Brown Algae Carbohydrates: Structures, Pharmaceutical Properties, and Research Challenges
Abstract
:1. Introduction
2. Alginate and Alginate Lyase
2.1. Antioxidant Activity
2.2. Antimicrobial Activity
2.3. Immunomodulatory and Antitumor Activity
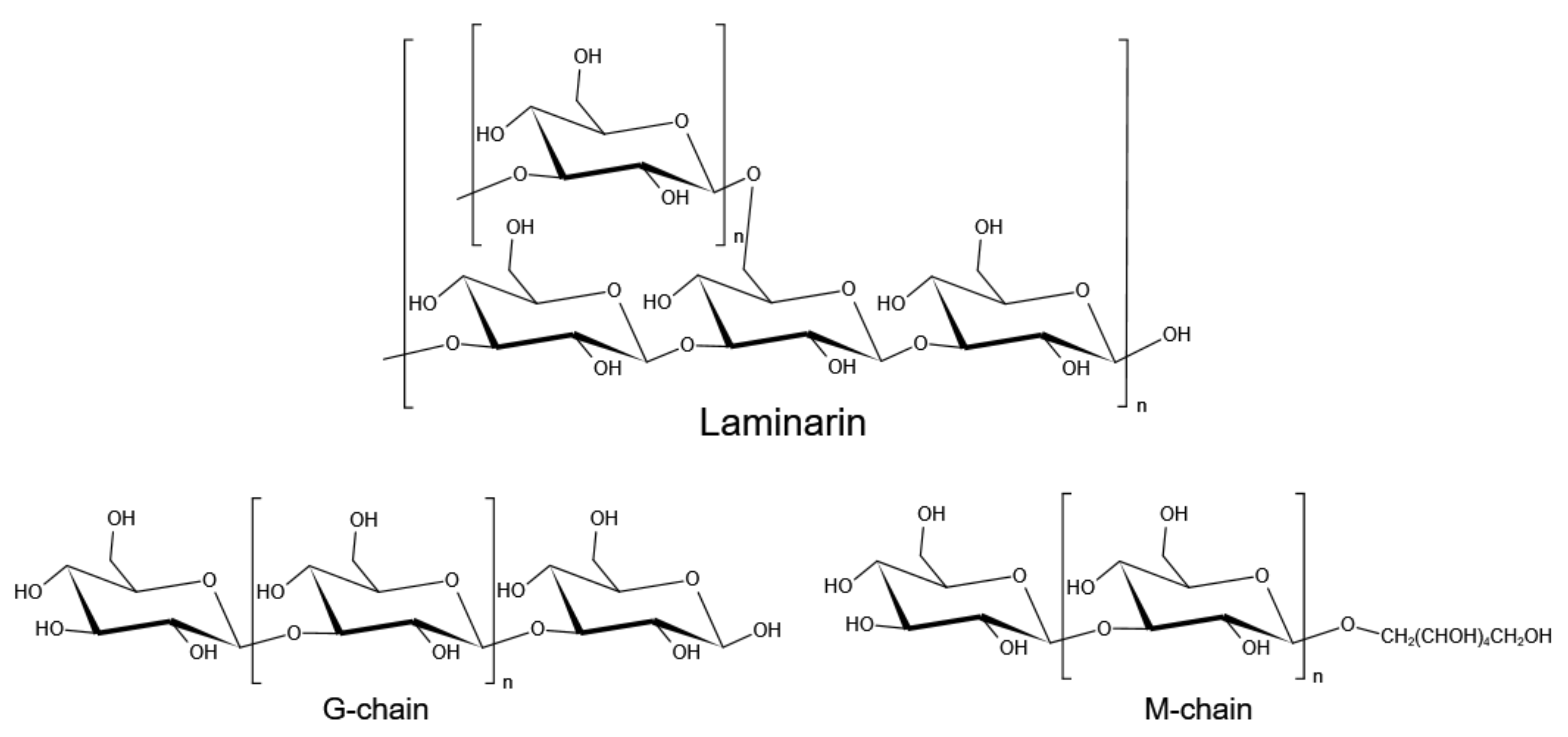
3. Laminarin
3.1. Antioxidant and Antimicrobial Activities
3.2. Antitumor and Anticoagulant Activity
3.3. Anti-Inflammatory and Immunostimulatory Activity
3.4. Prebiotic Activity
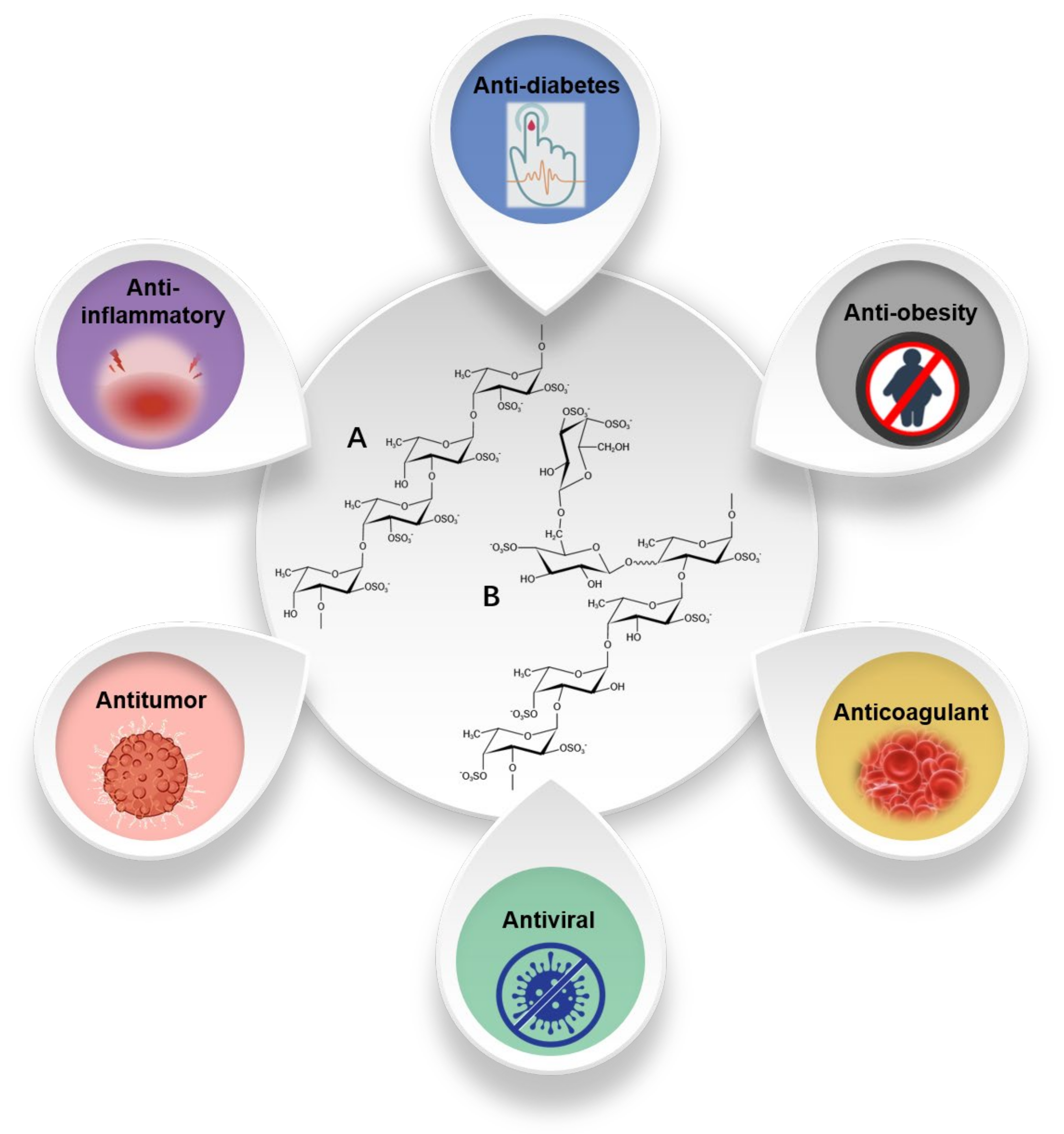
4. Fucoidan
4.1. Antitumor Activity
4.2. Antiviral and Anti-Inflammatory Activity
4.3. Antidiabetic Activity
4.4. Other Biological Activities
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Commercial Seaweeds Market Size, Share & Trends Analysis Report by Product (Brown Seaweeds, Red Seaweeds, Green Seaweeds), by Form (Liquid, Powdered, Flakes), by Application, by Region, and Segment Forecasts, 2020–2027. Available online: https://www.grandviewresearch.com/industry-analysis/commercial-seaweed-market (accessed on 25 April 2021).
