Marine Cyanobacteria and Microalgae Metabolites—A Rich Source of Potential Anticancer Drugs
Abstract
:1. Introduction
2. Literature Search Methodology
3. Various Classes of Secondary Metabolites of Marine Cyanobacterium and Microalgae
3.1. Alkaloids
3.2. Polyketides
3.3. Terpenes
3.4. Peptides
3.5. Nucleosides
3.6. Carbohydrates
4. Secondary Metabolites of Marine Cyanobacteria and Microalgae at Various Phases of Clinical Research
5. Marine Cyanobacteria Metabolites with Anticancer Property
5.1. Anthracyclines
5.2. Phenoxazin-3-One Compounds
5.3. Polyketides
5.4. Peptides
5.5. Macrolides
5.6. Lactones
5.7. Fatty Acid Amines
5.8. Pigment
5.9. Boron Containing Metabolite
5.10. Phenanthridine Alkaloids
6. Microalgae Metabolites as Anticancer Drugs with Their Mechanisms of Action
6.1. Polyunsaturated Aldehydes (PUAs)
6.2. Polysaccharide
6.2.1. Chrysolaminaran Polysaccharide
6.2.2. Sulfated Polysaccharide
6.2.3. Alginic Acid
6.2.4. Laminarin
6.3. Carotenoids
6.3.1. Violaxanthin
6.3.2. Neoxanthin
6.3.3. Fucoxanthin
6.3.4. Siphonaxanthin
6.3.5. Zeaxanthin and Lutein
6.4. Stigmasterol
6.5. Nonyl 8-Acetoxy-6-Methyloctanoate
6.6. Dinochrome A and B
6.7. Phaeophytins
6.8. Nigricanosides A (135) and B (136) and Methyl Esters of Nigricanosides A (137) and B (138)
7. Conclusions, Current Challenges and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sithranga-Boopathy, N.; Kathiresan, K. Anticancer drugs from marine flora: An overview. J. Oncol. 2010, 2010, 214186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.; Bose, S.; Mandal, S.C.; Dawn, S.; Sahoo, U.; Ramadan, M.A.; Mandal, S.K. Pharmacological Property of Pentacyclic Triterpenoids. Egypt J. Chem. 2019, 62, 13–35. [Google Scholar] [CrossRef]
- Grothaus, P.G.; Cragg, G.M.; Newman, D.J. Plant natural products in anticancer drug discovery. Curr. Org. Chem. 2010, 14, 1781–1791. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef] [Green Version]
- Cragg, G.M.; Kingston, D.G.I.; Newman, D.J. Anticancer Agents from Natural Products, 2nd ed.; CRC/Taylor & Francis: Boca Raton, FL, USA, 2012. [Google Scholar]
- Basmadjian, C.; Zhao, Q.; Djehal, A.; Bentouhami, E.; Nebigil, C.G.; Johnson, R.A.; Serova, M.; De Gramont, A.; Faivre, S.; Raymond, E.; et al. Cancer wars: Natural products strike back. Front. Chem. 2014, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, S.X.; Guan, H.S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine natural products: A source of novel anticancer drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [Green Version]
- Faulkner, D.J. Marine pharmacology. Antonie Leeuwenhoek 2000, 77, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Schwartsmann, G.; Brondani, A.; Berlinck, R.G.S.; Jimeno, J. Marine organisms and other novel natural sources of new cancer drugs. Ann. Oncol. 2000, 11, 235–243. [Google Scholar] [CrossRef] [PubMed]
- White, M.C.; Holman, D.M.; Boehm, J.E.; Peipins, L.A.; Grossman, M.; Henley, S.J. Age and cancerrisk: A potentially modifiable relationship. Am. J. Prev. Med. 2014, 46, S7–S15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.; Cragg, G. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar. Drugs 2014, 12, 255–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Townsend, M.; Davies, K.; Hanley, N.; Hewitt, J.E.; Lundquist, C.J.; Lohrer, A.M. The challenge of implementing the marine ecosystem services concept. Front. Mar. Sci. 2018, 5, 359. [Google Scholar] [CrossRef] [Green Version]
- Snelgrove, P.V.R. Getting to the bottom of marine biodiversity: Sedimentary habitats: Ocean bottom are the most widespread habitats on earth and support high biodiversity and key ecosystem services. BioScience 1999, 49, 129–138. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes and antibacterial activities. Front. Mar. Sci. 2016, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Torres, V.; Encinar, J.A.; Herranz-López, M.; Pérez-Sánchez, A.; Galiano, V.; Barrajón-Catalán, E.; Micol, V. An Updated Review on Marine Anticancer Compounds: The Use of Virtual Screening for the Discovery of Small-Molecule Cancer Drugs. Molecules 2017, 22, 1037. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Prakash, V.; Ranjan, N. Marine Fungi: A Source of Potential Anticancer Compounds. Front. Microbiol. 2018, 8, 2536. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.A.; Pomin, V.H. Marine Carbohydrate-Based Compounds with Medicinal Properties. Mar. Drugs 2018, 16, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahapatra, G.P.; Raman, S.; Nayak, S.; Gouda, S.; Das, G.; Patra, J.K. Metagenomics approaches in discovery and development of new bioactive compounds from marine actinomycetes. Curr. Microbiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef]
- Costa, M.; Costa-Rodrigues, J.; Fernandes, M.H.; Barros, P.; Vasconcelos, V.; Martins, R. Marine cyanobacteria compounds with anticancer properties: A review on the implication of apoptosis. Mar. Drugs 2012, 10, 2181–2207. [Google Scholar] [CrossRef] [Green Version]
- Boopathy, N.S.; Kathiresan, K. Anticancer agents derived from marine algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Dominguez, H., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Elsevier: Sawston, UK; Cambridge, UK, 2013; Chapter 9; pp. 307–337. [Google Scholar]
- Bajpai, V.K.; Shukla, S.; Kang, S.M.; Hwang, S.K.; Song, X.; Huh, Y.S.; Han, Y.K. Developments of cyanobacteria for nano-marine drugs: Relevance of nanoformulations in cancer therapies. Mar. Drugs 2018, 16, 179. [Google Scholar] [CrossRef] [Green Version]
- Raja, R.; Hemaiswarya, S.; Ganesan, V.; Carvalho, I.S. Recent developments in therapeutic applications of cyanobacteria. Crit. Rev. Microbiol. 2016, 42, 394–405. [Google Scholar] [CrossRef]
- Kang, K.H.; Kim, S.K. Beneficial effect of peptides from microalgae on anticancer. Curr. Protein Pept. Sci. 2013, 14, 212–217. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [Green Version]
- Mondal, A.; Gandhi, A.; Fimognari, C.; Atanasov, A.G.; Bishayee, A. Alkaloids for cancer prevention and therapy: Current progress and future perspectives. Eur. J. Pharmacol. 2019, 858, 172472. [Google Scholar] [CrossRef]
- Aniszewski, T. Alkaloids—Secrets of Life: Alkaloids Chemistry, Biological Significance, Applications and Ecological Role; Elsevier: Amsterdam, The Netherlands, 2007; p. 334. [Google Scholar]
- Güven, K.C.; Coban, B.; Sezik, E.; Erdugan, H.; Kaleağasıoğlu, F. Alkaloids of Marine Macroalgae. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 25–37. [Google Scholar]
- Choi, E.J.; Nam, S.J.; Paul, L.; Beatty, D.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Previously uncultured marine bacteria linked to novel alkaloid production. Chem. Biol. 2015, 22, 12709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R. Marine pigmented bacteria: A prospective source of antibacterial compounds. J. Nat. Sci. Biol. Med. 2019, 10, 104–113. [Google Scholar] [CrossRef]
- Picott, K.J.; Deichert, J.A.; deKemp, E.M.; Schatte, G.; Sauriol, F.; Ross, A.C. Isolation and characterization of tambjamine MYP1, a macrocyclic tambjamine analogue from marine bacterium Pseudoalteromonas citrea. Med. Chem. Comm. 2019, 10, 478–483. [Google Scholar] [CrossRef]
- El-Hack, M.E.A.; Abdelnour, S.; Alagawany, M.; Abdo, M.; Sakr, M.A.; Khafaga, A.F.; Mahgoub, S.A.; Elnesr, S.S.; Gebriel, M.G. Microalgae in modern cancer therapy: Current knowledge. Biomed. Pharmacother. 2019, 111, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Demay, J.; Bernard, C.; Reinhardt, A.; Marie, B. Natural Products from Cyanobacteria: Focus on Beneficial Activities. Mar. Drugs 2019, 17, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997; ISBN 0-9678550-9-8. [Google Scholar] [CrossRef]
- Sadaka, C.; Ellsworth, E.; Hansen, P.; Ewin, R.; Damborg, P.; Watts, J. Review on abyssomicins: Inhibitors of the chorismate pathway and folate biosynthesis. Molecules 2018, 23, 1371–1396. [Google Scholar] [CrossRef] [Green Version]
- Davidson, B.S. New dimensions in natural products research: Cultured marine microorganisms. Curr. Opin. Biotechnol. 1995, 6, 284–291. [Google Scholar] [CrossRef]
- Kandi, S.; Godishala, V.; Rao, P.; Ramana, K.V. Biomedical significance of terpenes: An insight. Biomed. Biotechnol. 2015, 3, 8–10. [Google Scholar]
- Kitts, D.D.; Weiler, K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr. Pharm. Des. 2003, 9, 1309–1323. [Google Scholar] [CrossRef]
- Sarmadi, B.; Ismail, A.; Hamid, M. Antioxidant and angiotensin converting enzyme (ACE) inhibitory activities of cocoa (Theobroma cacao L.) autolysates. Food Res. Int. 2011, 44, 290–296. [Google Scholar] [CrossRef]
- Ghanbari, R. Review on the bioactive peptides from marine sources: Indication for health effects. Int. J. Pept. Res. Ther. 2018, 25, 1187–1199. [Google Scholar] [CrossRef]
- Slizyte, R.; Mozuraityte, R.; Martinez-Alvarez, O. Functional, bioactive and antioxidative properties of hydrolysates obtained from cod (Gadusmorhua) backbones. Proc. Biochem. 2009, 44, 668–677. [Google Scholar] [CrossRef]
