2. Mice and Cell Lines
4.3. Preparation of CFP
4.4. Antibodies
4.5. Flow Cytometric Analysis
4.6. Splenic NK Cell Analysis
4.7. Intracellular Cytokine Analysis
4.8. Isolation of NK Cells
4.9. ELISA
4.10. Tumor Treatment
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zaharoff, D.A.; Rogers, C.J.; Hance, K.W.; Schlom, J.; Greiner, J.W. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine 2007, 25, 2085–2094. [Google Scholar] [PubMed] [Green Version]
- Xu, L.; Kwak, M.; Zhang, W.; Zeng, L.; Lee, P.C.; Jin, J.O. Rehmannia glutinosa polysaccharide induces toll-like receptor 4 dependent spleen dendritic cell maturation and anti-cancer immunity. Oncoimmunology 2017, 6, e1325981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.-O. Fucoidan from Macrocystis pyrifera has powerful immune-modulatory effects compared to three other fucoidans. Mar. Drugs 2015, 13, 1084–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Du, J.Y.; Jiang, Z.; Okimura, T.; Oda, T.; Yu, Q.; Jin, J.O. Ascophyllan purified from Ascophyllum nodosum induces Th1 and Tc1 immune responses by promoting dendritic cell maturation. Mar. Drugs 2014, 12, 4148–4164. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-B.; Lim, S.-M.; Hwang, J.; Zhang, W.; You, S.; Jin, J.-O. Cancer immunotherapy using a polysaccharide from Codium fragile in a murine model. Oncoimmunology 2020, 9, 1772663. [Google Scholar]
- Tabarsa, M.; Park, G.-M.; Shin, I.-S.; Lee, E.; Kim, J.-K.; You, S. Structure-activity relationships of sulfated glycoproteins from Codium fragile on nitric oxide releasing capacity from RAW264. 7 cells. Mar. Biotechnol. 2015, 17, 266–276. [Google Scholar]
- Heffernan, N.; Smyth, T.; Soler-Villa, A.; Fitzgerald, R.; Brunton, N.J. Phenolic content and antioxidant activity of fractions obtained from selected Irish macroalgae species (Laminaria digitata, Fucus serratus, Gracilaria gracilis and Codium fragile). J. Appl. Phycol. 2015, 27, 519–530. [Google Scholar]
- Ortiz, J.; Uquiche, E.; Robert, P.; Romero, N.; Quitral, V.; Llantén, C. Functional and nutritional value of the Chilean seaweeds Codium fragile, Gracilaria chilensis and Macrocystis pyrifera. Eur. J. Lipid Sci. Technol. 2009, 111, 320–327. [Google Scholar]
- Surayot, U.; You, S. Structural effects of sulfated polysaccharides from Codium fragile on NK cell activation and cytotoxicity. Int. J. Biol. Macromol. 2017, 98, 117–124. [Google Scholar]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar]
- Brown, M.G.; Scalzo, A.A.; Matsumoto, K.; Yokoyama, W.M. The natural killer gene complex: A genetic basis for understanding natural killer cell function and innate immunity. Immunol. Rev. 1997, 155, 53–65. [Google Scholar] [PubMed]
- Cerwenka, A.; Lanier, L.L. Natural killer cell memory in infection, inflammation and cancer. Nat. Rev. Immunol. 2016, 16, 112. [Google Scholar]
- Zamai, L.; Ponti, C.; Mirandola, P.; Gobbi, G.; Papa, S.; Galeotti, L.; Cocco, L.; Vitale, M. NK cells and cancer. J. Immunol. 2007, 178, 4011–4016. [Google Scholar] [PubMed] [Green Version]
- Cooper, M.A.; Fehniger, T.A.; Fuchs, A.; Colonna, M.; Caligiuri, M.A. NK cell and DC interactions. Trends Immunol. 2004, 25, 47–52. [Google Scholar] [PubMed]
- Mahrus, S.; Craik, C.S. Selective chemical functional probes of granzymes A and B reveal granzyme B is a major effector of natural killer cell-mediated lysis of target cells. Chem. Biol. 2005, 12, 567–577. [Google Scholar] [PubMed] [Green Version]