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. Metabolites from Macroalgae and Its Applications in the Cosmetic Industry: A Circular Economy Approach. Resources 2020, 9, 101. [Google Scholar] [CrossRef]
- Radulovich, R.; Neori, A.; Valderrama, D.; Reddy, C.R.K.; Cronin, H.; Forster, J. Chapter 3—Farming of seaweeds. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 27–59. [Google Scholar]
- Lü, J.; Sheahan, C.; Fu, P. Metabolic engineering of algae for fourth generation biofuels production. Energ. Environ. Sci. 2011, 4, 2451–2466. [Google Scholar] [CrossRef]
- Sudhakar, K.; Mamat, R.; Samykano, M.; Azmi, W.H.; Ishak, W.F.W.; Yusaf, T. An overview of marine macroalgae as bioresource. Renew. Sustain. Energy Rev. 2018, 91, 165–179. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Turgeon, S.L. Chapter 7—Seaweed carbohydrates. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 141–192. [Google Scholar]
- Salmeán, A.; Duffieux, D.; Harholt, J.; Qin, F.; Michel, G.; Czjzek, M.; Willats, W.; Hervé, C. Insoluble (1→3), (1→4)-β-D-glucan is a component of cell walls in brown algae (Phaeophyceae) and is masked by alginates in tissues. Sci. Rep. 2017, 7, 2880. [Google Scholar] [CrossRef]
- Lewis, D.H.; Smith, D.C. Sugar alcohols (polyols) in fungi and green plants. I. Distribution, physiology and metabolism. New Phytol. 1967, 66, 143–184. [Google Scholar] [CrossRef]
- Tada, B.; Bv, A.; Am, B. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar]
- Black, W.A.P. The seasonal variation in the cellulose content of the common Scottish Laminariaceae and Fucaceae. J. Mar. Biol. Assoc. UK 2010, 29, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Kloareg, B.; Quattrano, R.S. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr. Mar. Biol. 1988, 26, 259–315. [Google Scholar]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Extraction, structure and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Bursać Kovačević, D.; Elez Garofulić, I.; Dragović-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef] [Green Version]
- Olsson, J.; Toth, G.B.; Albers, E. Biochemical composition of red, green and brown seaweeds on the Swedish west coast. J. Appl. Phycol. 2020, 32, 3305–3317. [Google Scholar] [CrossRef]
- Cronshaw, J.; Myers, A.; Preston, R.D. A chemical and physical investigation of the cell walls of some marine algae. Biochim. Biophys. Acta 1958, 27, 89–103. [Google Scholar] [CrossRef]
- Brownlee, I.A.; Allen, A.; Pearson, J.P.; Dettmar, P.W.; Havler, M.E.; Atherton, M.R.; Onsøyen, E. Alginate as a source of dietary fiber. Crit. Rev. Food Sci. Nutr. 2005, 45, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.; Khalid, S.; Usman, A.; Hussain, Z.; Wang, Y. Chapter 5—Algal Polysaccharides, Novel Application, and Outlook. In Algae Based Polymers, Blends, and Composites; Zia, K.M., Zuber, M., Ali, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 115–153. [Google Scholar]
- Moen, E.; Larsen, B.; Østgaard, K.; Jensen, A. Alginate Stability during High Salt Preservation of Ascophyllum nodosum. In Proceedings of the Sixteenth International Seaweed Symposium, Cebu City, Philippines, 12–17 April 1998; Kain, J.M., Brown, M.T., Lahaye, M., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 535–539. [Google Scholar]
- Jiménez-Escrig, A.; Sánchez-Muniz, F.J. Dietary fibre from edible seaweeds: Chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutr. Res. 2000, 20, 585–598. [Google Scholar] [CrossRef]
- Radwan, A.; Davies, G.; Fataftah, A.; Ghabbour, E.A.; Jansen, S.A.; Willey, R.J. Isolation of humic acid from the brown algae Ascophyllum nodosum, Fucus vesiculosus, Laminaria saccharina and the marine angiosperm Zostera marina. J. Appl. Phycol. 1996, 8, 553–562. [Google Scholar] [CrossRef]
- Cheng, D.; Jiang, C.; Xu, J.; Liu, Z.; Mao, X. Characteristics and applications of alginate lyases: A review. Int. J. Biol. Macromol. 2020, 164, 1304–1320. [Google Scholar] [CrossRef] [PubMed]
- Gacesa, P. Alginate-modifying Enzymes—A proposed unified mechanism of action for the lyases and epimerases. FEBS Lett. 1987, 212, 199–202. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Jiang, X.; Guan, H.; Wang, P. Preparation, purification and characterization of alginate oligosaccharides degraded by alginate lyase from Pseudomonas sp. HZJ 216. Carbohydr. Res. 2011, 346, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.T.; Ko, H.-J.; Kim, N.; Kim, D.; Lee, D.; Choi, I.-G.; Woo, H.C.; Kim, M.D.; Kim, K.H. Characterization of a recombinant endo-type alginate lyase (Alg7D) from Saccharophagus degradans. Biotechnol. Lett. 2012, 34, 1087–1092. [Google Scholar] [CrossRef]
- Zhu, B.; Tan, H.; Qin, Y.; Xu, Q.; Du, Y.; Yin, H. Characterization of a new endo-type alginate lyase from Vibrio sp. W13. Int. J. Biol. Macromol. 2015, 75, 330–337. [Google Scholar] [CrossRef]
- Zhu, B.; Chen, M.; Yin, H.; Du, Y.; Ning, L. Enzymatic Hydrolysis of Alginate to Produce Oligosaccharides by a New Purified Endo-Type Alginate Lyase. Mar. Drugs 2016, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Che, J.; Li, X.; Li, J.; Wang, J.; Xu, Y. In vitro non-specific immunostimulatory effect of alginate oligosaccharides with different molecular weights and compositions on sea cucumber (Apostichopus japonicus) coelomocytes. Aquaculture 2014, 434, 434–441. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Zhang, L.; Wang, Y.; Wang, S.; Zhang, Y.; Guo, H.; Ji, D.; Wang, Y. Alginate Oligosaccharide DP5 Exhibits Antitumor Effects in Osteosarcoma Patients following Surgery. Front. Pharmacol. 2017, 8, 623. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Bi, D.; Zheng, R.; Cai, N.; Xu, H.; Zhou, R.; Lu, J.; Wan, M.; Xu, X. Identification and activation of TLR4-mediated signalling pathways by alginate-derived guluronate oligosaccharide in RAW264.7 macrophages. Sci. Rep. 2017, 7, 1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, J.; Hao, C.; Zhang, L.; Liu, X.; Zhou, X.; Dun, Y.; Li, H.; Li, G.; Zhao, X.; An, Y.; et al. OM2, a Novel Oligomannuronate-Chromium(III) Complex, Promotes Mitochondrial Biogenesis and Lipid Metabolism in 3T3-L1 Adipocytes via the AMPK-PGC1α Pathway. PLoS ONE 2015, 10, e0131930. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Feng, Z.; Feng, W.; Hu, T.; Guan, H.; Mao, Y. AOS ameliorates monocrotaline-induced pulmonary hypertension by restraining the activation of P-selectin/p38MAPK/NF-κB pathway in rats. Biomed. Pharmacother. 2019, 109, 1319–1326. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Han, F.; Gong, Q.; Yu, W. Cloning and characterization of the first polysaccharide lyase family 6 oligoalginate lyase from marine Shewanella sp. Kz7. J. Biochem. 2016, 159, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, M.F.; Jack, A.A.; Powell, L.C.; Sadh, H.; Rye, P.D.; Hill, K.E.; Thomas, D.W. Alginate oligosaccharides modify hyphal infiltration of Candida albicans in an in vitro model of invasive human candidosis. J. Appl. Microbiol. 2017, 123, 625–636. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, L.; Chen, Y.; Ni, H.; Xiao, A.; Cai, H. Characterization of an extracellular biofunctional alginate lyase from marine Microbulbifer sp. ALW1 and antioxidant activity of enzymatic hydrolysates. Microbiol. Res. 2016, 182, 49–58. [Google Scholar] [CrossRef]
- Falkeborg, M.; Cheong, L.-Z.; Gianfico, C.; Sztukiel, K.M.; Kristensen, K.; Glasius, M.; Xu, X.; Guo, Z. Alginate oligosaccharides: Enzymatic preparation and antioxidant property evaluation. Food Chem. 2014, 164, 185–194. [Google Scholar] [CrossRef]
- Liu, M.; Liu, L.; Zhang, H.-f.; Yi, B.; Everaert, N. Alginate oligosaccharides preparation, biological activities and their application in livestock and poultry. J. Integr. Agric. 2021, 20, 24–34. [Google Scholar] [CrossRef]
- Wang, P.; Jiang, X.; Jiang, Y.; Hu, X.; Mou, H.; Li, M.; Guan, H. In vitro antioxidative activities of three marine oligosaccharides. Nat. Prod. Res. 2007, 21, 646–654. [Google Scholar] [CrossRef]
- Zhao, X.; Li, B.; Xue, C.; Sun, L. Effect of molecular weight on the antioxidant property of low molecular weight alginate from Laminaria japonica. J. Appl. Phycol. 2012, 24, 295–300. [Google Scholar] [CrossRef]
- Tusi, S.K.; Khalaj, L.; Ashabi, G.; Kiaei, M.; Khodagholi, F. Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials 2011, 32, 5438–5458. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-J.; Ma, L.-L.; Shi, H.-T.; Zhu, J.-B.; Wu, J.; Ding, Z.-W.; An, Y.; Zou, Y.-Z.; Ge, J.-B. Alginate Oligosaccharide Prevents Acute Doxorubicin Cardiotoxicity by Suppressing Oxidative Stress and Endoplasmic Reticulum-Mediated Apoptosis. Mar. Drugs 2016, 14, 231. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, C.; Sørensen, A.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Source, Extraction, Characterization, and Applications of Novel Antioxidants from Seaweed. Annu. Rev. Food Sci. Technol. 2019, 10, 541–568. [Google Scholar] [CrossRef]
- Şen, M. Effects of molecular weight and ratio of guluronic acid to mannuronic acid on the antioxidant properties of sodium alginate fractions prepared by radiation-induced degradation. Appl. Radiat. Isot. 2011, 69, 126–129. [Google Scholar] [CrossRef]
- Ueno, M.; Hiroki, T.; Takeshita, S.; Jiang, Z.; Kim, D.; Yamaguchi, K.; Oda, T. Comparative study on antioxidative and macrophage-stimulating activities of polyguluronic acid (PG) and polymannuronic acid (PM) prepared from alginate. Carbohydr. Res. 2012, 352, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Jiang, X.; Gong, J.; Hwang, H.; Liu, Y.; Guan, H. Antibacterial activity of lyase-depolymerized products of alginate. J. Appl. Phycol. 2005, 17, 57–60. [Google Scholar] [CrossRef]
- Hengzhuang, W.; Song, Z.; Ciofu, O.; Onsøyen, E.; Rye, P.D.; Høiby, N. OligoG CF-5/20 Disruption of Mucoid Pseudomonas aeruginosa Biofilm in a Murine Lung Infection Model. Antimicrob. Agents Chemother. 2016, 60, 2620–2626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, L.C.; Sowedan, A.; Khan, S.; Wright, C.J.; Hawkins, K.; Onsøyen, E.; Myrvold, R.; Hill, K.E.; Thomas, D.W. The effect of alginate oligosaccharides on the mechanical properties of Gram-negative biofilms. Biofouling 2013, 29, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Nordgård, C.T.; Draget, K.I. Oligosaccharides as modulators of rheology in complex mucous systems. Biomacromolecules 2011, 12, 3084–3090. [Google Scholar] [CrossRef]
- Pritchard, M.F.; Powell, L.C.; Menzies, G.E.; Lewis, P.D.; Hawkins, K.; Wright, C.; Doull, I.; Walsh, T.R.; Onsøyen, E.; Dessen, A.; et al. A New Class of Safe Oligosaccharide Polymer Therapy to Modify the Mucus Barrier of Chronic Respiratory Disease. Mol. Pharm. 2016, 13, 863–872. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Hwang, H.M.; Aker, W.G.; Wang, P.; Lin, Y.; Jiang, X.; He, X. Synergistic combination of marine oligosaccharides and azithromycin against Pseudomonas aeruginosa. Microbiol. Res. 2014, 169, 759–767. [Google Scholar] [CrossRef]
- Pritchard, M.F.; Powell, L.C.; Jack, A.A.; Powell, K.; Beck, K.; Florance, H.; Forton, J.; Rye, P.D.; Dessen, A.; Hill, K.E.; et al. A Low-Molecular-Weight Alginate Oligosaccharide Disrupts Pseudomonal Microcolony Formation and Enhances Antibiotic Effectiveness. Antimicrob. Agents Chemother. 2017, 61, e00762-17. [Google Scholar] [CrossRef] [Green Version]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Tøndervik, A.; Sletta, H.; Klinkenberg, G.; Emanuel, C.; Powell, L.C.; Pritchard, M.F.; Khan, S.; Craine, K.M.; Onsøyen, E.; Rye, P.D.; et al. Alginate Oligosaccharides Inhibit Fungal Cell Growth and Potentiate the Activity of Antifungals against Candida and Aspergillus spp. PLoS ONE 2014, 9, e112518. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, Y.; Xu, X.; Tamura, T.; Oda, T.; Muramatsu, T. Enzymatically depolymerized alginate oligomers that cause cytotoxic cytokine production in human mononuclear cells. Biosci. Biotechnol. Biochem. 