- Shukla, S. Therapeutic importance of peptides from marine source: A mini review. Ind. J. Geo Mar. Sci. 2016, 45, 1422–1431. [Google Scholar]
- Aneiros, A.; Garateix, A. Bioactive peptides from marine sources: Pharmacological properties and isolation procedures. J. Chromatogr. B 2004, 803, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine Peptides: Bioactivities and Applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zou, B.; Cai, G.; Hu, X.; Liu, J.O. Total synthesis of the cyclodepsipeptideapratoxin A and its analogues and assessment of their biological activities. Chem. Eur. J. 2006, 12, 7615–7626. [Google Scholar] [CrossRef] [PubMed]
- Bhakuni, D.; Rawat, D. Bioactive marine nucleosides. In Bioactive Marine Natural Products; Springer: Dordrecht, The Netherlands, 2005; pp. 208–234. [Google Scholar]
- Huang, R.M.; Chen, Y.N.; Zeng, Z.; Gao, C.H.; Su, X.; Peng, Y. Marine nucleosides: Structure, bioactivity, synthesis and biosynthesis. Mar. Drugs 2014, 12, 5817–5838. [Google Scholar] [CrossRef] [Green Version]
- Isono, K. Nucleoside antibiotics-Structure, biologicalactivity, and biosynthesis. J. Antibiot. 1988, 41, 1711–1739. [Google Scholar] [CrossRef]
- Isono, K. Current progress on nucleoside antibiotics. Pharmacol. Ther. 1991, 52, 269–286. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1993, 10, 497–539. [Google Scholar] [CrossRef]
- Sable, R.; Parajuli, P.; Jois, S. Peptides, Peptidomimetics, and Polypeptides from Marine Sources: A Wealth of Natural Sources for Pharmaceutical Applications. Mar. Drugs 2017, 15, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Advanced preclinical and clinical trials of natural products and related compounds from marine sources. Curr. Med. Chem. 2004, 11, 1693–1731. [Google Scholar] [CrossRef] [PubMed]
- Machella, N.; Regoli, F.; Cambria, A.; Santella, R.M. Oxidative damage to DNA: An immunohistochemical approach for detection of 7,8-dihydro-8-oxodeoxyguanosine in marine organisms. Mar. Environ. Res. 2004, 58, 725–729. [Google Scholar] [CrossRef]
- Schwartsmann, G.; Da Rocha, A.B.; Mattei, J.; Lopes, R. Marine-derived anticancer agents in clinical trials. Expert Opin Investig. Drugs 2003, 12, 1367–1383. [Google Scholar] [CrossRef] [PubMed]
- Helbert, W. Marine polysaccharide sulfatases. Front. Mar. Sci. 2017, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, J.A.; Rodríguez-Amado, I.; Montemayor, M.I.; Fraguas, J.; González Mdel, P.; Murado, M.A. Chondroitin sulfate, hyaluronic acid and chitin/chitosan production using marine waste sources: Characteristics, applications and eco-friendly processes: A review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef] [Green Version]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial exopolysaccharides from extreme marine habitats: Production, characterization and biological activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef]
- Pomin, V.H. Marine medicinal glycomics. Front. Cell Infect. Microbiol. 2014, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.K.; Seo, C.H.; Park, Y. The effects of marine carbohydrates and glycosylated compounds on human health. Int. J. Mol. Sci. 2015, 16, 6018–6056. [Google Scholar] [CrossRef] [Green Version]
- Thomas, N.V.; Kim, S.K. Beneficial effects of marine algal compounds in cosmeticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boisson-Vidal, C.; Zemani, F.; Caligiuri, G.; Galy-Fauroux, I.; Colliec-Jouault, S.; Helley, D.; Fischer, A.M. Neoangiogenesis induced by progenitor endothelial cells: Effect of fucoidan from marine algae. Cardiovasc. Hematol. Agents Med. Chem. 2007, 5, 67–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aisa, Y.; Miyakawa, Y.; Nakazato, T.; Shibata, H.; Saito, K.; Ikeda, Y.; Kizaki, M. Fucoidan induces apoptosis of human HS-sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways. Am. J. Hematol. 2005, 78, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. 2009, 59, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Thacker, R.W.; Paul, V.J. Morphological, chemical, and genetic diversity of tropical marine cyanobacteria Lyngbya spp. and Symploca spp. (Oscillatoriales). Appl. Environ. Microbiol. 2004, 70, 3305–3312. [Google Scholar] [CrossRef] [Green Version]
- Bai, R.; Pettit, G.R.; Hamel, E. Dolastatin 10, a powerful cytostatic peptide derived from a marine animal. Inhibition of tubulin polymerization mediated through the vinca alkaloid binding domain. Biochem. Pharmacol. 1990, 39, 1941–1949. [Google Scholar] [CrossRef]
- Madden, T.; Tran, H.T.; Beck, D.; Huie, R.; Newman, R.A.; Pusztai, L.; Wright, J.J.; Abbruzzese, J.L. Novel marine-derived anticancer agents: A phase I clinical, pharmacological, and pharmacodynamic study of dolastatin 10 (NSC 376128) in patients with advanced solid tumors. Clin. Cancer Res. 2000, 6, 1293–1301. [Google Scholar]
- Pereira, R.B.; Evdokimov, N.M.; Lefranc, F.; Valentão, P.; Kornienko, A.; Pereira, D.M.; Andrade, P.B.; Gomes, N.G.M. Marine-Derived Anticancer Agents: Clinical Benefits, Innovative Mechanisms, and New Targets. Mar. Drugs 2019, 17, 329. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Natsume, T.; Tamaoki, S.; Watanabe, J.; Asano, H.; Mikami, T.; Miyasaka, K.; Miyazaki, K.; Gondo, M.; Sakakibara, K.; et al. Antitumor activity of TZT-1027, a novel dolastatin 10 derivative. Jpn. J. Cancer Res. 1997, 88, 316–327. [Google Scholar] [CrossRef]
- Otani, M.; Natsume, T.; Watanabe, J.I.; Kobayashi, M.; Murakoshi, M.; Mikami, T.; Nakayama, T. TZT-1027, an antimicrotubule agent, attacks tumor vasculature and induces tumor cell death. Jpn. J. Cancer Res. 2000, 91, 837–844. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Hammond, L.A.; Ruvuna, F.; Cunningham, C.C.; Ebbinghaus, S.; Rubin, E.; Hersh, A.M.E.; Eder, J.P.; Weiss, J.; Rowinsky, E.K. Phase (Ph) I evaluation of the dolastatin analogue synthadotin (SYN-D; ILX651): Pooled data analysis of three alternate schedules in patients (pts) with advanced solid tumors. J. Clin. Oncol. 2004, 22, 3068. [Google Scholar] [CrossRef]
- Ebbinghaus, S.; Hersh, E.; Cunningham, C.C.; O’Day, S.; McDermott, D.; Richards, J.S.D.A.; Eckardt, J.; Haider, O.L.; Hammond, L.A. Phase II study of synthadotin (SYN-D; ILX651) administered daily for 5 consecutive days once every 3 weeks (qdx5q3w) in patients (Pts) with inoperable locally advanced or metastatic melanoma. J. Clin. Oncol. 2004, 22, 7530. [Google Scholar] [CrossRef]
- Gulder, T.A.; Moore, B.S. Salinosporamide natural products: Potent 20 S proteasome inhibitors as promising cancer chemotherapeutics. Angew. Chem. Int. Ed. Engl. 2010, 49, 9346–9367. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Catley, L.; Li, G.; Podar, K.; Hideshima, T.; Velankar, M.; Mitsiades, C.; Mitsiades, N.; Yasui, H.; Letai, A.; et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell 2005, 8, 407–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, S.J.; Mainwaring, P.; Price, T.; Millward, M.J.; Padrik, P.; Underhill, C.R.; Cannell, P.K.; Reich, S.D.; Trikha, M.; Spencer, A. Phase I clinical trial of marizomib (NPI-0052) in patients with advanced malignancies including multiple myeloma: Study NPI-0052-102 final results. Clin. Cancer Res. 2016, 22, 4559–4566. [Google Scholar] [CrossRef] [Green Version]
- Itoh, T.; Kinoshita, M.; Aoki, S.; Kobayashi, M. Komodoquinone A, a novel neuritogenic anthracycline, from marine Streptomyces sp. KS3. J. Nat. Prod. 2003, 66, 1373–1377. [Google Scholar] [CrossRef]
- Maskey, R.P.; Li, F.; Qin, S.; Fiebig, H.H.; Laatsch, H. Chandrananimycins A approximately C: Production of novel anticancer antibiotics from a marine Actinomadura sp. isolate M048 by variation of medium composition and growth conditions. J. Antibiot. (Tokyo) 2003, 56, 622–629. [Google Scholar] [CrossRef] [Green Version]
- Andrianasolo, E.H.; Gross, H.; Goeger, D.; Musafija-Girt, M.; McPhail, K.; Leal, R.M.; Mooberry, S.L.; Gerwick, W.H. Isolation of swinholide A and related glycosylated derivatives from two field collections of marine cyanobacteria. Org. Lett. 2005, 7, 1375–1378. [Google Scholar] [CrossRef]
- Mooberry, S.L.; Leal, R.M.; Tinley, T.L.; Luesch, H.; Moore, R.E.; Corbett, T.H. The molecular pharmacology of symplostatin 1: A new antimitotic dolastatin 10 analog. Int. J. Cancer 2003, 104, 512–521. [Google Scholar] [CrossRef]
- Kwan, J.C.; Rocca, J.R.; Abboud, K.A.; Paul, V.J.; Luesch, H. Total Structure Determination of Grassypeptolide, a New Marine Cyanobacterial Cytotoxin. Org. Lett. 2008, 10, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.C.; Ratnayake, R.; Abboud, K.A.; Paul, V.J.; Luesch, H. Grassypeptolides A-C, cytotoxic bis-thiazoline containing marine cyclodepsipeptides. J. Org. Chem. 2010, 75, 8012–8023. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H.; Proteau, P.J.; Nagle, D.G.; Hamel, E.; Blokhin, A.; Slate, D. Structure of curacin A, a novel antimitotic, antiproliferative, and brine shrimp toxic natural product from the marine cyanobacterium Lyngbya majuscule. J. Org. Chem. 1994, 59, 1243–1245. [Google Scholar] [CrossRef]