- De Maria, A.; Bozzano, F.; Cantoni, C.; Moretta, L. Revisiting human natural killer cell subset function revealed cytolytic CD56dimCD16+ NK cells as rapid producers of abundant IFN-γ on activation. Proc. Natl. Acad. Sci. USA 2011, 108, 728–732. [Google Scholar] [PubMed] [Green Version]
- Smyth, M.J.; Thia, K.Y.; Cretney, E.; Kelly, J.M.; Snook, M.B.; Forbes, C.A.; Scalzo, A. Perforin is a major contributor to NK cell control of tumor metastasis. J. Immunol. 1999, 162, 6658–6662. [Google Scholar]
- Ziegler, S.F.; Ramsdell, F.; Alderson, M.R. The activation antigen CD69. Stem Cells 1994, 12, 456–465. [Google Scholar]
- Shresta, S.; MacIvor, D.M.; Heusel, J.W.; Russell, J.H.; Ley, T.J. Natural killer and lymphokine-activated killer cells require granzyme B for the rapid induction of apoptosis in susceptible target cells. Proc. Natl. Acad. Sci. USA 1995, 92, 5679–5683. [Google Scholar]
- Osińska, I.; Popko, K.; Demkow, U. Perforin: An important player in immune response. Cent. Eur. J. Immunol. 2014, 39, 109. [Google Scholar]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Cretney, E.; Kelly, J.M.; Westwood, J.A.; Street, S.E.; Yagita, H.; Takeda, K.; Van Dommelen, S.L.; Degli-Esposti, M.A.; Hayakawa, Y.J. Activation of NK cell cytotoxicity. Mol. Immunol. 2005, 42, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kwak, M.; Lee, P.C.; Jin, J.O. Rehmannia glutinosa polysaccharide promoted activation of human dendritic cells. Int. J. Biol. Macromol. 2018, 116, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, W.; Zeng, L.; Jin, J.-O. Rehmannia glutinosa polysaccharide induced an anti-cancer effect by activating natural killer cells. Int. J. Biol. Macromol. 2017, 105, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-B.; Hwang, J.; Lim, S.-M.; Zhang, W.; Jin, J.-O. Dendritic cell-mediated cancer immunotherapy with Ecklonia cava fucoidan. Int. J. Biol. Macromol. 2020, 159, 941–947. [Google Scholar] [CrossRef]
- Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H.J. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003, 65, 173–179. [Google Scholar] [CrossRef]
- Li, X.; Min, M.; Du, N.; Gu, Y.; Hode, T.; Naylor, M.; Chen, D.; Nordquist, R.E.; Chen, W.R.; Immunology, D. Chitin, chitosan, and glycated chitosan regulate immune responses: The novel adjuvants for cancer vaccine. Clin. Dev. Immunol. 2013, 2013, 387023. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [Green Version]
- Cella, M.; Fuchs, A.; Vermi, W.; Facchetti, F.; Otero, K.; Lennerz, J.K.; Doherty, J.M.; Mills, J.C.; Colonna, M.J.N. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 2009, 457, 722–725. [Google Scholar] [CrossRef]
- Esplugues, E.; Vega-Ramos, J.; Cartoixà, D.; Vazquez, B.N.; Salaet, I.; Engel, P.; Lauzurica, P.J.B. Induction of tumor NK-cell immunity by anti-CD69 antibody therapy. Blood 2005, 105, 4399–4406. [Google Scholar] [CrossRef] [Green Version]