2003, 67, 258–263. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Kurachi, M.; Yamaguchi, K.; Oda, T. Induction of multiple cytokine secretion from RAW264.7 cells by alginate oligosaccharides. Biosci. Biotechnol. Biochem. 2007, 71, 238–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uno, T.; Hattori, M.; Yoshida, T. Oral Administration of Alginic Acid Oligosaccharide Suppresses IgE Production and Inhibits the Induction of Oral Tolerance. Biosci. Biotechnol. Biochem. 2006, 70, 3054–3057. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kurachi, M.; Yamaguchi, K.; Oda, T. Stimulation of multiple cytokine production in mice by alginate oligosaccharides following intraperitoneal administration. Carbohydr. Res. 2007, 342, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Cho, K.; Nakazono, S.; Isaka, S.; Abu, R.; Takeshita, S.; Yamaguchi, K.; Kim, D.; Oda, T. Alginate oligomer induces nitric oxide (NO) production in RAW264.7 cells: Elucidation of the underlying intracellular signaling mechanism. Biosci. Biotechnol. Biochem. 2015, 79, 1787–1793. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Bi, D.-C.; Li, C.; Fang, W.-S.; Zhou, R.; Li, S.-M.; Chi, L.-L.; Wan, M.; Shen, L.-M. Morphological and Proteomic Analyses Reveal that Unsaturated Guluronate Oligosaccharide Modulates Multiple Functional Pathways in Murine Macrophage RAW264.7 Cells. Mar. Drugs 2015, 13, 1798–1818. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Bi, D.; Wu, X.; Wang, Q.; Wei, G.; Chi, L.; Jiang, Z.; Oda, T.; Wan, M. Unsaturated guluronate oligosaccharide enhances the antibacterial activities of macrophages. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2014, 28, 2645–2654. [Google Scholar] [CrossRef]
- Xu, X.; Wu, X.; Wang, Q.; Cai, N.; Zhang, H.; Jiang, Z.; Wan, M.; Oda, T. Immunomodulatory Effects of Alginate Oligosaccharides on Murine Macrophage RAW264.7 Cells and Their Structure-Activity Relationships. J. Agric. Food Chem. 2014, 62, 3168–3176. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, X.; Hwang, H.; Liu, S.; Guan, H. Antitumour activities of alginate-derived oligosaccharides and their sulphated substitution derivatives. Eur. J. Phycol. 2004, 39, 67–71. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, L.; Yu, X.; Wang, S.; Xu, C.; Yin, H.; Wang, S. Alginate oligosaccharide attenuates α2,6-sialylation modification to inhibit prostate cancer cell growth via the Hippo/YAP pathway. Cell Death Dis. 2019, 10, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peat, S.; Whelan, W.J.; Lawley, H.G. 141. The structure of laminarin. Part I. The main polymeric linkage. J. Chem. Soc. 1958, 724–728. [Google Scholar] [CrossRef]
- Stark, J.R. A new method fot the analysis of laminarins and for preparative-scale fractionation of their components. Carbohydr. Res. 1976, 47, 176–178. [Google Scholar] [CrossRef]
- Shin, H.J.; Oh, S.J.; Kim, S.I.; Won Kim, H.; Son, J.-H. Conformational characteristics of β-glucan in laminarin probed by terahertz spectroscopy. Appl. Phys. Lett. 2009, 94, 111911. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, P.-J.; Kim, E.-K.; Park, J.-S.; Yoon, H.-D.; Kim, K.-R.; Ahn, C.-B. Antioxidant activity of enzymatic extracts from the brown seaweed Undaria pinnatifida by electron spin resonance spectroscopy. LWT—Food Sci. Technol. 2009, 42, 874–878. [Google Scholar] [CrossRef]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Stiger-Pouvreau, V.; Bourgougnon, N.; Deslandes, E. Chapter 8—Carbohydrates from Seaweeds. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 223–274. [Google Scholar]
- Nelson, T.E.; Lewis, B.A. Separation and characterization of the soluble and insoluble components of insoluble laminaran. Carbohydr. Res. 1974, 33, 63–74. [Google Scholar] [CrossRef]
- Alderkamp, A.C.; van Rijssel, M.; Bolhuis, H. Characterization of marine bacteria and the activity of their enzyme systems involved in degradation of the algal storage glucan laminarin. FEMS Microbiol. Ecol. 2007, 59, 108–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, G.; Henrissat, B. Structures and Mechanisms of Glycosyl Hydrolases. Structure 1995, 3, 853–859. [Google Scholar] [CrossRef] [Green Version]
- Bara, M.T.F.; Lima, A.L.; Ulhoa, C.J. Purification and characterization of an exo-beta-1,3-glucanase produced by Trichoderma asperellum. Fems Microbiol. Lett. 2003, 219, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Kim, D.H.; Yun, E.J.; Park, Y.-C.; Seo, J.-H.; Kim, K.H. The first bacterial beta-1,6-endoglucanase from Saccharophagus degradans 2-40T for the hydrolysis of pustulan and laminarin. Appl. Microbiol. Biotechnol. 2017, 101, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Correia, M.A.S.; Pires, V.M.R.; Dhillon, A.; Sharma, K.; Rajulapati, V.; Fontes, C.M.G.A.; Carvalho, A.L.; Goyal, A. Novel insights into the degradation of beta-1,3-glucans by the cellulosome of Clostridium thermocellum revealed by structure and function studies of a family 81 glycoside hydrolase. Int. J. Biol. Macromol. 2018, 117, 890–901. [Google Scholar] [CrossRef]
- Badur, A.H.; Ammar, E.M.; Yalamanchili, G.; Hehemann, J.-H.; Rao, C.V. Characterization of the GH16 and GH17 laminarinases from Vibrio breoganii 1C10. Appl. Microbiol. Biotechnol. 2020, 104, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Kim, D.H.; Seo, N.; Yun, E.J.; An, H.J.; Kim, J.H.; Kim, K.H. A Novel Glycoside Hydrolase Family 5 β-1,3-1,6-Endoglucanase from Saccharophagus degradans 2-40T and Its Transglycosylase Activity. Appl. Environ. Microbiol. 2016, 82, 4340–4349. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.L. Overview of (1→3)-beta-D-glucan immunobiology. Mediat. Inflamm. 1997, 6, 247–250. [Google Scholar] [CrossRef]
- Cheung, N.K.; Modak, S.; Vickers, A.; Knuckles, B. Orally administered beta-glucans enhance anti-tumor effects of monoclonal antibodies. Cancer Immunol. Immunother. 2002, 51, 557–564. [Google Scholar] [CrossRef]