- Yoo, H.D.; Nagle, D.G.; Geralds, R.S.; Gerwick, W.H.; Kim, T.S.; Nambu, M.; White, J.D. Absolute configuration of curacin A, a novel antimitotic agent from the tropical marine cyanobacterium Lyngbyamajuscula. Tetrahedron Lett. 1995, 36, 1189–1192. [Google Scholar]
- Catassi, A.; Cesario, A.; Arzani, D.; Menichini, P.; Alama, A.; Bruzzo, C.; Imperatori, A.; Rotolo, N.; Granone, P.; Russo, P. Characterization of apoptosis induced by marine natural products in non small cell lung cancer A549 cells. Cell Mol. Life Sci. 2006, 63, 2377–2386. [Google Scholar] [CrossRef]
- Blokhin, A.V.; Yoo, H.D.; Geralds, R.S.; Nagle, D.G.; Gerwick, W.H.; Hamel, E. Characterization of the interaction of the marine cyanobacterial natural product curacin A with the colchicine site of tubulin and initial structure-activity studies with analogues. Mol. Pharmacol. 1995, 48, 523–531. [Google Scholar] [PubMed]
- Verdier-Pinard, P.; Lai, J.Y.; Yoo, H.D.; Yu, J.; Marquez, B.; Nagle, D.G.; Nambu, M.; White, J.D.; Falck, J.R.; Gerwick, W.H.; et al. Structure-activity analysis of the interaction of curacin A, the potent colchicine site antimitotic agent, with tubulin and effects of analogs on the growth of MCF-7 breast cancer cells. Mol. Pharmacol. 1998, 53, 62–76. [Google Scholar] [CrossRef]
- Wipf, P.; Reeves, J.; Day, B. Chemistry and Biology of Curacin A. Curr. Pharm. Des. 2004, 10, 1417–1437. [Google Scholar] [CrossRef]
- Williams, P.G.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. The isolation and structure elucidation of Tasiamide B, a 4-amino-3-hydroxy-5-phenylpentanoic acid containing peptide from the marine cyanobacterium Symploca sp. J. Nat. Prod. 2003, 66, 1006–1009. [Google Scholar] [CrossRef]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. New apratoxins of marine cyanobacterial origin from Guam and Palau. Bioorg. Med. Chem. 2002, 10, 1973–1978. [Google Scholar] [CrossRef]
- Paatero, A.O.; Kellosalo, J.; Dunyak, B.M.; Almaliti, J.; Gestwicki, J.E.; Gerwick, W.H.; Taunton, J.; Paavilainen, V.O. Apratoxin kkills cells by direct blockade of the Sec61 protein translocation channel. Cell Chem. Biol. 2016, 23, 561–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthew, S.; Schupp, P.J.; Luesch, H. Apratoxin E, a cytotoxic peptolide from a Guamanian collection of the marine cyanobacterium Lyngbya bouillonii. J. Nat. Prod. 2008, 71, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Tidgewell, K.; Engene, N.; Byrum, T.; Media, J.; Doi, T.; Valeriote, F.A.; Gerwick, W.H. Evolved diversification of a modular natural product pathway: Apratoxins F and G, two cytotoxic cyclic depsipeptides from a Palmyra collection of Lyngbya bouillonii. Chem. Biochem. 2010, 11, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Gross, H.; Goeger, D.E.; Mooberry, S.L.; Gerwick, W.H. Aurilides B and C, Cancer cell toxins from a Papua New Guinea collection of the marine cyanobacterium Lyngb yamajuscula. J. Nat. Prod. 2006, 69, 572–575. [Google Scholar] [CrossRef]
- Sato, S.; Murata, A.; Orihara, T.; Shirakawa, T.; Suenaga, K.; Kigoshi, H.; Uesugi, M. Marine natural product aurilide activates the OPA1-mediated apoptosis by binding to prohibitin. Chem. Biol. 2011, 18, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Medina, R.A.; Goeger, D.E.; Hills, P.; Mooberry, S.L.; Huang, N.; Romero, L.I.; Ortega-Barría, E.; Gerwick, W.H.; McPhail, K.L. Coibamide A, a potent antiproliferative cyclic depsipeptide from the Panamanian marine cyanobacterium Leptolyngbya sp. J. Am. Chem. Soc. 2008, 130, 6324–6325. [Google Scholar] [CrossRef] [Green Version]
- Serrill, J.D.; Wan, X.; Hau, A.M.; Jang, H.S.; Coleman, D.J.; Indra, A.K.; Alani, A.W.; McPhail, K.L.; Ishmael, J.E. Coibamide A, a natural lariat depsipeptide, inhibits VEGFA/VEGFR2 expression and suppresses tumor growth in glioblastoma xenografts. Investig. New Drugs 2016, 34, 24–40. [Google Scholar] [CrossRef]
- Hau, A.M.; Greenwood, J.A.; Löhr, C.V.; Serrill, J.D.; Proteau, P.J.; Ganley, I.G.; McPhail, K.L.; Ishmael, J.E. Coibamide A induces mTOR-independent autophagy and cell death in human glioblastoma cells. PLoS ONE 2013, 8, e65250. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Xu, X.; Li, T.; Liu, Z. Shellfish toxins targeting voltage-gated sodium channels. Mar. Drugs 2013, 11, 4698–4723. [Google Scholar] [CrossRef] [Green Version]
- Davies-Coleman, M.T.; Dzeha, T.M.; Gray, C.A.; Hess, S.; Pannell, L.K.; Hendricks, D.T.; Arendse, C.E. Isolation of homodolastatin 16, a new cyclic depsipeptide from a Kenyancollection of Lyngbya majuscula. J. Nat. Prod. 2003, 66, 712–715. [Google Scholar] [CrossRef]
- Taori, K.; Paul, V.J.; Luesch, H. Structure and activity of largazole, a potent antiproliferative agent from the Floridian marine cyanobacterium Symploca sp. J. Am. Chem. Soc. 2008, 130, 1806–1807. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Salvador, L.A.; Byeon, S.; Ying, Y.; Kwan, J.C.; Law, B.K.; Hong, J.; Luesch, H. Anticolon cancer activity of largazole, a marine-derived tunable histone deacetylase inhibitor. J. Pharmacol. Exp. Ther. 2010, 335, 351–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.K.; Choi, M.C.; Seo, C.H.; Park, Y. Therapeutic properties and biological benefits of marine-derived anticancer peptides. Int. J. Mol. Sci. 2018, 19, 919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, B.N.; McPhail, K.L.; Gross, H.; Goeger, D.E.; Mooberry, S.L.; Gerwick, W.H. Isolation and structure of five lyngbyabellin derivatives from a Papua New Guinea collection of the marine cyanobacterium Lyngbya majuscula. Tetrahedron 2005, 61, 11723–11729. [Google Scholar] [CrossRef]
- Choi, H.; Mevers, E.; Byrum, T.; Valeriote, F.A.; Gerwick, W.H. Lyngbyabellins K–N from Two Palmyra Atoll Collections of the marine cyanobacterium Moorea bouillonii. Eur. J. Org. Chem. 2012, 2012, 5141–5150. [Google Scholar] [CrossRef]
- Pettit, G.R.; Hogan, F.; Xu, J.P.; Tan, R.; Nogawa, T.; Cichacz, Z.; Pettit, R.K.; Du, J.; Ye, Q.H.; Cragg, G.M.; et al. Antineoplastic agents. 536. New sources of naturally occurring cancer cellgrowth inhibitors from marine organisms, terrestrial plants, and microorganisms. J. Nat. Prod. 2008, 71, 438–444. [Google Scholar] [CrossRef]
- Williams, P.G.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Isolation and structure determination of obyanamide, a novel cytotoxic cyclic depsipeptide from the marine cyanobacterium Lyngbya confervoides. J. Nat. Prod. 2002, 65, 29–31. [Google Scholar] [CrossRef]
- Williams, P.G.; Yoshida, W.Y.; Quon, M.K.; Moore, R.E.; Paul, V.J. The structure of palau’amide, a potent cytotoxin from a species of the marine cyanobacterium Lyngbya. J. Nat. Prod. 2003, 66, 1545–1549. [Google Scholar] [CrossRef]
- Taniguchi, M.; Nunnery, J.K.; Engene, N.; Esquenazi, E.; Byrum, T.; Dorrestein, P.C.; Gerwick, W.H. Palmyramide A, a cyclic depsipeptide from a Palmyra Atoll collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 393–398. [Google Scholar] [CrossRef] [Green Version]
- Montaser, R.; Paul, V.J.; Luesch, H. Pitipeptolides C–F, antimycobacterial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula from Guam. Phytochemistry 2011, 72, 2068–2074. [Google Scholar] [CrossRef] [Green Version]
- Luesch, H.; Pangilinan, R.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Pitipeptolides A and B, new cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2001, 64, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Montaser, R.; Abboud, K.A.; Paul, V.J.; Luesch, H. Pitiprolamide, a proline-rich dolastatin 16 analogue from the marine cyanobacterium Lyngbya majuscula from Guam. J. Nat. Prod. 2011, 74, 109–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, P.G.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Tasipeptins A and B: New cytotoxicdepsipeptides from the marine cyanobacterium Symploca sp. J. Nat. Prod. 2003, 66, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.G.; Yoshida, W.Y.; Quon, M.K.; Moore, R.E.; Paul, V.J. Ulongapeptin, a cytotoxiccyclicdepsipeptide from a Palauan marine cyanobacterium Lyngbya sp. J. Nat. Prod. 2003, 66, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Salvador, L.A.; Biggs, J.S.; Paul, V.J.; Luesch, H. Veraguamides A−G, cyclic hexadepsipeptides from a dolastatin 16-producing cyanobacterium Symploca cf. hydnoides from Guam. J. Nat. Prod. 2011, 74, 917–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mevers, E.; Liu, W.T.; Engene, N.; Mohimani, H.; Byrum, T.; Pevzner, P.A.; Dorrestein, P.C.; Spadafora, C.; Gerwick, W.H. Cytotoxic veraguamides, alkynyl bromide-containing cyclicdepsipeptides from the marine cyanobacterium cf. Oscillatoria margaritifera. J. Nat. Prod. 2011, 74, 928–936. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Goeger, D.; Maier, C.S.; Gerwick, W.H. The wewakpeptins, cyclic depsipeptides from aPapua New Guinea collection of the marine cyanobacterium Lyngbya semiplena. J. Org. Chem. 2005, 70, 3133–3139. [Google Scholar] [CrossRef]