- Kalinski, P.; Mailliard, R.B.; Giermasz, A.; Zeh, H.J.; Basse, P.; Bartlett, D.L.; Kirkwood, J.M.; Lotze, M.T.; Herberman, R.B. Natural killer–dendritic cell cross-talk in cancer immunotherapy. Expert Opin. Biol. Ther. 2005, 5, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, L.; Park, H.-B.; Hwang, J.; Kwak, M.; Lee, P.C.; Liang, G.; Zhang, X.; Xu, J.; Jin, J.-O. Escherichia coli adhesion portion FimH functions as an adjuvant for cancer immunotherapy. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, W.; Xu, L.; Jin, J.-O. Porphyromonas gingivalis lipopolysaccharide induced proliferation and activation of natural killer cells in vivo. Molecules 2016, 21, 1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Zhang, W.; Park, H.B.; Kwak, M.; Oh, J.; Lee, P.C.W.; Jin, J.O. Indocyanine green and poly I:C containing thermo-responsive liposomes used in immune-photothermal therapy prevent cancer growth and metastasis. J. Immunother. Cancer 2019, 7, 220. [Google Scholar] [CrossRef] [Green Version]
- Kodama, T.; Takeda, K.; Shimozato, O.; Hayakawa, Y.; Atsuta, M.; Kobayashi, K.; Ito, M.; Yagita, H.; Okumura, K.J.E. Perforin-dependent NK cell cytotoxicity is sufficient for anti-metastatic effect of IL-12. Eur. J. Immunol. 1999, 29, 1390–1396. [Google Scholar] [CrossRef]
- Borg, C.; Jalil, A.; Laderach, D.; Maruyama, K.; Wakasugi, H.; Charrier, S.; Ryffel, B.; Cambi, A.; Figdor, C.; Vainchenker, W.J.B. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood 2004, 104, 3267–3275. [Google Scholar] [CrossRef]
- Trapani, J.A.; Smyth, M.J. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2002, 2, 735–747. [Google Scholar] [CrossRef]
- Trapani, J.A.; Sutton, V.R. Granzyme B: Pro-apoptotic, antiviral and antitumor functions. Adv. Anti-Cancer Drugs 2003, 15, 533–543. [Google Scholar] [CrossRef]
- Ross, G.; Větvička, V.J.C.; Immunology, E. CR3 (CD11b, CD18): A phagocyte and NK cell membrane receptor with multiple ligand specificities and functions. Clin. Exp. Immunol. 1993, 92, 181–184. [Google Scholar] [CrossRef]
- Wagner, C.; Hänsch, G.M.; Stegmaier, S.; Denefleh, B.; Hug, F.; Schoels, M.J.E. The complement receptor 3, CR3 (CD11b/CD18), on T lymphocytes: Activation-dependent up-regulation and regulatory function. Eur. J. Immunol. 2001, 31, 1173–1180. [Google Scholar] [CrossRef]
- Sándor, N.; Kristóf, K.; Paréj, K.; Pap, D.; Erdei, A.; Bajtay, Z.J.I. CR3 is the dominant phagocytotic complement receptor on human dendritic cells. Immunobiology 2013, 218, 652–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, G.; Cain, J.; Lachmann, P.J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J. Immunol. 1985, 134, 3307–3315. [Google Scholar] [PubMed]
- Dillon, S.; Agrawal, S.; Banerjee, K.; Letterio, J.; Denning, T.L.; Oswald-Richter, K.; Kasprowicz, D.J.; Kellar, K.; Pare, J.; van Dyke, T.J. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Investig. 2006, 116, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, R.H.; Bazhanov, N.; Oh, J.Y.; Prockop, D.J. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Bloods 2011, 118, 330–338. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-B.; Hwang, J.; Zhang, W.; Go, S.; Kim, J.; Choi, I.; You, S.; Jin, J.-O. Polysaccharide from Codium fragile Induces Anti-Cancer Immunity by Activating Natural Killer Cells. Mar. Drugs 2020, 18, 626. https://doi.org/10.3390/md18120626
Park H-B, Hwang J, Zhang W, Go S, Kim J, Choi I, You S, Jin J-O. Polysaccharide from Codium fragile Induces Anti-Cancer Immunity by Activating Natural Killer Cells. Marine Drugs. 2020; 18(12):626. https://doi.org/10.3390/md18120626
Chicago/Turabian StylePark, Hae-Bin, Juyoung Hwang, Wei Zhang, Seulgi Go, Jihoe Kim, Inho Choi, SangGuan You, and Jun-O Jin. 2020. "Polysaccharide from Codium fragile Induces Anti-Cancer Immunity by Activating Natural Killer Cells" Marine Drugs 18, no. 12: 626. https://doi.org/10.3390/md18120626