- Hong, F.; Yan, J.; Baran, J.T.; Allendorf, D.J.; Hansen, R.D.; Ostroff, G.R.; Xing, P.X.; Cheung, N.K.; Ross, G.D. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J. Immunol. 2004, 173, 797–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, D.L.; Mueller, A.; Browder, W. Glucan-Based Macrophage Stimulators. Clin. Immunother. 1996, 5, 392–399. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, L.; Williams, D.L.; Browder, I.W. Glucan stimulates human dermal fibroblast collagen biosynthesis through a nuclear factor-1 dependent mechanism. Wound Repair Regen. 2002, 10, 161–168. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, Y.-J.; Kim, H.J.; Kim, Y.-S.; Park, W. Immunostimulatory Effect of Laminarin on RAW 264.7 Mouse Macrophages. Molecules 2012, 17, 5404–5411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devillé, C.; Damas, J.; Forget, P.; Dandrifosse, G.; Peulen, O. Laminarin in the dietary fibre concept. J. Sci. Food Agric. 2004, 84, 1030–1038. [Google Scholar] [CrossRef]
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish Brown Seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound Assisted Extraction, Characterization and Bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Kim, I.H.; Kim, J.; Nam, T.J. Induction of apoptosis by laminarin, regulating the insulin-like growth factor-IR signaling pathways in HT-29 human colon cells. Int. J. Mol. Med. 2012, 30, 734–738. [Google Scholar] [CrossRef] [Green Version]
- Zargarzadeh, M.; Amaral, A.J.R.; Custódio, C.A.; Mano, J.F. Biomedical applications of laminarin. Carbohyd. Polym. 2020, 232, 115774. [Google Scholar] [CrossRef]
- Ji, Y.B.; Ji, C.F.; Zhang, H. Laminarin induces apoptosis of human colon cancer LOVO cells through a mitochondrial pathway. Molecules 2012, 17, 9947–9960. [Google Scholar] [CrossRef]
- Ji, C.F.; Ji, Y.B. Laminarin-induced apoptosis in human colon cancer LoVo cells. Oncol. Lett. 2014, 7, 1728–1732. [Google Scholar] [CrossRef]
- Ermakova, S.; Men’shova, R.; Vishchuk, O.; Kim, S.-M.; Um, B.-H.; Isakov, V.; Zvyagintseva, T. Water-soluble polysaccharides from the brown alga Eisenia bicyclis: Structural characteristics and antitumor activity. Algal Res. 2013, 2, 51–58. [Google Scholar] [CrossRef]
- Usoltseva Menshova, R.V.; Anastyuk, S.D.; Shevchenko, N.M.; Zvyagintseva, T.N.; Ermakova, S.P. The comparison of structure and anticancer activity in vitro of polysaccharides from brown algae Alaria marginata and A. angusta. Carbohydr. Polym. 2016, 153, 258–265. [Google Scholar] [CrossRef]
- Pang, Z.; Otaka, K.; Maoka, T.; Hidaka, K.; Ishijima, S.; Oda, M.; Ohnishi, M. Structure of beta-glucan oligomer from laminarin and its effect on human monocytes to inhibit the proliferation of U937 cells. Biosci. Biotechnol. Biochem. 2005, 69, 553–558. [Google Scholar] [CrossRef]
- Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Isakov, V.V.; Dubrovskaya, Y.V.; Kusaykin, M.I.; Um, B.H.; Zvyagintseva, T.N. Structure, enzymatic transformation and anticancer activity of branched high molecular weight laminaran from brown alga Eisenia bicyclis. Carbohydr. Polym. 2014, 99, 101–109. [Google Scholar] [CrossRef]
- Ji, C.F.; Ji, Y.B.; Meng, D.Y. Sulfated modification and anti-tumor activity of laminarin. Exp. Ther. Med. 2013, 6, 1259–1264. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Jiang, H.; Mao, X.; Ci, F. Laminarin and Laminarin Oligosaccharides Originating from Brown Algae: Preparation, Biological Activities, and Potential Applications. J. Ocean Univ. China 2021, 20, 641–653. [Google Scholar] [CrossRef]
- Shanmugam, M.; Mody, K.H. Heparinoid-active sulphated polysaccharides from marine algae as potential blood anticoagulant agents. Curr. Sci. 2000, 79, 1672–1683. [Google Scholar]
- Hoffman, J.; Larm, O.; Larsson, K.; Andersson, L.O.; Holmer, E.; Söderström, G. Studies on the blood-anticoagulant activity of sulphated polysaccharides with different uronic acid content. Carbohyd. Polym. 1982, 2, 115–121. [Google Scholar] [CrossRef]
- O’Neill, A.N. Sulphated derivatives of laminarin. Can. J. Chem. 1955, 33, 1097–1101. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, W.W.; O’Neill, A.N. The anticoagulant action in blood of sulphated derivatives of laminarin. Can. J. Biochem. Physiol. 1955, 33, 545–552. [Google Scholar] [CrossRef]
- Adams, S.S.; Thorpe, H.M. The anticoagulant activity and toxicity of laminarin sulphate K. J. Pharm. Pharmacol. 1957, 9, 459–463. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. Beta-glucan recognition by the innate immune system. Immunol. Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef]
- Ozanne, H.; Toumi, H.; Roubinet, B.; Landemarre, L.; Lespessailles, E.; Daniellou, R.; Cesaro, A. Laminarin Effects, a β-(1,3)-Glucan, on Skin Cell Inflammation and Oxidation. Cosmetics 2020, 7, 66. [Google Scholar] [CrossRef]
- Ramnani, P.; Chitarrari, R.; Tuohy, K.; Grant, J.; Hotchkiss, S.; Philp, K.; Campbell, R.; Gill, C.; Rowland, I. In vitro fermentation and prebiotic potential of novel low molecular weight polysaccharides derived from agar and alginate seaweeds. Anaerobe 2012, 18, 1–6. [Google Scholar] [CrossRef]
- Walsh, A.M.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; ’Doherty, J.V.O. Effect of supplementing varying inclusion levels of laminarin and fucoidan on growth performance, digestibility of diet components, selected faecal microbial populations and volatile fatty acid concentrations in weaned pigs. Anim. Feed Sci. Technol. 2013, 183, 151–159. [Google Scholar] [CrossRef]
- Devillé, C.; Gharbi, M.; Dandrifosse, G.; Peulen, O. Study on the effects of laminarin, a polysaccharide from seaweed, on gut characteristics. J. Sci. Food Agric. 2007, 87, 1717–1725. [Google Scholar] [CrossRef]
- Nguyen, S.G.; Kim, J.; Guevarra, R.B.; Lee, J.H.; Kim, E.; Kim, S.I.; Unno, T. Laminarin favorably modulates gut microbiota in mice fed a high-fat diet. Food Funct. 2016, 7, 4193–4201. [Google Scholar] [CrossRef]