- Golakoti, T.; Yoshida, W.Y.; Chaganty, S.; Moore, R.E. Isolation and structure determination of nostocyclopeptides A1 and A2 from the terrestrial cyanobacterium Nostoc sp. ATCC53789. J. Nat. Prod. 2001, 64, 54–59. [Google Scholar] [CrossRef]
- Williams, P.G.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Tasiamide, a cytotoxic peptide from the marine cyanobacterium Symploca sp. J Nat. Prod. 2002, 65, 1336–1339. [Google Scholar] [CrossRef]
- Simmons, T.L.; McPhail, K.L.; Ortega-Barria, E.; Mooberry, S.L.; Gerwick, W.H. Belamide A, a new antimitotic tetrapeptide from a Panamanian marine cyanobacterium. Tetrahedron Lett. 2006, 47, 3387–3390. [Google Scholar] [CrossRef]
- Teruya, T.; Sasaki, H.; Fukazawa, H.; Suenaga, K. Bisebromoamide, a potent cytotoxic peptide from the marine cyanobacterium Lyngbya sp.: Isolation, stereostructure, and biological activity. Org. Lett. 2009, 11, 5062–5065. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Teruya, T.; Fukazawa, H.; Suenaga, K. Revised structure and structure–activity relationship of bisebromoamide and structure of norbisebromoamide from the marine cyanobacterium Lyngbya sp. Tetrahedron 2011, 67, 990–994. [Google Scholar] [CrossRef]
- Jimenez, J.I.; Scheuer, P.J. New lipopeptides from the Caribbean cyanobacterium Lyngbyamajuscula. J. Nat. Prod. 2001, 64, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.B.; Liu, Y.; Coothankandaswamy, V.; Mahdi, F.; Jekabsons, M.B.; Gerwick, W.H.; Valeriote, F.A.; Zhou, Y.D.; Nagle, D.G. Kalkitoxin inhibits angiogenesis, disrupts cellular hypoxic signaling, and blocks mitochondrial electron transport in tumor cells. Mar. Drugs 2015, 13, 1552–1568. [Google Scholar] [CrossRef]
- Wrasidlo, W.; Mielgo, A.; Torres, V.A.; Barbero, S.; Stoletov, K.; Suyama, T.L.; Klemke, R.L.; Gerwick, W.H.; Carson, D.A.; Stupack, D.G. The marine lipopeptide somocystinamide a triggers apoptosis via caspase 8. Proc. Natl. Acad. Sci. USA 2008, 105, 2313–2318. [Google Scholar] [CrossRef] [Green Version]
- Malloy, K.L.; Villa, F.A.; Engene, N.; Matainaho, T.; Gerwick, L.; Gerwick, W.H. Malyngamide 2, an oxidized lipopeptide with nitric oxide inhibiting activity from a Papua New Guinea marine cyanobacterium. J. Nat. Prod. 2011, 74, 95–98. [Google Scholar] [CrossRef] [Green Version]
- Gross, H.; McPhail, K.L.; Goeger, D.E.; Valeriote, F.A.; Gerwick, W.H. Two cytotoxic stereo isomers of malyngamide C, 8-epi-malyngamide C and 8-O-acetyl-8-epi-malyngamide C, from the marine cyanobacterium Lyngbya majuscula. Phytochemistry 2010, 71, 1729–1735. [Google Scholar] [CrossRef] [Green Version]
- Horgen, F.D.; Kazmierski, E.B.; Westenburg, H.E.; Yoshida, W.Y.; Scheuer, P.J. Malevamide D: Isolation and structure determination of an isodolastatin H analogue from the marine cyanobacterium Symploca hydnoides. J. Nat. Prod. 2002, 65, 487–491. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Tidgewell, K.; Capson, T.L.; Engene, N.; Almanza, A.; Schemies, J.; Jung, M.; Gerwick, W.H. Malyngolide Dimer, a bioactive symmetric cyclodepside from the Panamanian marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 709–711. [Google Scholar] [CrossRef] [Green Version]
- Foster, B.J.; Fortuna, M.; Wiegand, R.A.; Valeriote, F.A. Cryptophycin 1 cellular levels and effects in vitro using L1210 cells. Investig. New Drugs 1998, 16, 199–204. [Google Scholar] [CrossRef]
- Kerksiek, K.; Mejillano, M.R.; Schwartz, R.E.; Georg, G.I.; Himes, R.H. Interaction of cryptophycin 1 with tubulin and microtubules. FEBS Lett. 1995, 377, 59–61. [Google Scholar] [CrossRef] [Green Version]
- Weiss, C.; Figueras, E.; Borbely, A.N.; Sewald, N. Cryptophycins: Cytotoxic cyclodepsipeptides with potential for tumor targeting. J. Pept. Sci. 2017, 23, 514–531. [Google Scholar] [CrossRef]
- Mooberry, S.L.; Busquets, L.; Tien, G. Induction of apoptosis by cryptophycin 1, a new antimicrotubule agent. Int. J. Cancer 1997, 73, 440–448. [Google Scholar] [CrossRef]
- Field, J.J.; Kanakkanthara, A.; Miller, J.H. Microtubule-targeting agents are clinically successful due to both mitotic and interphase impairment of microtubule function. Bioorg. Med. Chem. 2014, 22, 5050–5059. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Rottmann, M.; Tan, L.T. Lagunamides A and B: Cytotoxic and antimalarial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 1810–1814. [Google Scholar] [CrossRef]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Rottmann, M.; Chan, K.P.; Chen, D.Y.K.; Tan, L.T. Lagunamide C, a cytotoxic cyclodepsipeptide from the marine cyanobacterium Lyngbya majuscula. Phytochemistry 2011, 72, 2369–2375. [Google Scholar] [CrossRef]
- Teruya, T.; Sasaki, H.; Kitamura, K.; Nakayama, T.; Suenaga, K. Biselyngbyaside, a macrolideglycoside from the marine cyanobacterium Lyngbya sp. Org. Lett. 2009, 11, 2421–2424. [Google Scholar] [CrossRef]
- Morita, M.; Ohno, O.; Teruya, T.; Yamori, T.; Inuzuka, T.; Suenaga, K. Isolation and structures of biselyngbyasides B, C, and D from the marine cyanobacterium Lyngbya sp., and the biological activities of biselyngbyasides. Tetrahedron 2012, 68, 5984–5990. [Google Scholar] [CrossRef]
- Watanabe, A.; Ohno, O.; Morita, M.; Inuzuka, T.; Suenaga, K. Structures and biological activities of novel biselyngbyaside analogs isolated from the marine cyanobacterium Lyngbya sp. Bull. Chem. Soc. Jpn. 2015, 88, 1256–1264. [Google Scholar] [CrossRef]
- Luesch, H.; Yoshida, W.Y.; Harrigan, G.G.; Doom, J.P.; Moore, R.E.; Paul, V.J. Lyngbyaloside B, a new glycoside macrolide from a Palauan marine cyanobacterium, Lyngbya sp. J. Nat. Prod. 2002, 65, 1945–1948. [Google Scholar] [CrossRef]
- Matthew, S.; Salvador, L.A.; Schupp, P.J.; Paul, V.J.; Luesch, H. Cytotoxic halogenated macrolides and modified peptides from the apratoxin-producing marine cyanobacterium Lyngbya bouillonii from Guam. J. Nat. Prod. 2010, 73, 1544–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohno, O.; Watanabe, A.; Morita, M.; Suenaga, K. Biselyngbyolide B, a novel ER stress-inducer isolated from the marine cyanobacterium Lyngbya sp. Chem. Lett. 2014, 43, 287–289. [Google Scholar] [CrossRef]
- Iwasaki, A.; Teruya, T.; Suenaga, K. Isolation and structure of koshikalide, a 14-membered macrolide from the marine cyanobacterium Lyngbya sp. Tetrahedron Lett. 2010, 51, 959–960. [Google Scholar] [CrossRef]
- Barchi, J.J.; Moore, R.E.; Patterson, G.M.L. Acutiphycin and 20,21-didehydroacutiphycin, new antineoplastic agents from the cyanophyte Oscillatoria acutissima. J. Am. Chem. Soc. 1984, 106, 8193–8197. [Google Scholar] [CrossRef]
- Tan, L.T.; Márquez, B.L.; Gerwick, W.H. Lyngbouilloside, a novel glycosidic macrolide from the marine cyanobacterium Lyngbya bouillonii. J. Nat. Prod. 2002, 65, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Cummings, S.; Lee, J.; Moss, N.; Glukhov, E.; Valeriote, F.A.; Gerwick, L.; Gerwick, W.H. Isolation of polycavernoside D from a marine cyanobacterium. Environ. Sci. Technol. Lett. 2015, 2, 166–170. [Google Scholar] [CrossRef]
- Patterson, G.M.L.; Carmeli, S. Biological effects of tolytoxin (6-hydroxy-7-O-methyl-scytophycin b), a potent bioactive metabolite from cyanobacteria. Arch. Microbiol. 1992, 157, 406–410. [Google Scholar] [CrossRef]
- Carmeli, S.; Moore, R.E.; Patterson, G.M.L. Tolytoxin and new scytophycins from three species of Scytonema. J. Nat. Prod. 1990, 53, 1533–1542. [Google Scholar] [CrossRef]
- MacMillan, J.B.; Molinski, T.F. Caylobolide A, a unique 36-membered macrolactone from a Bahamian Lyngbyamajuscula. Org. Lett. 2002, 4, 1535–1538. [Google Scholar] [CrossRef]
- Salvador, L.A.; Paul, V.J.; Luesch, H. Caylobolide B, a macrolactone from symplostatin1-producing marine cyanobacteria Phormidium sp., from Florida. J. Nat. Prod. 2010, 73, 1606–1609. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.T.; More, S.V.; Lu, I.H.; Hsu, J.C.; Chen, T.J.; Jen, Y.C.; Lu, C.K.; Li, W.S. Isomalyngamide A, A-1 and their analogs suppress cancer cell migration in vitro. Eur. J. Med. Chem. 2011, 46, 3810–3819. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.J.; Marquez, B.L.; Nogle, L.M.; McPhail, K.; Goeger, D.E.; Roberts, M.A.; Gerwick, W.H. Structure and biosynthesis of the jamaicamides, new mixed polyketide-peptide neurotoxins from the marine cyanobacterium Lyngbyamajuscula. Chem. Biol. 2004, 11, 817–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, C.S.; Capper, E.A.; Roshak, A.K.; Marquez, B.; Grace, K.; Gerwick, W.H.; Jacobs, R.S.; Marshall, L.A. Scytonemin—A marine natural product inhibitor of kinases key in hyperproliferative inflammatory diseases. Inflam. Res. 2002, 51, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, C.S.; Capper, E.A.; Roshak, A.K.; Marquez, B.; Eichman, C.; Jackson, J.R.; Mattern, M.; Gerwick, W.H.; Jacobs, R.S.; Marshall, L.A. The identification and characterization of the marine natural product scytonemin as a novel antiproliferative pharmacophore. J. Pharmacol. Exp. Ther. 2002, 303, 858–866. [Google Scholar] [CrossRef]
- Banker, R.; Carmeli, S. Tenuecyclamides A-D, cyclic hexapeptides from the cyanobacterium Nostoc spongiaeforme var.tenue. J. Nat. Prod. 1998, 61, 1248–1251. [Google Scholar] [CrossRef]
- Rickards, R.W.; Rothschild, J.M.; Willis, A.C.; de Chazal, N.M.; Kirk, J.; Kirk, K.; Saliba, K.J.; Smith, G.D. Calothrixins A and B, novel pentacyclic metabolites from Calothrix cyanobacteria with potent activity against malaria parasites and human cancer cells. Tetrahedron 1999, 55, 13513–13520. [Google Scholar] [CrossRef]
- Xu, S.; Nijampatnam, B.; Dutta, S.; Velu, S.E. Cyanobacterial metabolite calothrixins: Recent advances in synthesis and biological evaluation. Mar. Drugs 2016, 14, 17. [Google Scholar] [CrossRef] [Green Version]
- Khan, Q.A.; Lu, J.; Hecht, S.M. Calothrixins, a new class of human DNA topoisomerase Ipoisons. J. Nat. Prod. 2009, 72, 438–442. [Google Scholar] [CrossRef]
- Lavrentyev, P.J.; Franzè, G.; Pierson, J.J.; Stoecker, D.K. The effect of dissolved polyunsaturated aldehydes on microzooplankton growth rates in the Chesapeake Bay and Atlantic coastal waters. Mar. Drugs 2015, 13, 2834–2856. [Google Scholar] [CrossRef] [Green Version]
- Miralto, A.; Barone, G.; Romano, G.; Poulet, S.A.; Ianora, A.; Russo, G.L.; Buttino, I.; Mazzarella, G.; Laabir, M.; Cabrini, M.; et al. The insidious effect of diatoms on copepod reproduction. Nature 1999, 402, 173–176. [Google Scholar] [CrossRef]
- Sansone, C.; Braca, A.; Ercolesi, E.; Romano, G.; Palumbo, A.; Casotti, R.; Francone, M.; Ianora, A. Diatom-derived polyunsaturated aldehydes activate cell death in human cancer cell lines but not normal cells. PLoS ONE 2014, 9, e101220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanda, M.; Merghoub, N.; EL Arroussi, H. Microalgae polysaccharides: The new sustainable bioactive products for the development of plant bio-stimulants? World J. Microbiol. Biotechnol. 2019, 35, 177. [Google Scholar] [CrossRef] [PubMed]
- Kusaikin, M.I.; Ermakova, S.P.; Shevchenko, N.M.; Isakov, V.V.; Gorshkov, A.G.; Vereshchagin, A.L.; Grachev, M.A.; Zvyagintseva, T.N. Structural characteristics and antitumor activity of a new chrysolaminaran from the diatom alga Synedra acus. Chem. Nat. Compd. 2010, 46, 1–4. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.J.; Kim, S.M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Park, Y.I. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, S.; Sokolova, R.; Kim, S.M.; Um, B.H.; Isakov, V.; Zvyagintseva, T. Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: Structural characteristics and anticancer activity. Appl. Biochem. Biotechnol. 2011, 164, 841–850. [Google Scholar] [CrossRef]
- Alekseyenko, T.V.; Zhanayeva, S.Y.; Venediktova, A.A.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Besednova, N.N.; Korolenko, T.A. Antitumor and antimetastatic activity of fucoidan, a sulfated polysaccharide isolated from the Okhotsk sea Fucus evanescens brown alga. Bull. Exp. Biol. Med. 2007, 143, 730–732. [Google Scholar] [CrossRef]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Induction of apoptosis by low-molecular-weight fucoidan through calcium-and caspase-dependent mitochondrial pathways in MDA-MB-231 breast cancer cells. Biosci. Biotechnol. Biochem. 2013, 77, 235–242. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.S.; Kim, E. Fucoidan from seaweed Fucusvesiculosus inhibits migration and invasion of human lung cancer cell via PI3K-Akt-mTOR pathways. PLoS ONE 2012, 7, e50624. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ermakova, S.P.; Zvyagintseva, T.N.; Kang, K.W.; Dong, Z.; Choi, H.S. Inhibitory effects of fucoidan on activation of epidermal growth factor receptor and cell transformation in JB6 Cl41 cells. Food Chem. Toxicol. 2008, 46, 1793–1800. [Google Scholar] [CrossRef]
- Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003, 65, 173–179. [Google Scholar] [CrossRef]
- Nagamine, T.; Hayakawa, K.; Kusakabe, T.; Takada, H.; Nakazato, K.; Hisanaga, E.; Iha, M. Inhibitory effect of fucoidan on Huh7 hepatoma cells through downregulation of CXCL12. Nutr. Cancer 2009, 61, 340–347. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Lin, T.Y.; Hwang, P.A.; Chen, R.H.; Tsao, S.M.; Hsu, J. Fucoidan induces changes in the epithelial-mesenchymal transition and decreases metastasis by enhancing ubiquitin dependent TGFβ receptor degradation in breast cancer. Carcinogenesis 2013, 34, 874–884. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Ermakova, S.P.; Zvyagintseva, T.N.; Stonik, V.A. Anticancer and cancer preventive properties of marine polysaccharides: Some results and prospects. Mar. Drugs 2013, 11, 4876–4901. [Google Scholar] [CrossRef] [Green Version]
- Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Isakov, V.V.; Dubrovskaya, Y.V.; Kusaykin, M.I.; Um, B.H.; Zvyagintseva, T.N. Structure, enzymatic transformation and anticancer activity of branched high molecular weight laminaran from brown alga Eisenia bicyclis. Carbohydr. Polym. 2014, 99, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Song, G.; Lee, J.Y.; Hong, T.; Chang, M.J.; Lim, W. Laminarin-derived from brown algae suppresses the growth of ovarian cancer cells via mitochondrial dysfunction and ER stress. Mar. Drugs 2020, 18, 152. [Google Scholar] [CrossRef] [Green Version]
- Malyarenko, O.S.; Usoltseva, R.V.; Zvyagintseva, T.N.; Ermakova, S.P. Laminaran from brown alga Dictyota dichotoma and its sulfated derivative as radioprotectors and radiosensitizers in melanoma therapy. Carbohydr. Polym. 2019, 206, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Menshova, R.V.; Anastyuk, S.D.; Ermakova, S.P.; Shevchenko, N.M.; Isakov, V.I.; Zvyagintseva, T.N. Structure and anticancer activity in vitro of sulfated galactofucan from brown alga Alaria angusta. Carbohydr. Polym. 2015, 132, 118–125. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Anastyuk, S.D.; Shevchenko, N.M.; Zvyagintseva, T.N.; Ermakova, S.P. The comparison of structure and anticancer activity in vitro of polysaccharides from brown algae Alaria marginata and A. angusta. Carbohydr. Polym. 2016, 153, 258–265. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Shevchenko, N.M.; Malyarenko, O.S.; Ishina, I.A.; Ivannikova, S.I.; Ermakova, S.P. Structure and anticancer activity of native and modified polysaccharides from brown alga Dictyota dichotoma. Carbohydr. Polym. 2018, 180, 21–28. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Anastyuk, S.D.; Shevchenko, N.M.; Surits, V.V.; Silchenko, A.S.; Isakov, V.V.; Zvyagintseva, T.N.; Thinh, P.D.; Ermakova, S.P. Polysaccharides from brown algae Sargassum duplicatum: The structure and anticancer activity In vitro. Carbohydr. Polym. 2017, 175, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.F.; Ji, Y.B. Laminarin-induced apoptosis in human colon cancer LoVo cells. Oncol. Lett. 2014, 7, 1728–1732. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.B.; Ji, C.F.; Zhang, H. Laminarin induces apoptosis of human colon cancer LoVocells through a mitochondrial pathway. Molecules 2012, 17, 9947–9960. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.F.; Ji, Y.B.; Meng, D.Y. Sulfated modification and anti-tumor activity of laminarin. Exp. Ther. Med. 2013, 6, 1259–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.K.; Kim, I.H.; Kim, J.; Nam, T.J. Induction of apoptosis and the regulation of ErbB signaling by laminarin in HT-29 human colon cancer cells. Int. J. Mol. Med. 2013, 32, 291–295. [Google Scholar] [CrossRef] [Green Version]
- Henríquez, V.; Escobar, C.; Galarza, J.; Gimpel, J. Carotenoids in Microalgae. In Carotenoids in Nature; Stange, C., Ed.; Springer: Cham, Switzerland, 2016; Volume 79. [Google Scholar]
- De Jesus Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef]
- Pasquet, V.; Morisset, P.; Ihammouine, S.; Chepied, A.; Aumailley, L.; Berard, J.B.; Serive, B.; Kaas, R.; Lanneluc, I.; Thiery, V.; et al. Antiproliferative activity of violaxanthin isolated from bioguided fractionation of Dunaliella tertiolecta extracts. Mar. Drugs 2011, 9, 819–831. [Google Scholar] [CrossRef] [Green Version]
- Molnár, J.; Engi, H.; Hohmann, J.; Molnar, P.; Deli, J.; Wesolowska, O.; Michalak, K.; Wang, Q. Reversal of multidrug resistance by natural substances from plants. Curr. Top. Med. Chem. 2010, 10, 1757–1768. [Google Scholar]
- Gyémánt, N.; Tanaka, M.; Molnár, P.; Deli, J.; Mándoky, L.; Molnár, J. Reversal of multidrug resistance of cancer cells in vitro: Modification of drug resistance by selected carotenoids. Anticancer Res. 2006, 26, 367–374. [Google Scholar]