- Leal, B.E.S.; Prado, M.R.; Grzybowski, A.; Tiboni, M.; Koop, H.S.; Scremin, L.B.; Sakuma, A.C.; Takamatsu, A.A.; Santos, A.F.d.; Cavalcanti, V.F.; et al. Potential prebiotic oligosaccharides from aqueous thermopressurized phosphoric acid hydrolysates of microalgae used in treatment of gaseous steakhouse waste. Algal Res. 2017, 24, 138–147. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from Fucoidan: An Update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phull, A.-R.; Majid, M.; Haq, I.-u.; Khan, M.R.; Kim, S.J. In vitro and in vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017, 97, 468–480. [Google Scholar] [CrossRef]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and Cancer: A Multifunctional Molecule with Anti-Tumor Potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, W.A.P. The seasonal variation in the combined L-fucose content of the common British Laminariaceae and fucaceae. J. Sci. Food Agric. 1954, 5, 445–448. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Nishino, T.; Nishioka, C.; Ura, H.; Nagumo, T. Isolation and partial characterization of a novel amino sugar-containing fucan sulfate from commercial Fucus vesiculosus fucoidan. Carbohydr. Res. 1994, 255, 213–224. [Google Scholar] [CrossRef]
- Lahrsen, E.; Schoenfeld, A.K.; Alban, S. Size-dependent pharmacological activities of differently degraded fucoidan fractions from Fucus vesiculosus. Carbohydr. Polym. 2018, 189, 162–168. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. A highly regular fraction of a fucoidan from the brown seaweed Fucus distichus L. Carbohydr. Res. 2004, 339, 511–517. [Google Scholar] [CrossRef]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef] [Green Version]
- Chevolot, L.; Foucault, A.; Chaubet, F.; Kervarec, N.; Sinquin, C.; Fisher, A.M.; Boisson-Vidal, C. Further data on the structure of brown seaweed fucans: Relationships with anticoagulant activity. Carbohydr. Res. 1999, 319, 154–165. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Ermakova, S.P.; Zvyagintseva, T.N.; Stonik, V.A. Anticancer and cancer preventive properties of marine polysaccharides: Some results and prospects. Mar. Drugs 2013, 11, 4876–4901. [Google Scholar] [CrossRef] [Green Version]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Kelly, M.; Sanderson, C.J.; Nifantiev, N.E.; Usov, A.I. Further studies on the composition and structure of a fucoidan preparation from the brown alga Saccharina latissima. Carbohydr. Res. 2010, 345, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Chevolot, L.; Mulloy, B.; Ratiskol, J.; Foucault, A.; Colliec-Jouault, S. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydr. Res. 2001, 330, 529–535. [Google Scholar] [CrossRef]
- Lim, S.J.; Wan Aida, W.M.; Schiehser, S.; Rosenau, T.; Böhmdorfer, S. Structural elucidation of fucoidan from Cladosiphon okamuranus (Okinawa mozuku). Food Chem. 2019, 272, 222–226. [Google Scholar] [CrossRef]
- Menshova, R.V.; Anastyuk, S.D.; Ermakova, S.P.; Shevchenko, N.M.; Isakov, V.I.; Zvyagintseva, T.N. Structure and anticancer activity in vitro of sulfated galactofucan from brown alga Alaria angusta. Carbohydr. Polym. 2015, 132, 118–125. [Google Scholar] [CrossRef]
- Shevchenko, N.M.; Anastyuk, S.D.; Menshova, R.V.; Vishchuk, O.S.; Isakov, V.I.; Zadorozhny, P.A.; Sikorskaya, T.V.; Zvyagintseva, T.N. Further studies on structure of fucoidan from brown alga Saccharina gurjanovae. Carbohydr. Polym. 2015, 121, 207–216. [Google Scholar] [CrossRef]
- Sun, Q.-L.; Li, Y.; Ni, L.-Q.; Li, Y.-X.; Cui, Y.-S.; Jiang, S.-L.; Xie, E.-Y.; Du, J.; Deng, F.; Dong, C.-X. Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr. Polym. 2020, 229, 115487. [Google Scholar] [CrossRef] [PubMed]
- Zvyagintseva, T.N.; Usoltseva, R.V.; Shevchenko, N.M.; Surits, V.V.; Imbs, T.I.; Malyarenko, O.S.; Besednova, N.N.; Ivanushko, L.A.; Ermakova, S.P. Structural diversity of fucoidans and their radioprotective effect. Carbohydr. Polym. 2021, 273, 118551. [Google Scholar] [CrossRef] [PubMed]
- Kusaykin, M.I.; Silchenko, A.S.; Zakharenko, A.M.; Zvyagintseva, T.N. Fucoidanases. Glycobiology 2016, 26, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, B.; Zhao, X.; Piao, M. Isolation and characterization of a fucoidan-degrading bacterium from Laminaria japonica. J. Ocean Univ. China 2014, 13, 153–156. [Google Scholar] [CrossRef]
- Dong, S.; Chang, Y.; Shen, J.; Xue, C.; Chen, F. Purification, expression and characterization of a novel alpha-L-fucosidase from a marine bacteria Wenyingzhuangia fucanilytica. Protein Expr. Purif. 2017, 129, 9–17. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Jeon, Y.-J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Vo, T.-S.; Ngo, D.-H.; Kim, S.-K. Marine algae as a potential pharmaceutical source for anti-allergic therapeutics. Process Biochem. 2012, 47, 386–394. [Google Scholar] [CrossRef]
- Yamasaki-Miyamoto, Y.; Yamasaki, M.; Tachibana, H.; Yamada, K. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. J. Agric. Food Chem. 2009, 57, 8677–8682. [Google Scholar] [CrossRef]
- Xie, J.H.; Liu, X.; Shen, M.Y.; Nie, S.P.; Zhang, H.; Li, C.; Gong, D.M.; Xie, M.Y. Purification, physicochemical characterisation and anticancer activity of a polysaccharide from Cyclocarya paliurus leaves. Food Chem. 2013, 136, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Zong, A.; Cao, H.; Wang, F. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Aisa, Y.; Miyakawa, Y.; Nakazato, T.; Shibata, H.; Saito, K.; Ikeda, Y.; Kizaki, M. Fucoidan induces apoptosis of human HS-Sultan cells accompanied by activation of caspase-3 and down-regulation of ERK Pathways. Am. J. Hematol. 2005, 78, 7–14. [Google Scholar] [CrossRef]
- Haneji, K.; Matsuda, T.; Tomita, M.; Kawakami, H.; Ohshiro, K.; Uchihara, J.-N.; Masuda, M.; Takasu, N.; Tanaka, Y.; Ohta, T.; et al. Fucoidan Extracted From Cladosiphon Okamuranus Tokida Induces Apoptosis of Human T-Cell Leukemia Virus Type 1-Infected T-Cell Lines and Primary Adult T-Cell Leukemia Cells. Nutr. Cancer 2005, 52, 189–201. [Google Scholar] [CrossRef]