- Saini, R.K.; Moon, S.H.; Gansukh, E.; Keum, Y.S. An efficient one-step scheme for the purification of major xanthophyll carotenoids from lettuce, and assessment of their comparative anticancer potential. Food Chem. 2018, 266, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Wanezaki, S.; Miyauchi, K.; Kurihara, H.; Kohno, H.; Kawabata, J.; Odashima, S.; Takahashi, K. Apoptosis-inducing effect of fucoxanthin on human leukemia cell line HL-60. Food Sci. Technol. Res. 1999, 5, 243–246. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.R.; Hosokawa, M.; Miyashita, K. Fucoxanthin: A marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar. Drugs 2013, 11, 5130–5147. [Google Scholar] [CrossRef] [Green Version]
- Kotake-nara, E.; Kushiro, M.; Zhang, H.; Sugawara, T.; Miyashita, K.; Nagao, A. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr. 2001, 131, 3303–3306. [Google Scholar] [CrossRef] [PubMed]
- Kadekaru, T.; Toyama, H.; Yasumoto, T. Safety evaluation of Fucoxanthin purified from Undaria pinnatifida. J. Jpn. Soc. Food Sci. 2008, 55, 304–308. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, C.; Tafuku, S.; Kadekaru, T.; Sawada, S.; Tomita, M.; Okudaira, T.; Nakazato, T.; Toda, T.; Uchihara, J.N.; Taira, N.; et al. Antiadult T-cell leukemia effects of brown algae fucoxanthin and its deacetylated product, fucoxanthinol. Int. J. Cancer 2008, 123, 2702–2712. [Google Scholar] [CrossRef]
- Kong, Z.; Kao, N.; Hu, J.; Wu, C. Fucoxanthin richbrown algae extract decreases inflammation and attenuates colitis-associated colon cancer in mice. J. Food Nutr. Res. 2016, 4, 137–147. [Google Scholar]
- Ganesan, P.; Noda, K.; Manabe, Y.; Ohkubo, T.; Tanaka, Y.; Maoka, T.; Sugawara, T.; Hirata, T. Siphonaxanthin, a marine algal carotenoids from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. Biochim. Biophys. Acta 2011, 1810, 497–503. [Google Scholar] [CrossRef]
- Ganesan, P.; Matsubara, K.; Ohkubo, T.; Tanaka, Y.; Noda, K.; Sugawara, T.; Hirata, T. Anti-angiogenic effect of siphonaxanthin from green alga, Codium fragile. Phytomedicine 2010, 17, 1140–1144. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, P.; Matsubara, K.; Sugawara, T.; Hirata, T. Marine algal carotenoids inhibit angiogenesis by down-regulating FGF-2-mediated intracellular signals in vascular endothelial cells. Mol. Cell Biochem. 2013, 380, 1–9. [Google Scholar] [CrossRef]
- Sugawara, T.; Ganesan, P.; Li, Z.; Manabe, Y.; Hirata, T. Siphonaxanthin, a green algal carotenoid, as a novel functional compound. Mar. Drugs 2014, 12, 3660–3668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Lena, G.; Casini, I.; Lucarini, M.; Lombardi-Boccia, G. Carotenoid profiling of five microalgae species from large-scale production. Food Res. Int. 2019, 120, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Grudzinski, W.; Piet, M.; Luchowski, R.; Reszczynska, E.; Welc, R.; Paduch, R.; Gruszecki, W.I. Different molecular organization of two carotenoids, lutein and zeaxanthin, in human colon epithelial cells and colon adenocarcinoma cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 188, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Galasso, C.; Gentile, A.; Orefice, I.; Ianora, A.; Bruno, A.; Noonan, D.M.; Sansone, C.; Albini, A.; Brunet, C. Microalgal derivatives as potential nutraceutical and food supplements for human health: A focus on cancer prevention and interception. Nutrients 2019, 11, 1226. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Li, X.F.; Kang, K.H.; Ryu, B.; Kim, S.K. Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB Rep. 2014, 47, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Hussein, H.A.; Abdullah, M.A. Anticancer compounds derived from marine diatoms. Mar. Drugs 2020, 18, 356. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, K.W.; Ko, J.Y.; Lee, J.H.; Kwon, O.N.; Kim, S.W.; Jeon, Y.J. Apoptotic anticancer activity of a novel fatty alcohol ester isolated from cultured marine diatom, Phaeodactylum tricornutum. J. Funct. Foods 2014, 6, 231–240. [Google Scholar] [CrossRef]
- Maoka, T.; Tsushima, M.; Nishino, H. Isolation and characterization of dinochrome A and B, anti-carcinogenic active carotenoids from the fresh water red tide Peridinium bipes. Chem. Pharm. Bull. 2002, 50, 1630–1633. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Li, M.; Xu, B.; Zhu, X.; Deng, Z.; Lin, W. Proteasome and NF-κB inhibiting phaeophytins from the green alga Cladophora fascicularis. Molecules 2007, 12, 582–592. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.E.; Sturgeon, C.M.; Roberge, M.; Andersen, R.J. Nigricanosides A and B, antimitotic glycolipids isolated from the green alga Avrainvillea nigricans collected in Dominica. J. Am. Chem. Soc. 2007, 129, 5822–5823. [Google Scholar] [CrossRef]










| Class | Secondary Metabolite | Biological Source | Cell Lines Used | Effects and Mechanisms | IC50 Values | References |
|---|---|---|---|---|---|---|
| Anthracycline | Komodoquinone A(1) | Streptomyces sp. KS3 | Neuro 2A neuroblastoma cell | Neuritogenic activity, ↑cell differentiation | 1 μg/mL | [84] |
| Phenoxazin-3-one | Chandrananimycins A, B, C (2,3,4) | Actinomadura sp. | CCL HT29 (colon cancer); MEXF 514L (melanoma); LXFA 526L, LXFL 529L (lung cancer); CNCL SF268, LCL H460, MACL MCF-7 (breast cancer); PRCL PC3M, RXF 631L (kidney tumor cells) | Anti-tumor activity | ~1.4 μg/mL | [85] |
| Glycosilated polyketide | Ankaraholide A (5) | Geitlerinema sp. | NCI-H460; Neuro-2a; MDAMB- 435 cell lines | ┴ Proliferation; ↑cytotoxicity | 119; 262; 8.9 nM | [86] |
| Polyketide | Swinholide A (6) | Symploca cf. sp. | Several cancer cell lines | Antitumor activity; ┴ proliferation; ↑cytotoxicity; disruption of the actin cytoskeleton | 0.37 nM–1.0 μM | [86] |
| Pentapeptide | Symplostatin 1 (7) | Symploca hydnoides | MDA-MB-435 (breast cancer cell), SK-OV-3 (ovarian cancer cell), NCI/ADR (multidrug-resistance ovarian cancer cell), A-10 (smooth muscle cells), and HUVEC (Human umbilical vein endothelial cells); in vivo study (murine colon 38 and murine mammary 16/C carcinoma cells) | Antitumor activity; ↑phosphorylation of Bcl-2; ↑micronuclei formation, ↑caspase 3, ↑apoptosis, cell cycle arrest at G2/M Phase, ┴tubulin accumulation | 0.15 ± 0.03 nM; 0.09 ± 0.02 nM; 2.90 ± 0.64 nM; 1.8 ± 0.43 nM; 0.16 ± 0.02 nM | [72,87] |
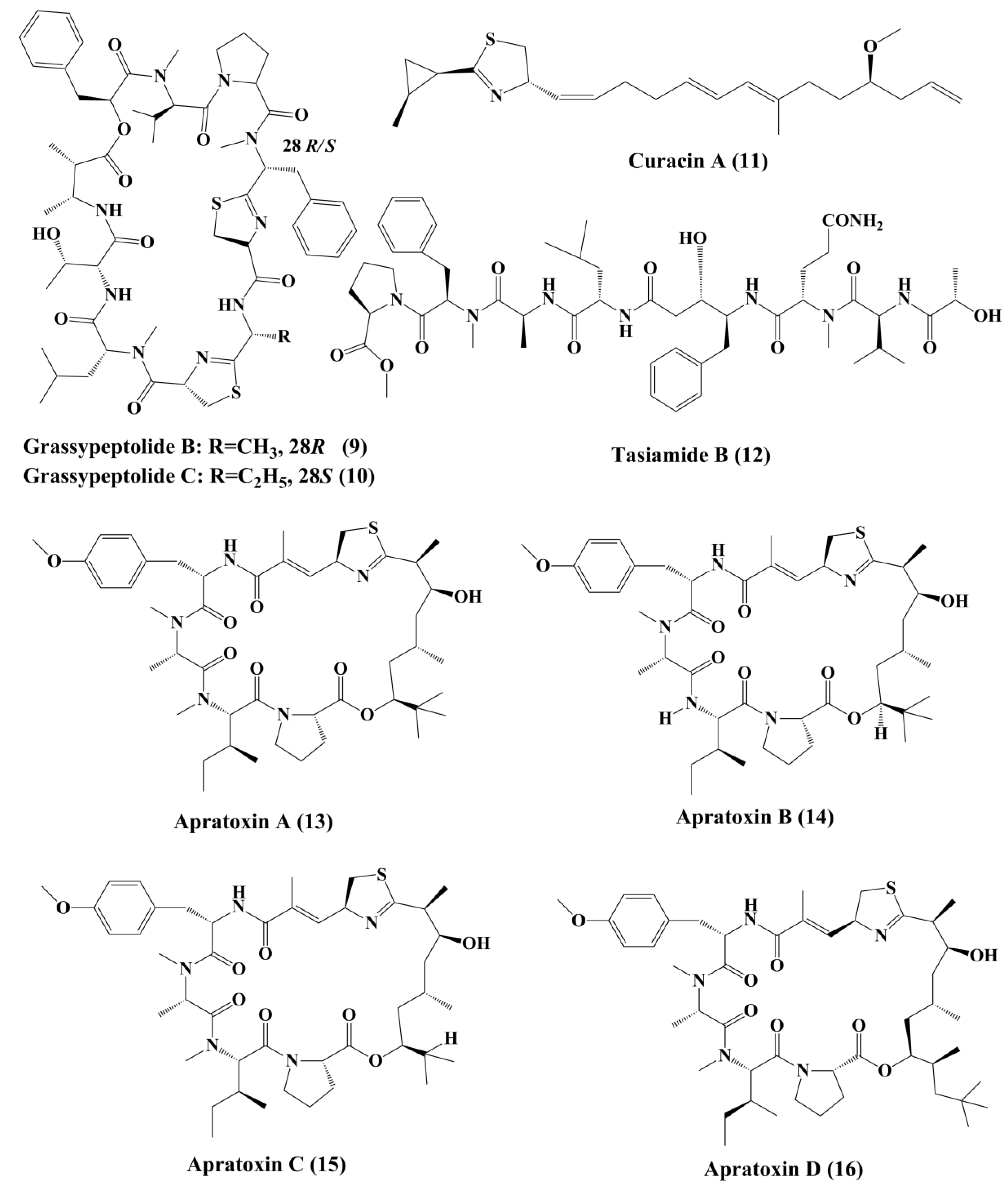
| Macrocyclic depsipeptide | Grassypeptolide, Grassypeptolide A, B and C (8, 9, 10) | Lyngbya confervoides | human osteosarcoma (U2OS), cervical carcinoma (HeLa), colorectal adenocarcinoma (HT29), and neuroblastoma (IMR-32); | Anticancer activity; ┴proliferation; Cell cycle arrest at G1 or G2/M Phase | 1–4.2 μM for grassypeptolide in all cell lines. Grassypeptolide A: 1.22 &1.01 μM in HT29 and Hela. Grassypeptolide B: 4.07 and 2.93 μM in HT29 and Hela. Grassypeptolide C: 76.7 and 44.6 nM HT29 and Hela. | [88,89] |
| ketopeptide | Curacin A (11) | Lyngbya majuscule | Non-small cell lung cancer cell line (A549) | Anticancer activity; ┴proliferation; ↑apoptosis; cell cycle arrest at G2/M Phase; binds to tubulin at colchicines binding site | 0.72 ± 0.02 μM | [90,91,92,93,94,95] |
| Linear peptide | Tasiamide B (12) | Symploca sp. | ĸB oral epidermoid cancer; human colon carcinoma (LoVo) cells | ┴Proliferation; ↑cytotoxicity | 0.48; 3.47 μg/mL | [96] |
| Cyclic depsipeptide | Apratoxin A (13) | Lyngbya majuscula | U2OS osteosarcoma; HeLa cervical carcinoma; in LoVo colon carcinoma; ĸB carcinoma cancer cells | ┴Secretory pathway; ┴cell cycle at G1 Phase; ↑cytotoxicity; ┴translocation of protein targeting Sec61α | 50; 2.2; 0.36; 0.52 nM | [97,98] |
| Apratoxin B (14) | Lyngbya sp. | ĸB oral epidermoid cancer and LoVo colon cancer lines | ↑Cytotoxicity | 21.3; 10.8 nM | [97] | |
| Apratoxin C (15) | Symploca cf. sp. | Several cancer cell lines | ↑Cytotoxicity | 1.0; 0.73 nM | [97] | |
| Apratoxin D (16) | Lyngbya majuscule; Lyngbya sordida | H-460 lung cancer | 2.6 nM | [98] | ||
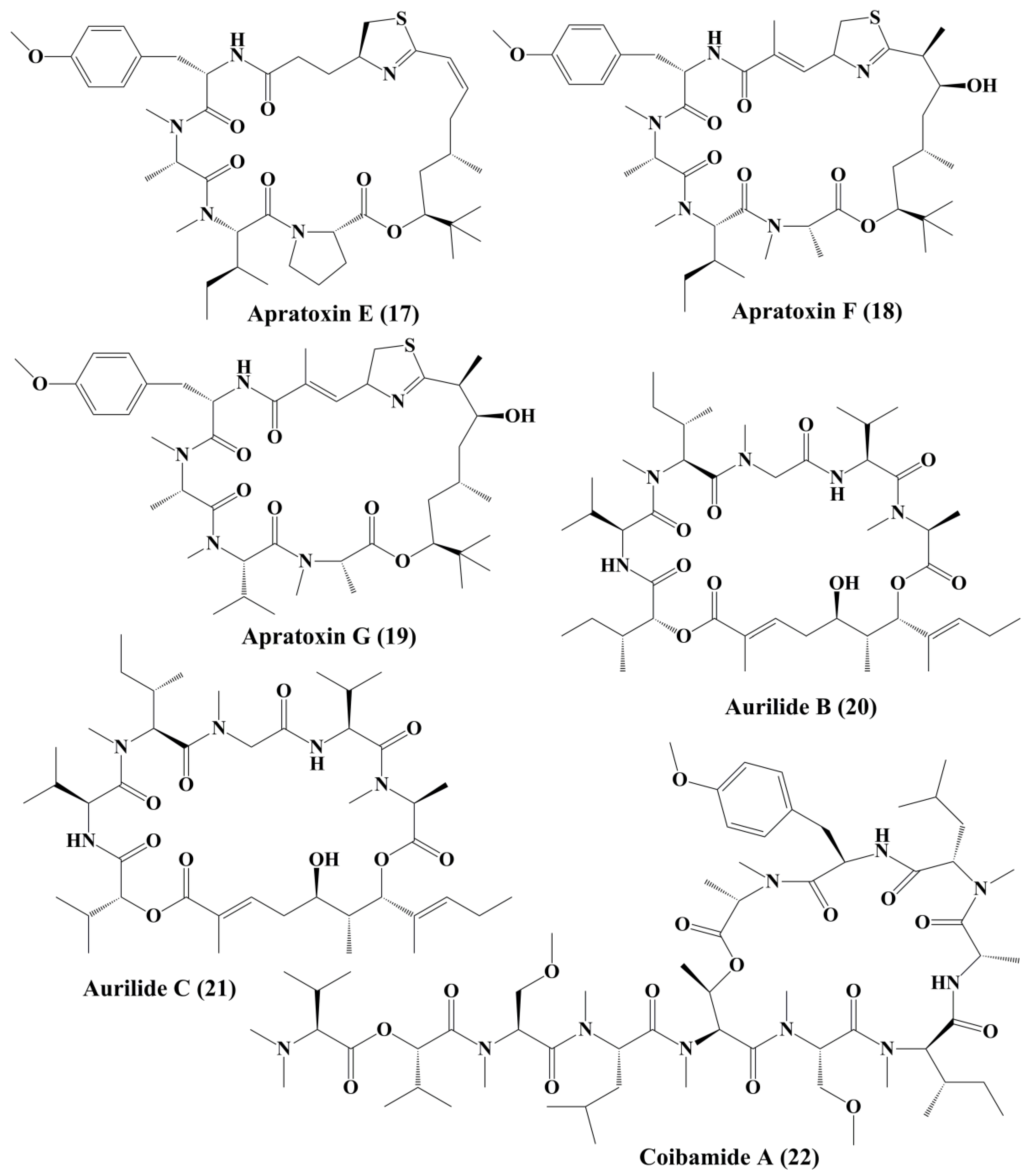
| Apratoxin E (17) | Lyngbya bouilloni | U2OS osteosarcoma, HT29 colon adenocarcinoma and HeLa epithelial carcinoma | ↑Antiproliferative Activity | 59; 21; 72 nM | [99] | |
| Apratoxin F (18) | Lyngbya sp. | H-460 lung cancer; HCT-116 colorectal cancer cells | ↑Cytotoxicity | 2; 36.7 nM | [100] | |
| Apratoxin G (19) | 14 nM; Not specified | |||||
| Aurilide B (20) | Lyngbya majuscula | NCI-H460 human lung tumor and neuro-2a mouse neuroblastoma cells | ↑Antiproliferative activity; ↑OPA1 synthesis, ↑apoptosis | 0.04; 0.01 µM | [101,102] | |
| Aurilide C (21) | Lyngbya majuscula | U2OS osteosarcoma, HT29 colon adenocarcinoma and HeLa epithelial carcinoma | 0.13; 0.05 µM | |||
| Coibamide A (22) | Leptolyngbya sp. | MDA-MB-231, melanoma LOX IMVI, leukemia HL-60 and astrocytoma SNB75 | ↑Cytotoxicity; ┴cell cycle at G1 Phase | 2.8; 7.4; 7.4 and 7.6 nM | [103] | |
| glioblastoma cell lines U87-MG and SF-295 | ↑Cytotoxicity | 20 nM | [104] | |||
| Normal human umbilical vein endothelial cells (HUVECs) | ┴Proliferation; ↓VEGFR2 | 0.3–3 nM | ||||
| Human U87-MG glioblastoma cells and SF-295 glioblastoma cells | ↑Cytotoxicity; ↑autophagy | 28.8, 96.2 nM | [105] | |||
| Hoiamide A (23) | Lyngbya majuscule, Phormidium gracile | H-460 lung cancer and neuro-2a mouse neuroblastoma | ↑Cytotoxicity; ↑neurotoxicity | 11.2; 2.1 μM | [106] | |
| Hoiamide B (24) | 8.3 μM; no effect on neuro-2a | |||||
| Homodolastatin 16 (25) | Lyngbya majuscule | WHCO1 and WHCO6 esophageal cancer; ME180 cervical cancer | ↑Apoptosis; ┴cell cycle at G2/M Phase; ↑cytotoxicity | 4.3 and 10.1; 8.3 µg/mL | [107] | |
| Largazole (26) | Symploca sp. | MDA-MB-23I breast cancer; U2OS osteosarcoma; colon HT29; neuroblastoma IMR-32; nontransformed murine mammary epithelial cells NMuMG; HCT-116 colorectal carcinoma | ↑Cytotoxicity; ┴tumor; cell cycle arrest at G2/M Phase; ┴HDAC | 7.7; 55; 12; 16; 122 nM; Not specified | [108,109] | |
| Lyngbyabellin A (27) | Lyngbya majuscula | ĸB nasopharyngeal carcinoma and LoVo colon adenocarcinoma | ↑Cytotoxicity; ┴tumor; cell cycle arrest at G2/M Phase; ↑actin polymerization | 0.03; 0.05 μg/mL | [110] | |
| Lyngbyabellin B (28) | Lyngbya majuscula | 0.10; 0.83 μg/mL | [110] | |||
| Lyngbyabellin E (29) | Lyngbya majuscula Symploca sp. | NCI-H460 human lung tumor and neuro-2a mouse neuroblastoma cells | ┴Tumor growth; ┴cell microfibrils network | 0.4; 1.2 μM | [111] | |
| Lyngbyabellin F (30) | Lyngbya majuscula | ↑Cytotoxicity | 1; 1.8 μM | |||
| Lyngbyabellin G (31) | Lyngbya majuscula | 2.2; 4.8 μM | [111] | |||
| Lyngbyabellin H (32) | 0.2; 1.4 μM | [111] | ||||
| Lyngbyabellin I (33) | Lyngbya majuscula | 1; 0.7 μM | [111] | |||
| Lyngbyabellin N (34) | Moorea bouillonii | HCT116 (colon cancer cell line) | Anticancer activity; ↑cytotoxicity | 40.9 ± 3.3 nM | [112] | |
| Majusculamide C (35) | Lyngbya majuscule | Ovarian carcinoma OVCAR-3, kidney cancer A498, lung cancer NCI-H460, colorectal cancer KM20L2; glioblastoma SF-295 | Anticancer activity; ↑cytotoxicity | 0.51; 0.058; 0.0032; 0.0013; 0.013 μg/mL | [110,113] | |
| Desmethoxymajusculamide C (36) | Lyngbya majuscule | HCT-116 human colon carcinoma cells | Selective antitumor activity | 20 nM | [110] | |
| Obyanamide (37) | Lyngbya confervoides | ĸB and LoVo cells | Anticancer activity | 0.58; 3.14 µg/mL | [114] | |
| Palau’amide (38) | Lyngbya confervoides | ĸB oral epidermoid cancer cells | Anticancer activity | 13 nM | [115] | |
| Palmyramide A (39) | Lyngbya majuscule | Neuro2a cells and human lung cell H-460 | Anticancer activity; ↑cytotoxicity; blocking the voltage regulated sodium channel | 17.2; 39.7 µM | [116] | |
| Pitipeptolide A (40) | Lyngbya majuscule | HT29 colon adenocarcinoma cancer cells, MCF-7 and LoVo colon cancer | Anticancer activity; ↑cytotoxicity | 13; 13 µM & 2.25 µg/mL | [117,118] | |
| Pitipeptolide B(41) | Lyngbya majuscula | HT29 colon adenocarcinoma cancer cells, MCF-7 and LoVo colon cancer | Anticancer activity; ↑cytotoxicity | 13; 11 µM; 1.95 µg/mL | [117,118] | |
| Pitiprolamide (42) | Lyngbya majuscula | HCT116 colorectal carcinoma and MCF7 breast adenocarcinoma | Anticancer activity, ↑cytotoxicity | 33; 33 µM | [119] | |
| Tasipeptins A (43) | Symploca sp. | ĸB oral epidermoid cancer | Anticancer activity, ↑cytotoxicity | 0.93 µM | [120] | |
| Tasipeptins B (44) | Symploca sp. | ĸB oral epidermoid | Anticancer activity, ↑cytotoxicity | 0.82 µM | [120] | |
| Ulongapeptin (45) | Lyngbya sp. | ĸB oral epidermoid cancer | Anticancer activity; ↑cytotoxicity | 0.63 µM | [121] | |
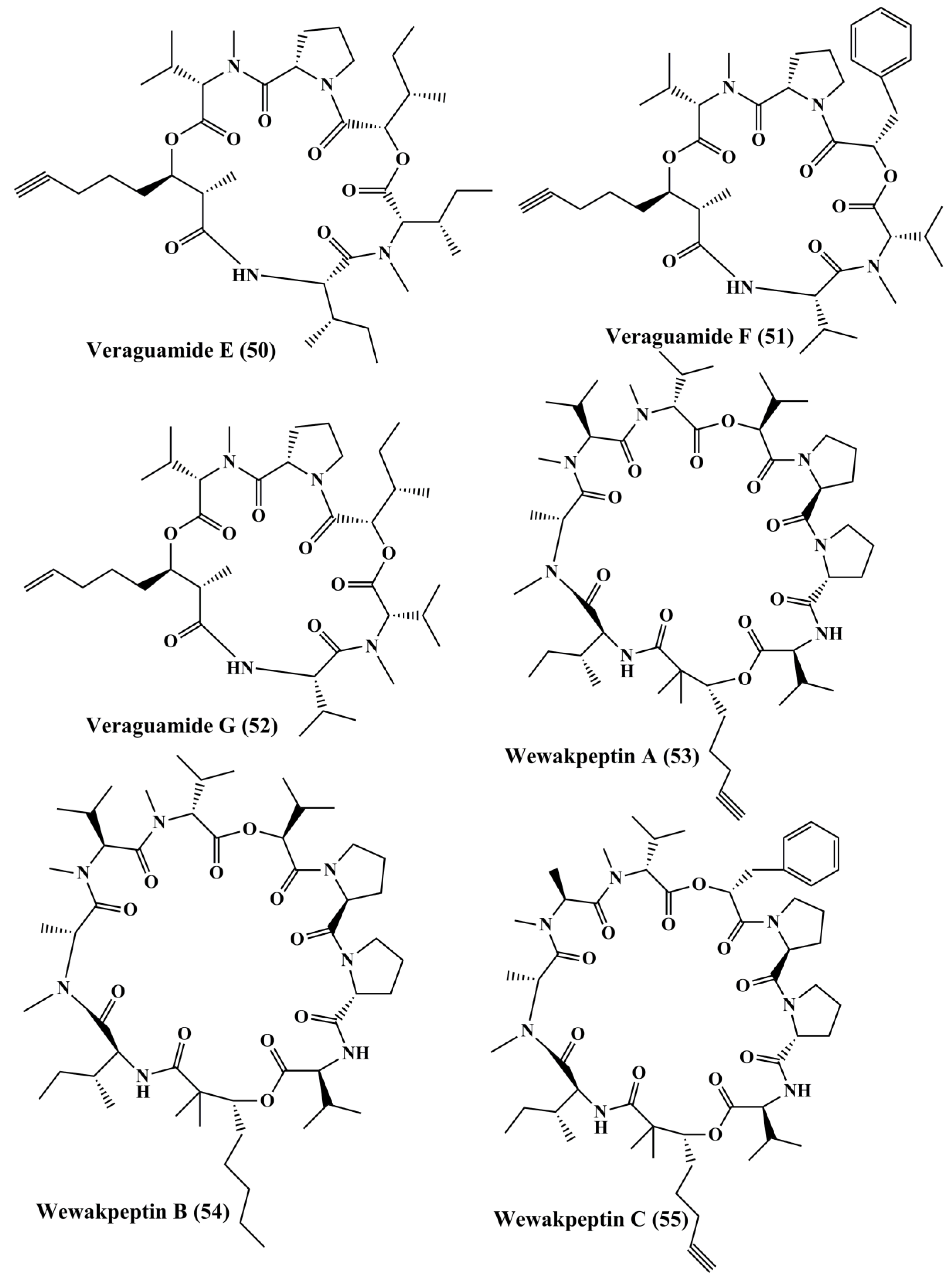
| Veraguamide A-G (46–52) | Symploca cf. hydnoides, Oscillatoria margaritifera | HT29 colon adenocarcinoma; HeLa cervical carcinoma | Anticancer activity; ↑cytotoxicity | 26; 2 µM & 141 nM; 30 & 17 µM; 5.8 & 6.1 µM; 0.84 & 0.54 µM; 1.5 & 0.83 µM; 49 & 49 µM; 2.7 & 2.3 µM | [122,123] | |
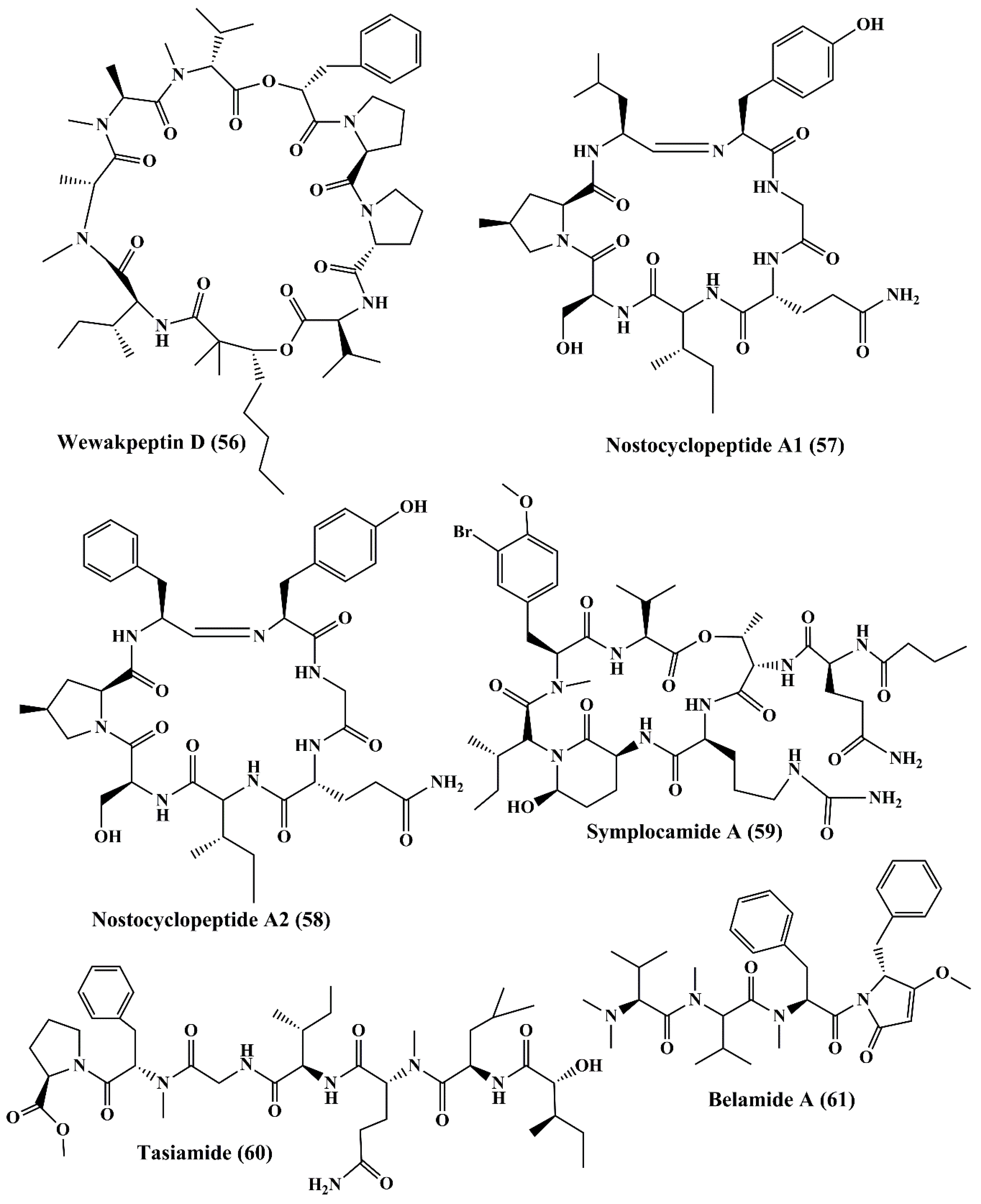
| Wewakpeptins A-D (53–56) | Lyngbya semiplena | H-460 lung cancer | Anticancer activity; ↑cytotoxicity | 0.4 µM | [124] | |
| Cyclic heptapeptides | Nostocyclopeptide A1 & A2 (57, 58) | Nostoc sp. | ĸB oral epidermoid cancer and LoVo colon carcinoma cell line | Anticancer activity; ↑cytotoxicity | 1 & 1 µM for both | [125] |
| Cyclopeptide | Symplocamide (59) | Symploca sp. | Non-small cell lung cancer cells H-460 and neuro-2a neuroblastoma cells | Anticancer activity; ↑cytotoxicity | 40; 29 nM | [110] |
| Cyclicpeptide | Tasiamide (60) | Symploca sp. | Human nasopharyngeal carcinoma (ĸB) and human colon carcinoma (LoVo) cells | Anticancer activity; ↑cytotoxicity | 0.48; 3.47 µg/mL | [126] |
| Linear tetrapeptide | Belamide A (61) | Symploca sp. | MCF7 breast cancer cell; HCT-116 colon cancer cell | Anticancer activity; ↑cytotoxicity; depolymerizing effect on microtubule in A-10 cells; antimitotic activity | 1.6 µM; 0.74 µM | [127] |
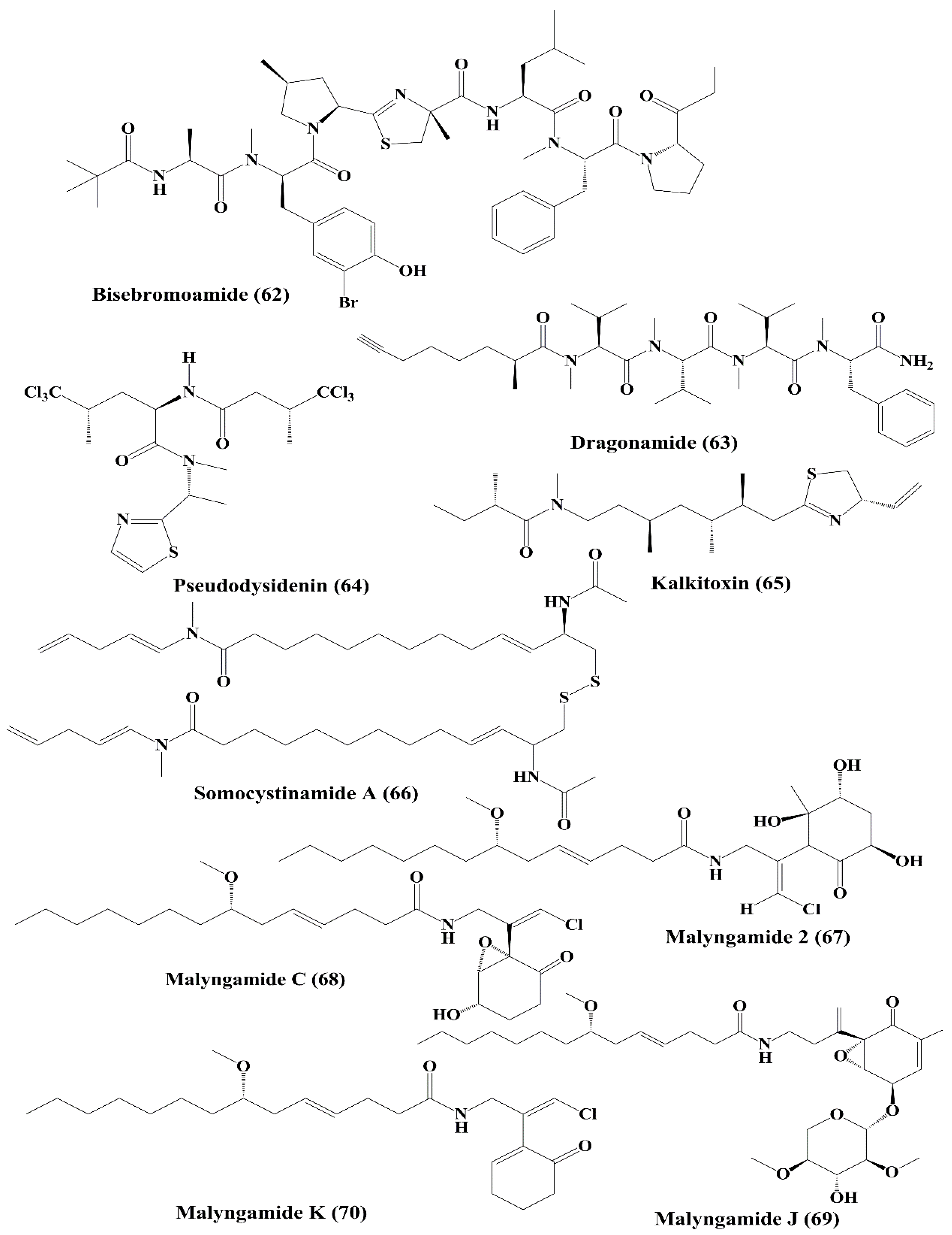
| Peptide | Bisebromoamide (62) | Lyngbya sp. | HeLa S3 cells; a panel of 39 human cancer cell lines of the Japanese Foundation for Cancer Research (JFCR39) Cancer Research | ↑Cytotoxicity; ┴protein kinases; ┴phosphorylation of ERK | 0.04 µg/mL; average 40 nM | [128,129] |
| Lipopeptides | Dragonamide, Pseudodysidenin (63, 64) | Lyngbya majuscula | P-388; A-549 lung epithelial adenocarcinoma, HT-29 colon adenocarcinoma; MEL-28 melanoma | Anticancer activity; ↑cytotoxicity | > 1 µg/mL | [130] |
| Lipopeptide | Kalkitoxin (65) | Phormidium sp. | HCT-116 colon cancer cell; T47D breast tumor cells | Anticancer activity; ↑cytotoxicity; ┴hypoxia-induced activation of HIF-1; ↓mitochondrial oxygen consumption at electron transport chain (ETC) complex I (NADH-ubiquinone oxidoreductase); blocking of VEGF | 2.7 nM; 5.6 nM | [131] |
| Lipopeptide | Somocystinamide A (66) | Lyngbya majuscula | Jurkat, CEM (leukemia), A549 (lung carcinoma), Molt4 (T cell leukemia), M21 melanoma, and U266 myeloma cell lines | ↑Cytotoxicity; ↑apoptosis via caspase 8 | 3; 14; 46; 60 nM; 1.3; 5.8 µM | [132] |
| Lipopeptide, Lyngbic acid derivative | Malyngamide 2 (67) | Lyngbya sordida | H-460 lung cancer | ↑Cytotoxicity | 27.3 µM | [133] |
| Malyngamide C, J, & K (68, 69, 70) | Lyngbya majuscula | NCI-H460, Neuro-2a, and HCT-116 | ↑Cytotoxicity | 1.4; 3.1; 0.2 µg/mL 10.8, 4 µg/mL, nd 1.1; 0.49 µg/mL, nd | [134] | |
| Peptide ester | Malevamide D (71) | Symploca hydnoides Kü tzing ex Gomont | P388, Lung cancer A-549, colon cancer HT-29 Melanoma MEL-28 | ↑Cytotoxicity | 0.3–0.7 nM 0.7 nM | [135] |
| Cyclodepside | Malyngolide dimer (72) | Lyngbya majuscule | NCI H-460 human lung tumor cell line | Moderate cytotoxicity; anticancer activity | Not specified | [136] |
| Macrolide depsipeptide | Cryptophycin 1 (73) | Nostoc sp. | L1210 murine leukemia cells | Anticancer activity; ↑disruption of microtubule assembly | Not specified | [137,138] |
| kB cells and LoVo cell | ↑Apoptosis | 4.58, 7.63 pM | [139] | |||
| MDA-MB-435 mammary adenocarcinoma; SKOV3 ovarian carcinoma cell lines | ┴Proliferation; ┴cell cycle at G2/M Phase | 50 pM | [140,141] | |||
| Cyclic depsipeptide | Lagunamides A, B (75, 76) | Lyngbya majuscule | P388 (a murine leukemia cell line) | ↑Cytotoxicity | 6.4 and 20.5 nM | [142] |
| Lagunamides C (77) | P388, A549, PC3, HCT8, and SK-OV3carcinoma cell lines | 2.1 to 24.4 nM | [143] | |||
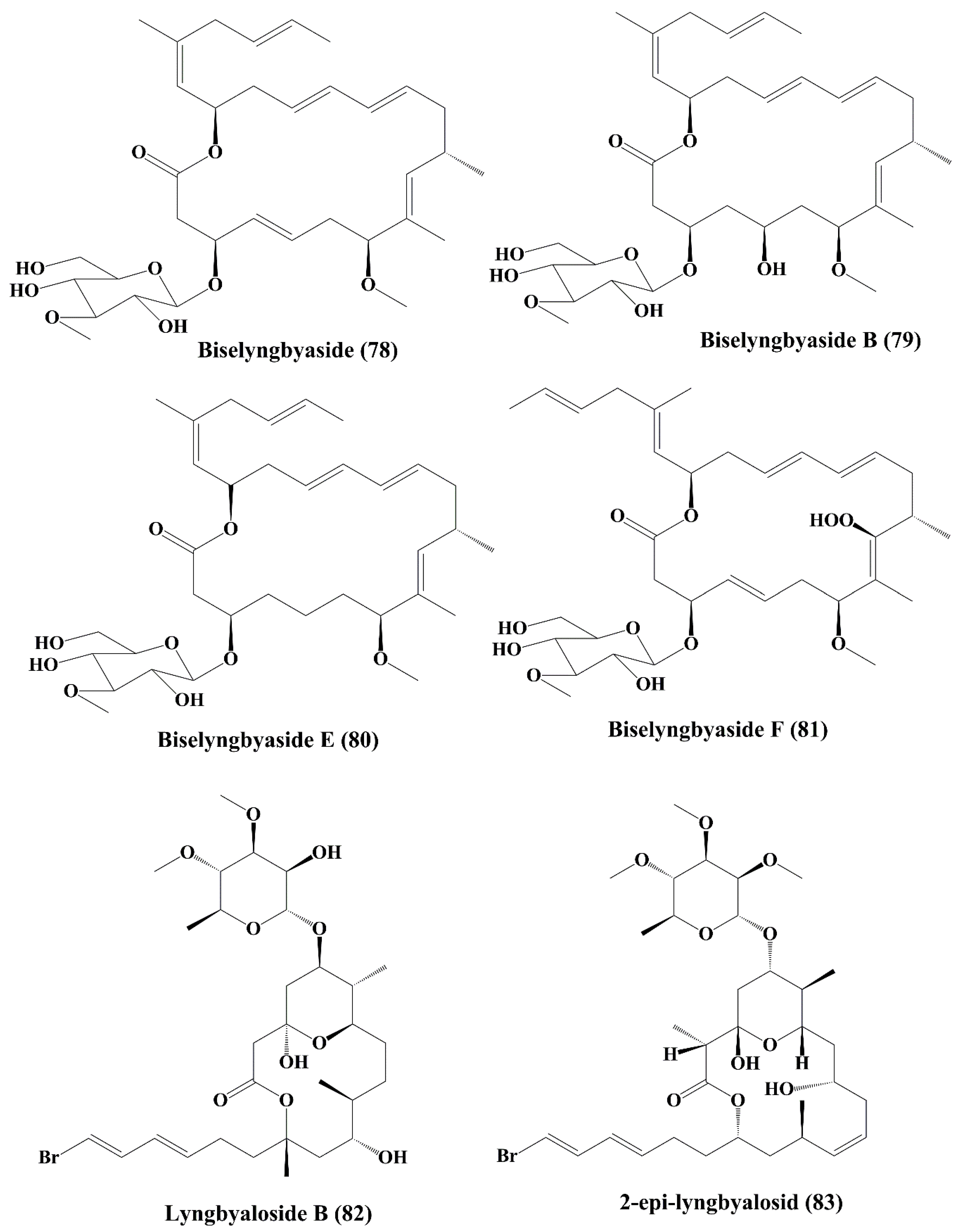
| Macrolide glycoside | Biselyngbyaside (78) | Lyngbya sp. | HeLa S3 epithelial carcinoma; SNB-78 central nervous system cancer; NCI H522 lung cancer | ┴Proliferation of cancer cell; induced cytotoxicity | 0.1 µg/mL; 0.036; 0.067 µM | [144] |
| Biselyngbyasid B (79) | Symploca hydnoides | HeLa S3 cells and HL60 cells | ┴Proliferation of cancer cell; induced cytotoxicity | 3.5 & 0.82 µM | [145] | |
| Biselyngbyasid E & F (80, 81) | Lyngbya sp. | HeLa and HL60 cells | ┴Proliferation of cancer cell; induced cytotoxicity | 0.19 & 0.071 µM; 3.1 & 0.66 µM | [146] | |
| Glycomacrolide | Lyngbyaloside B (82) | Lyngbya sp. | ĸB nasopharyngeal carcinoma and LoVo colon adenocarcinoma | ↑Cytotoxicity; anticancer activity | 4.3; 15 µM | [147] |
| 2-epi-lyngbyalosid (83) | Lyngbya bouillonii | HT29 colorectal adenocarcinoma and HeLa cells | Anticancer activity; ┴proliferation | 38 and 33 µM | [148] | |
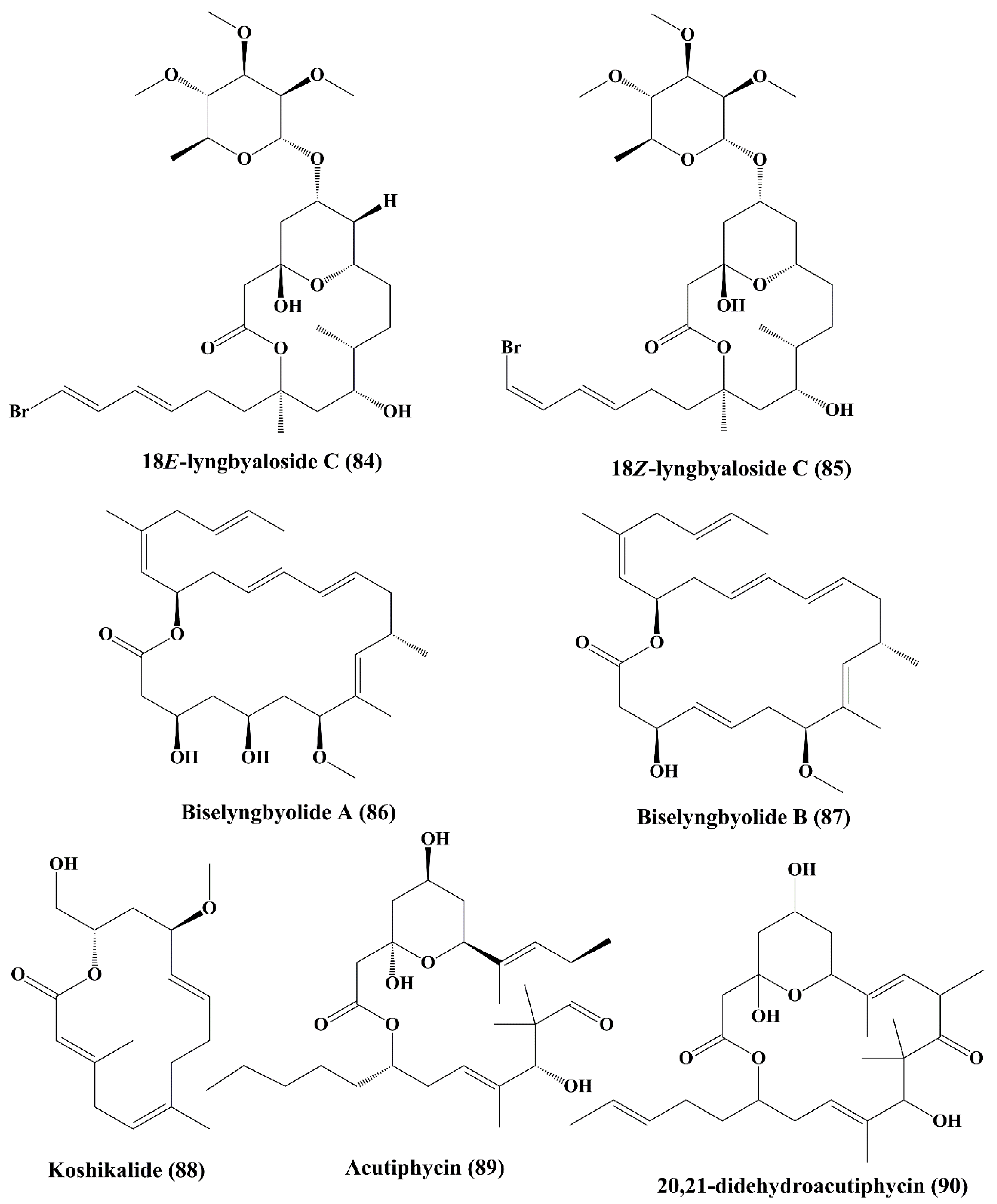
| 18E-lyngbyaloside C; 18Z-lyngbyaloside C (84, 85) | Lyngbya sp. | HT29 colorectal adenocarcinoma and HeLa cells | Anticancer activity; ┴proliferation; | 13 & 9.3 µM; >100 µM & 53 µM | [148] | |
| Macrolide | Biselyngbyolide A; Biselyngbyolide B (86, 87) | Lyngbya sp. | HeLa S3 cells and HL60 cells | Anticancer activity | 0.22 & 0.027 µM; 0.028 & 0.0027 µM | [149] |
| Macrolide | Koshikalide; Acutiphycin and 20, 21-didehydroacutiphycin (88, 89, 90) | Lyngbya sp., Oscillatoria acutissima | HeLa S3 cells; KB and NIH/3T3 cells | Anticancer activity; ↑cytotoxicity | 42 µg/mL, Not specified for Acutiphycin and 20, 21-didehydroacutiphycin | [150,151] |