- Hyun, J.-H.; Kim, S.-C.; Kang, J.-I.; Kim, M.-K.; Boo, H.-J.; Kwon, J.-M.; Koh, Y.-S.; Hyun, J.-W.; Park, D.-B.; Yoo, E.-S.; et al. Apoptosis Inducing Activity of Fucoidan in HCT-15 Colon Carcinoma Cells. Biol. Pharm. Bull. 2009, 32, 1760–1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.-O.; Song, M.-G.; Kim, Y.-N.; Park, J.-I.; Kwak, J.-Y. The mechanism of fucoidan-induced apoptosis in leukemic cells: Involvement of ERK1/2, JNK, glutathione, and nitric oxide. Mol. Carcinog. 2010, 49, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Park, S.-H.; Jeong, J.-W.; Yoon, D.; Han, M.H.; Lee, D.-S.; Choi, G.; Yim, M.-J.; Lee, J.M.; Kim, D.-H.; et al. Induction of p53-Independent Apoptosis and G1 Cell Cycle Arrest by Fucoidan in HCT116 Human Colorectal Carcinoma Cells. Mar. Drugs 2017, 15, 154. [Google Scholar] [CrossRef] [Green Version]
- Park, H.S.; Kim, G.-Y.; Nam, T.-J.; Deuk Kim, N.; Hyun Choi, Y. Antiproliferative Activity of Fucoidan Was Associated with the Induction of Apoptosis and Autophagy in AGS Human Gastric Cancer Cells. J. Food Sci. 2011, 76, T77–T83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Fucoidan extract induces apoptosis in MCF-7 cells via a mechanism involving the ROS-dependent JNK activation and mitochondria-mediated pathways. PLoS ONE 2011, 6, e27441. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.-J.; Kim, S.-M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Il Park, Y. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [Green Version]
- Queiroz, K.C.; Medeiros, V.P.; Queiroz, L.S.; Abreu, L.R.; Rocha, H.A.; Ferreira, C.V.; Jucá, M.B.; Aoyama, H.; Leite, E.L. Inhibition of reverse transcriptase activity of HIV by polysaccharides of brown algae. Biomed. Pharmacother. 2008, 62, 303–307. [Google Scholar] [CrossRef]
- Dinesh, S.; Menon, T.; Hanna, L.E.; Suresh, V.; Sathuvan, M.; Manikannan, M. In vitro anti-HIV-1 activity of fucoidan from Sargassum swartzii. Int. J. Biol. Macromol. 2016, 82, 83–88. [Google Scholar] [CrossRef]
- Feldman, S.C.; Reynaldi, S.; Stortz, C.A.; Cerezo, A.S.; Damont, E.B. Antiviral properties of fucoidan fractions from Leathesia difformis. Phytomedicine Int. J. Phytother. Phytopharm. 1999, 6, 335–340. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakano, T.; Hashimoto, M.; Kanekiyo, K.; Hayashi, T. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 2008, 8, 109–116. [Google Scholar] [CrossRef]
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.-O. Fucoidan from Macrocystis pyrifera Has Powerful Immune-Modulatory Effects Compared to Three Other Fucoidans. Mar. Drugs 2015, 13, 1084–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [Green Version]
- Oka, S.; Okabe, M.; Tsubura, S.; Mikami, M.; Imai, A. Properties of fucoidans beneficial to oral healthcare. Odontology 2020, 108, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.; Fernando, I.; Kim, E.; Kim, S.Y.; Jeon, Y.J. Anti-inflammatory activity of a fucose rich sulfated polysaccharide isolated from an enzymatic digest of brown seaweed Sargassum horneri. In Proceedings of the 2016 KFN International Symposium and Annual Meeting, Jeju, Korea, 31 October–2 November 2016; p. 476. [Google Scholar]
- Ln, A.; Lei, W.; Xf, A.; Ddd, E.; Yjjb, C.; Jx, A.; Xin, G.A. In vitro and in vivo anti-inflammatory activities of a fucose-rich fucoidan isolated from Saccharina japonica. Int. J. Biol. Macromol. 2020, 156, 717–729. [Google Scholar]
- Rm, A.; Dp, A.; Km, A.; Mb, B.; Ma, A.; Sj, A.; Spc, D.; Sgyc, D.; Nmp, E. Studies on isolation, characterization of fucoidan from brown algae Turbinaria decurrens and evaluation of it’s in vivo and in vitro anti-inflammatory activities. Int. J. Biol. Macromol. 2020, 160, 1263–1276. [Google Scholar]
- Jayawardena, T.U.; Fernando, I.; Lee, W.W.; Sanjeewa, K.; Kim, H.S.; Lee, D.S.; Jeon, Y.J. Isolation and purification of fucoidan fraction in Turbinaria ornata from the Maldives; Inflammation inhibitory potential under LPS stimulated conditions in in-vitro and in-vivo models. Int. J. Biol. Macromol. 2019, 131, 614–623. [Google Scholar] [CrossRef]
- Lee, S.H.; Ko, C.I.; Ahn, G.; You, S.G.; Kim, J.S.; Min, S.H.; Kim, J.I.; Jee, Y.; Jeon, Y.J. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef]
- Lim, J.D.; Lee, S.R.; Kim, T.; Jang, S.-A.; Kang, S.C.; Koo, H.J.; Sohn, E.; Bak, J.P.; Namkoong, S.; Kim, H.K.; et al. Fucoidan from Fucus vesiculosus Protects against Alcohol-Induced Liver Damage by Modulating Inflammatory Mediators in Mice and HepG2 Cells. Mar. Drugs 2015, 13, 1051–1067. [Google Scholar] [CrossRef] [Green Version]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.Y.; Kim, G.Y.; Choi, I.W.; Kim, N.D.; Nam, T.J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-kB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar] [CrossRef]
- Hwang, P.A.; Phan, N.N.; Lu, W.J.; Hieu, B.T.N.; Lin, Y.C. Low-molecular-weight fucoidan and high-stability fucoxanthin from brown seaweed exert prebiotics and anti-inflammatory activities in Caco-2 cells. Food Nutr. Res. 2016, 60, 32033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.-O.; Zhang, W.; Du, J.-Y.; Wong, K.-W.; Oda, T.; Yu, Q. Fucoidan Can Function as an Adjuvant In Vivo to Enhance Dendritic Cell Maturation and Function and Promote Antigen-Specific T Cell Immune Responses. PLoS ONE 2014, 9, e99396. [Google Scholar]
- Raghavendran, H.R.B.; Srinivasan, P.; Rekha, S. Immunomodulatory activity of fucoidan against aspirin-induced gastric mucosal damage in rats. Int. Immunopharmacol. 2011, 11, 157–163. [Google Scholar] [CrossRef]
- Li, C.; Gao, Y.; Xing, Y.; Zhu, H.; Shen, J.; Tian, J. Fucoidan, a sulfated polysaccharide from brown algae, against myocardial ischemia–reperfusion injury in rats via regulating the inflammation response. Food Chem. Toxicol. 2011, 49, 2090–2095. [Google Scholar] [CrossRef]