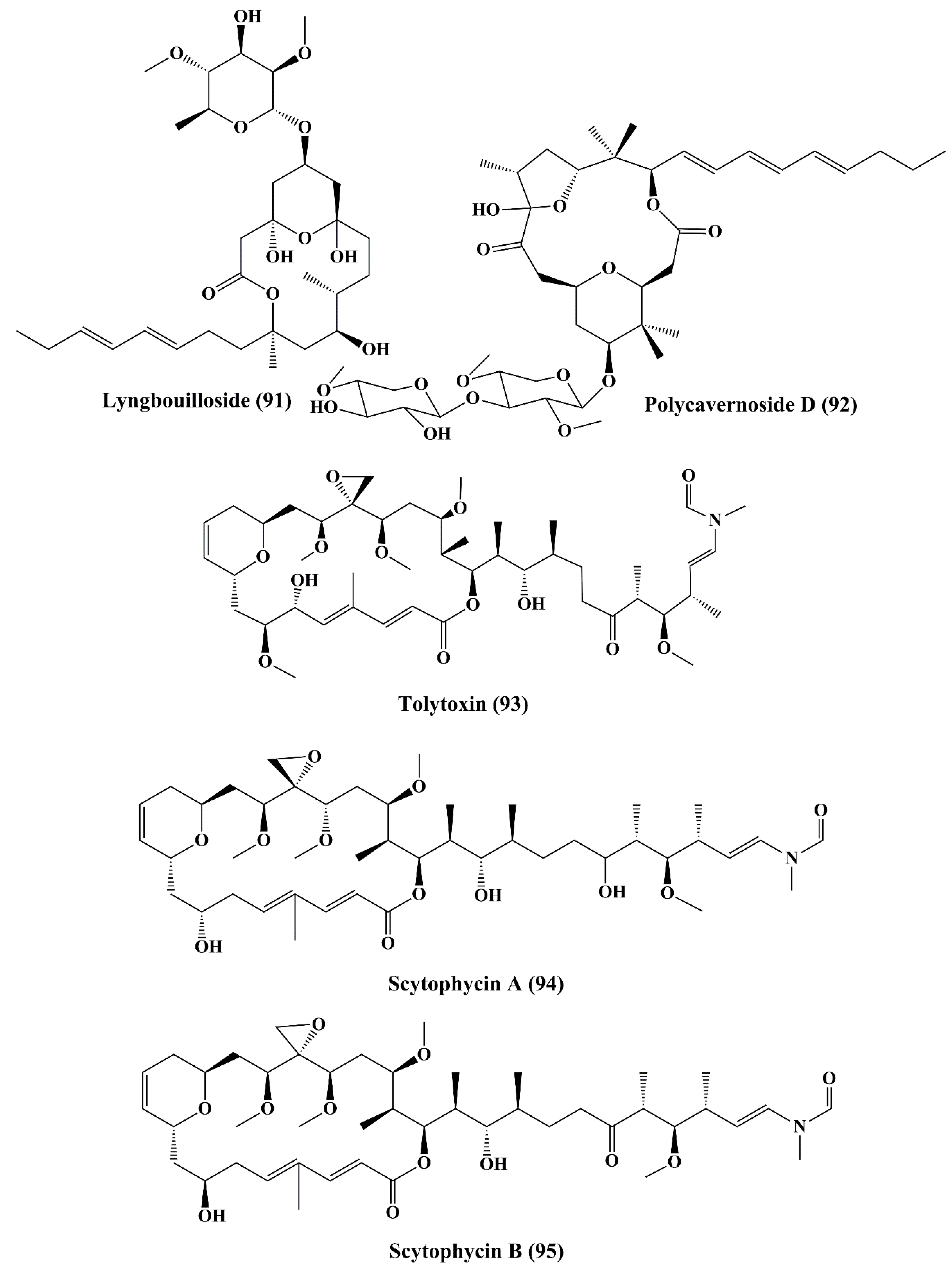
| Glycosylated macrolide | Lyngbouilloside (91) | Lyngbya bouillonii | Neuro-2a neuroblastoma cells | Anticancer activity; ↑cytotoxicity | 17 µM | [152] |
| Glycosylated macrolide | Polycavernoside D (92) | Okeania sp. | H-460 human lung cancer cell line | ┴Proliferation | EC50 = 2.5 µM | [153] |
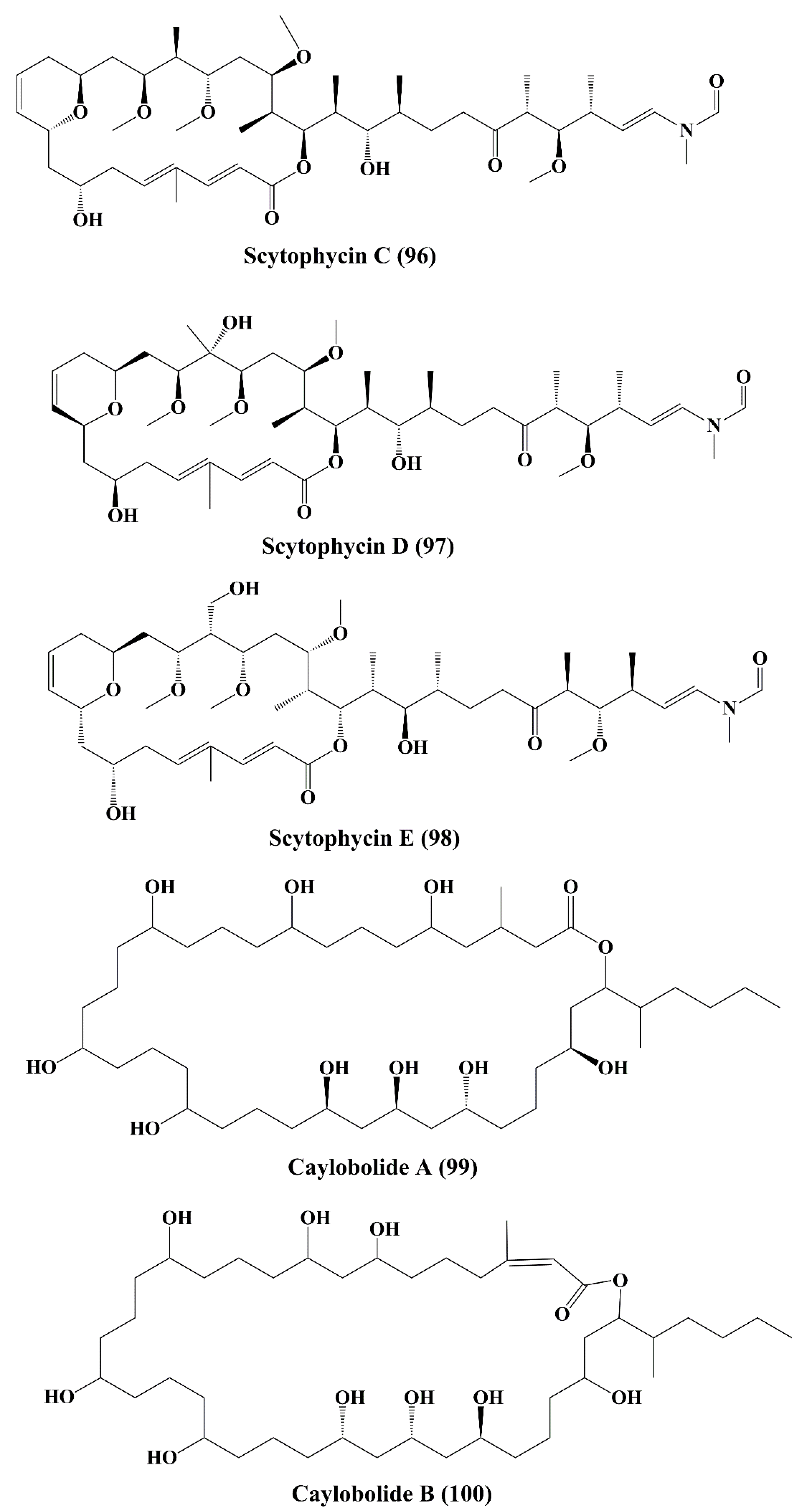
| Macrocyclic lactone | Tolytoxin(93) 6-hydroxyscytophycin B (95), 19-O-demethylscytophycin C (96), and 6-hydroxy-7-O-methylscytophycin E (98) | Seytonema ocellaturn Lyngbye ex Bornet and Flahault | L1210 (murine leukemia), LoVo, kB, HEp-2 (human epithelial type 2 cells), HL-60 (Human promyelocytic leukemia), HBL-100 (breast cancer cell), T47-D (human ductal carcinoma), COLO-201 (colon adenocarcinom), KATO-III (human gastric carcinoma) Nasopharynx cell (ĸB cells), &LoVo cells | Anticancer activity; ↑cytotoxicity; | 3.9, 8.4, 5.3, 2.3, 4.8, 2.4, 4.9, 0.52, and 0.78 nM >5 ng/mL | [154,155] |
| Macrolactone | Caylobolide A (99), Caylobolide B (100) | Lyngbya majuscula Phormidium sp. | HCT-116 colon tumor HT29 colorectal adenocarcinoma, and HeLa cervical carcinoma | Anticancer activity; ↑cytotoxicity | 9.9 µM (same for both caylobolide A & B) 4.5; 12.2 µM | [156,157] |
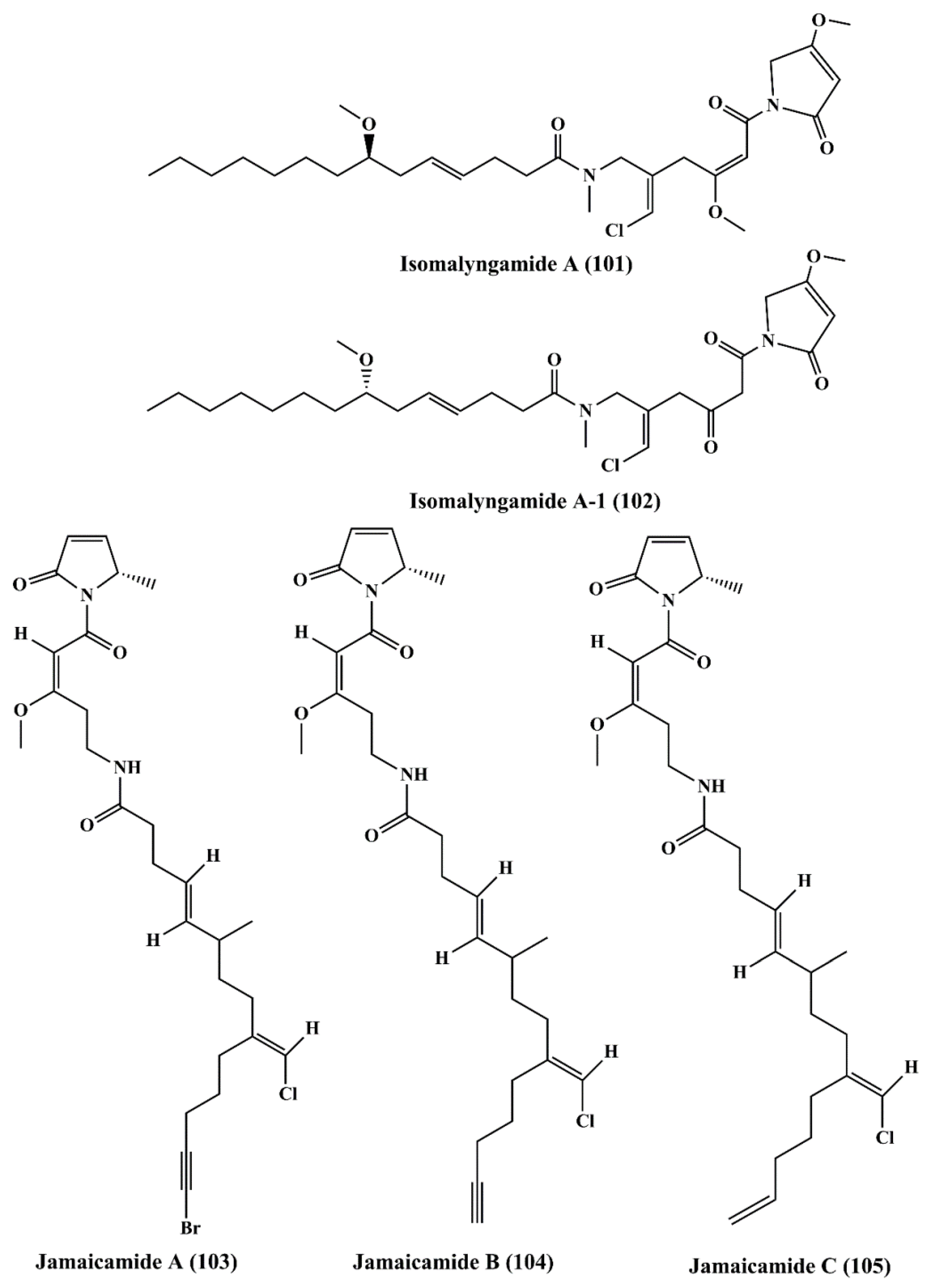
| Fatty acid amines | Isomalyngamide A (101), and Isomalyngamide A-1 (102) | Lyngbya majuscula | Breast cancer MCF-7 and MDA-MB-231 | ┴Proliferation; ┴apoptosis; ┴cell migration; antimetastatic activity | 4.6 & 2.8 µM; 12.7 µM & > 20 µM | [158] |
| Jamaicamides A, B, & C (103, 104, 105) | Lyngbya majuscula | H-460 lung cancer and Neuro-2a mouse neuro blastoma cell lines | ┴proliferation | LC50: 15 µM for all | [159] | |
| Pigment | Scytonemin (106) | Stigonema sp. | Jurkat T cells | ↑Apoptosis; ┴formation of mitotic spindle; ┴protein serine/threonine kinase activity | 7.8 μM | [160,161] |
| Boron containing metabolite | Borophycin (107) | Nostoc spongiaeforme, N. linckia | Human cancer cell lines ĸB colorectal adenocarcinoma and LoVo (human epidermoid carcinoma) | ┴Cancer; ┴cell cycle at G2/M Phase | Not specified | [48,162] |
| Phenanthridine alkaloids | Calothrixins A and B (108, 109) | Calothrix sp. | Human carcinoma cell line (HeLa) | ↑Cytotoxicity;┴proliferation | 40and 350 nM | [163,164] |
| CEM leukemia cells | ┴Proliferation;┴cell cycle at G1 and G2/M Phases | 0.20 to 5.13 µM | [165] |
| Class | Secondary Metabolite | Biological Source | Cell Lines | Effects and Mechanisms | IC50/Conc. | References |
|---|---|---|---|---|---|---|
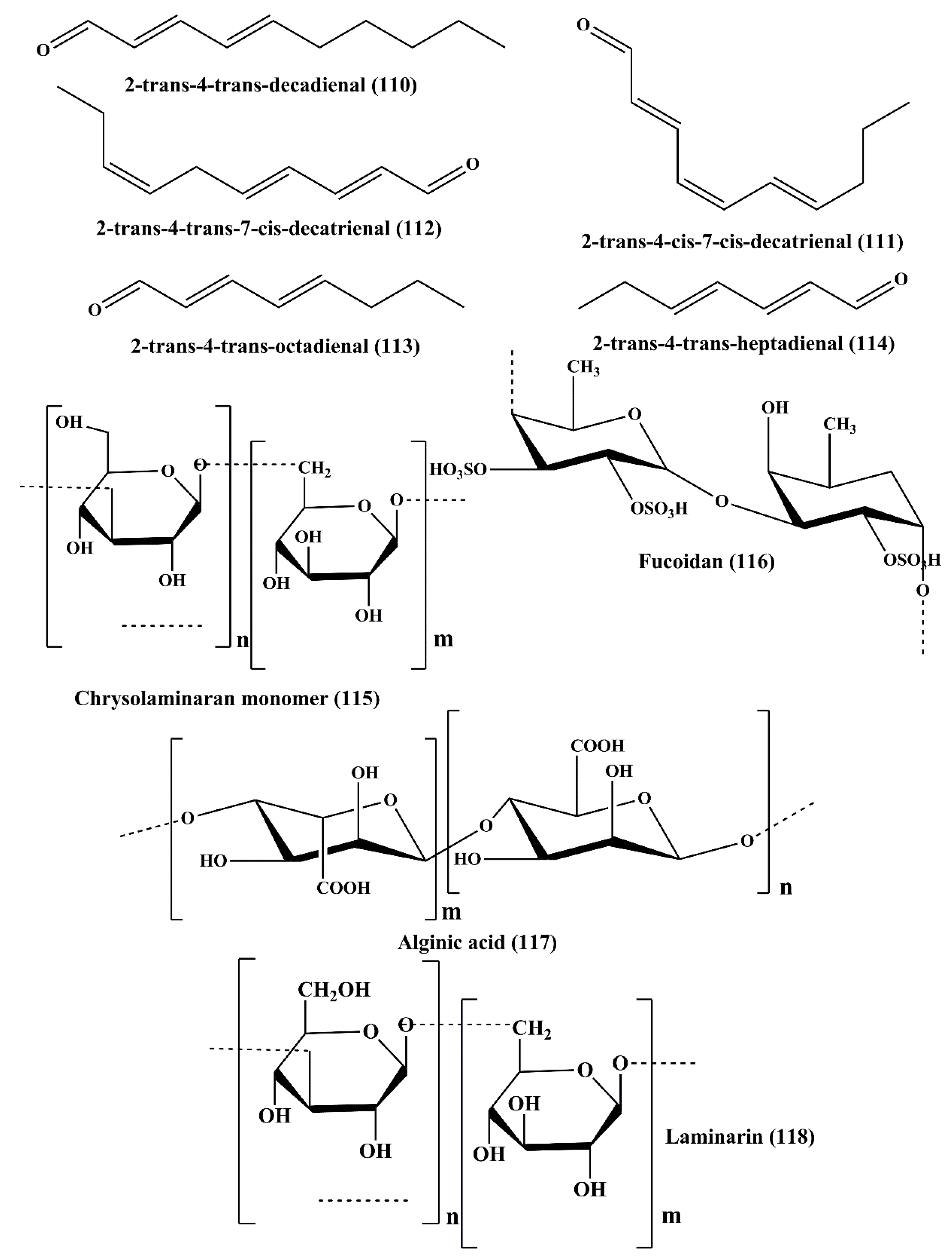
| Polyunsaturated aldehydes | 2-trans-4-trans-decadienal (110) | Thalassiosira rotula, Skeletonema costatum, Phaeocystis pouchetii and Pseudonitzschia delicatissima | Human colon adenocarcinoma cancer line Caco-2 | ┴Proliferation;↑cytotoxicity | 11–17 µg/mL | [166] |
| 2-trans-4-cis-7-cis-decatrienal (111) | ||||||
| 2-trans-4-trans-7-cis-decatrienal (112) | ||||||
| 2-trans,4-trans-heptadienal (113) | Skeletonema marinoi | Lung adenocarcinoma cell line A549, and colon COLO 205 | ↑Cytotoxicity; ┴cell cycle at either G1 or S Phase | 10 µM | [167,168] | |
| 2-trans,4-trans–octadienal (114) | Lung adenocarcinoma cell line A549 | ┴Cell cycle at either G1 or S Phase | 5 µM | |||
| Polysaccharide | Chrysolaminaran polysaccharide (115) | Synedra acus | Human colon cancer cell lines HTC-116 and DLD-1 | ┴Proliferation | 54.5 and 47.7 µg/mL | [169,170] |
| Sulfated polysaccharide | Fucoidans (116) | Sargassum hornery, Eclonia cava and Costaria costata | Human skin melanoma cell line (SK-MEL-28) and human colon cancer cell line (DLD-1) | ┴Cancer | 100 μg/mL | [171,172,173] |
| MDA-MB-231 cells | ↑Apoptosis | 820 μg/mL | [174,175] | |||
| Human lung cancer cells (A549) | ┴ERK1/2 pathway;┴Metastatic activity;┴PI3K/Akt/mTOR pathway | 400 μg/mL | [176] | |||
| Human hepatocellularcarcinoma cells (Huh7);HepG2 cells | ┴Proliferation | 2.0 and 4.0 mg/mL | [177,178,179,180] | |||
| Fucus evanescens | C57Bl/6 mice | ┴Growth of tumor | 10 mg/kg | [174] | ||
| Anionic polysaccharide | Alginic acid (117) | Sargassum wightii | H22 tumor-bearing mice | ┴Growth of tumor | Not specified | [41] |
| Polysaccharide | Laminarin (118) | Eisenia bicyclis | ES2 (ovarian clear cell carcinoma cells); OV90 (papillary serous adenocarcinoma cells) cell lines | ┴Proliferation;↑apoptosis;┴cell cycle at subG1 Phase | 2 mg/mL | [181,182] |
| JB6 Cl41 (normal mouse epidermal cells); SK-MEL-28 (human malignant melanoma) cells | ┴Cancer | Not specified | [183,184] | |||
| Human colon cancer cell lines, such as HCT-116, HT-29, and DLD-1 | ↑Cytotoxicity | 200 μg/mL | [182,185,186,187,188] | |||
| Human colon carcinoma cells (LoVo) | ↑Apoptosis | Not specified | [189] | |||
| Human colon cancer cell line (HT-29) | ↑Apoptosis, ┴cell cycle at subG1 and G2-M Phase | 5 mg/mL | [190,191,192] | |||
| Carotenoids | Violaxanthin (119) | Dunaliella tertiolecta | MCF-7 cancer cell line | ↑Apoptosis;↑cytotoxicity | 20 and 40 μg/mL | [193,194,195,196] |
| L1210 (human MDR1 gene-transfected mouse lymphoma cells); MDA-MB-231 (human breast cancer cells) | ┴P-glycoprotein (P-gp) and MRP1 | Not specified | [197] | |||
| Human MDR1 gene-transfected mouse lymphoma; MCF-7 (human breast cancer cell) | [198] | |||||
| Neoxanthin (120) | Tetraselmis suecica | HeLa; A549 cancer cells | ↑Cytotoxicity | Not specified | [199] | |
| Fucoxanthin (121) | Undaria pinnatifida | Human leukemia cell line (HL-60) | ┴Proliferation;↑apoptosis;┴cell cycle at G0/G1 Phase or G2/M Phase | 22.6 μM | [200,201,202,203,204,205] | |
| Siphonaxanthin (122) | Codium fragile, Caulerpa lentillifera and Umbraulva japonica | Human leukemia cell line (HL-60) | ↑Apoptosis; ↑chromatin condensation;↓Bcl-2;↑caspase-3;↑GADD5α;↑DR5 | 10 μM | [206] | |
| Human umbilical vein endothelial cells (HUVECs) | ┴Angiogenic effect;↓FGF-2;↓FGFR-1;↓EGR-1 | 2.5 μM | [207,208,209] | |||
| Zeaxanthin (123) | Porphyridium cruentum, Isochrysis galbana, Phaeodactylum tricornutum, Tetraselmis suecica and Nannochloropsis gaditana | Human colon adenocarcinoma cell line (HT-29) | ↑Cytotoxicity | 10 μM | [210,211] | |
| Xanthophyll carotenoids | Lutein (124) | Porphyridium cruentum, Isochrysis galbana, Phaeodactylum tricornutum, Tetraselmis suecica and Nannochloropsi sgaditana | Human colon adenocarcinoma cell line (HT-29) | ↑Cytotoxicity | Not specified | [211] |
| Sterol | Stigmasterol (125) | Navicula incerta | Human liver cancer cell line (HepG2) | ↑Cytotoxicity;┴proliferation;↑apoptosis;┴cell cycle at G0/G1 and G2/M Phase;↑caspase-8;↑caspase-9;↑Bax;↑p53;↓Bcl-2;↓XIAP | 20 μM | [213,214] |
| Fatty alcohol ester | Nonyl 8-acetoxy-6-methyloctanoate (126) | Phaeodactylum tricornutum | Human promyelocytic leukemia cell line (HL-60), a human lung carcinoma cell line (A549) and a mouse melanoma cell line (B16F10). | ↑Apoptosis;┴cell cycle at the sub G1 Phase | 65.15 μM, 50μg/mL, not specified | [215] |
| Epimeric carotenoids | Dinochrome A and B (127, 128) | Peridinium bipes | GOTO (neuroblastoma cells); OST (osteosarcoma cells) and HeLa cells | ┴Proliferation;┴TPA-stimulated 32P-incorporation into the phosholipids of HeLa cells | 5 μg/mL and 25 μg/mL | [216] |
| Porphyrin Phaeophytins | Porphyrinolactone (129) | Cladophora fascicularis | HeLa carcinoma cell line | ┴Proliferation;┴activation of NF-κB | 50 μM | [217] |
| 20-chlorinated (132-S)-hydroxyphaeophytin A (130) | ||||||
| (132-S)-hydroxyphaeophytin A (131) and B (132) | ||||||
| (132-R)-hydroxyphaeophytin A (133) and B (134) | ||||||
| Glycolipid | Nigricanosides A (135) and B (136) and methyl esters of nigricanosides A (137) and B (138) | Avrainvillea nigricans | Human breast cancer MCF-7 cells and human colon cancer HCT-116 cells | ┴Proliferation, antimitotic activity, ↑tubulin polymerization within the cell | Not specified | [218] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, A.; Bose, S.; Banerjee, S.; Patra, J.K.; Malik, J.; Mandal, S.K.; Kilpatrick, K.L.; Das, G.; Kerry, R.G.; Fimognari, C.; et al. Marine Cyanobacteria and Microalgae Metabolites—A Rich Source of Potential Anticancer Drugs. Mar. Drugs 2020, 18, 476. https://doi.org/10.3390/md18090476
Mondal A, Bose S, Banerjee S, Patra JK, Malik J, Mandal SK, Kilpatrick KL, Das G, Kerry RG, Fimognari C, et al. Marine Cyanobacteria and Microalgae Metabolites—A Rich Source of Potential Anticancer Drugs. Marine Drugs. 2020; 18(9):476. https://doi.org/10.3390/md18090476
Chicago/Turabian StyleMondal, Arijit, Sankhadip Bose, Sabyasachi Banerjee, Jayanta Kumar Patra, Jai Malik, Sudip Kumar Mandal, Kaitlyn L. Kilpatrick, Gitishree Das, Rout George Kerry, Carmela Fimognari, and et al. 2020. "Marine Cyanobacteria and Microalgae Metabolites—A Rich Source of Potential Anticancer Drugs" Marine Drugs 18, no. 9: 476. https://doi.org/10.3390/md18090476