- Senni, K.; Gueniche, F.; Foucault-Bertaud, A.; Igondjo-Tchen, S.; Fioretti, F.; Colliec-Jouault, S.; Durand, P.; Guezennec, J.; Godeau, G.; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006, 445, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Ley, K. The role of selectins in inflammation and disease. Trends Mol. Med. 2003, 9, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Bordone, L.; Motta, M.C.; Picard, F.; Robinson, A.; Jhala, U.S.; Apfeld, J.; McDonagh, T.; Lemieux, M.; McBurney, M.; Szilvasi, A.; et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006, 4, e31. [Google Scholar] [CrossRef] [Green Version]
- Lorenzati, B.; Zucco, C.; Miglietta, S.; Lamberti, F.; Bruno, G. Oral Hypoglycemic Drugs: Pathophysiological Basis of Their Mechanism of ActionOral Hypoglycemic Drugs: Pathophysiological Basis of Their Mechanism of Action. Pharmaceuticals 2010, 3, 3005–3020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structural Dependence of Sulfated Polysaccharide for Diabetes Management: Fucoidan From Undaria pinnatifida Inhibiting α-Glucosidase More Strongly Than α-Amylase and Amyloglucosidase. Front. Pharmacol. 2020, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Raghu, C.; Arjun, H.A.; Anantharaman, P. In vitro and in silico inhibition properties of fucoidan against α-amylase and α-D-glucosidase with relevance to type 2 diabetes mellitus. Carbohydr. Polym. 2019, 209, 350–355. [Google Scholar]
- Havale, S.H.; Pal, M. Medicinal chemistry approaches to the inhibition of dipeptidyl peptidase-4 for the treatment of type 2 diabetes. Bioorganic Med. Chem. 2009, 17, 1783–1802. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, A.; Jayawardena, T.U.; Kim, H.S.; Kim, S.Y.; Jeon, Y.J. Fucoidan isolated from Padina commersonii inhibit LPS-induced inflammation in macrophages blocking TLR/NF-κB signal pathway. Carbohydr. Polym. 2019, 224, 115195. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [Green Version]
- Hui, S.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of Bioactivities of Fucoidan from the Brown Seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.-C.; Chen, Y.-L.; Hwang, P.-A.; Chen, T.-H.; Chou, T.-C. Fucoidan ameliorates pancreatic β-cell death and impaired insulin synthesis in streptozotocin-treated β cells and mice via a Sirt-1-dependent manner. Mol. Nutr. Food Res. 2017, 61, 1700136. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, M.; Lu, Y.; Wang, R.; Li, J.; Yang, B.; Xia, M.; Zhang, H.; Li, X. Fucoidan exerts protective effects against diabetic nephropathy related to spontaneous diabetes through the NF-κB signaling pathway in vivo and in vitro. Int. J. Mol. Med. 2015, 35, 1067–1073. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Ren, D.; Song, Y.; Hu, Y.; Wu, L.; Wang, Q.; He, Y.; Zhou, H.; Liu, S.; Cong, H. Effects of a combined fucoidan and traditional Chinese medicine formula on hyperglycaemia and diabetic nephropathy in a type II diabetes mellitus rat model. Int. J. Biol. Macromol. 2020, 147, 408–419. [Google Scholar] [CrossRef]
- Lindahl, U. ‘Heparin’—From anticoagulant drug into the new biology. Glycoconj. J. 2000, 17, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Tolwani, A.J.; Wille, K.M. Anticoagulation for continuous renal replacement therapy. Semin. Dial. 2009, 22, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Mourão, P.A. Use of sulfated fucans as anticoagulant and antithrombotic agents: Future perspectives. Curr. Pharm. Des. 2004, 10, 967–981. [Google Scholar] [CrossRef]
- Mourão, P.A.; Pereira, M.S. Searching for alternatives to heparin: Sulfated fucans from marine invertebrates. Trends Cardiovasc. Med. 1999, 9, 225–232. [Google Scholar] [CrossRef]
- Nishino, T.; Nagumo, T. Anticoagulant and antithrombin activities of oversulfated fucans. Carbohydr. Res. 1992, 229, 355–362. [Google Scholar] [CrossRef]
- Usov, A.I.; Bilan, M.I. Fucoidans—Sulfated polysaccharides of brown algae. Russ. Chem. Rev. 2009, 78, 846–862. [Google Scholar] [CrossRef]
- Grauffel, V.; Kloareg, B.; Mabeau, S.; Durand, P.; Jozefonvicz, J. New natural polysaccharides with potent antithrombic activity: Fucans from brown algae. Biomaterials 1989, 10, 363–368. [Google Scholar] [CrossRef]
- Kim, M.-J.; Chang, U.-J.; Lee, J.-S. Inhibitory Effects of Fucoidan in 3T3-L1 Adipocyte Differentiation. Mar. Biotechnol. 2009, 11, 557–562. [Google Scholar] [CrossRef]
- Park, M.K.; Jung, U.; Roh, C. Fucoidan from Marine Brown Algae Inhibits Lipid Accumulation. Mar. Drugs 2011, 9, 1359–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanino, Y.; Hashimoto, T.; Ojima, T.; Mizuno, M. F-fucoidan from Saccharina japonica is a novel inducer of galectin-9 and exhibits anti-allergic activity. J. Clin. Biochem. Nutr. 2016, 59, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Brown Seaweed | Monosaccharide Composition | Molecular Weight | Concentration | Sulphate Content (%) | Mechanisms | References |
|---|---|---|---|---|---|---|
| Cladosiphon novae-caledoniae | Fucose 73 mol%, Xylose 12 mol% Mannose 7 mol% | - | 19.35 ng/µL; 80.64 ng/µL | 14.5% | Inhibited COX-1 and COX-2 | [153] |
| Sargassum horneri | Polyphenols 3.9% | Mw > 30 kDa | 25–100 µg/mL | 12% | Decreased production of TNF-α, IL-6, NO and PGE2 | [154] |
| Laminaria japonica | Fucose 79.49% Xylose 1.08% Mannose 1.84% Galactose 16.76% Rhamnose 0.82% | 104.3 kDa | 25 µg/mL | 30.72% | Decreased production of TNF-α, IL-1β, IL-6, NO, iNOS, and COX-2 expression; downregulation of MAPK and NF-κB signaling pathways | [155] |
| Turbinaria decurrens | Fucose 59.3% Xylose 11.4% Galactose 12.6% Mannose 9.6% | - | 50 mg/kg | 23.51% | Reduced the expression of genes of COX-2, IL-1β, the NF-κB signaling pathway | [156] |
| Turbinaria ornata | Fucose 86.4 mol% Rhamnose 0.4 mol% Galactose 1.7 mol% Glucose 0.8 mol% | - | 25–100 µg/mL | 38.3% | Suppressed the expression of COX-2 and pro-inflammatory cytokines in LPS-induced RAW 264.7 macrophages | [157] |
| Undaria pinnatifida | Fucose 50.9% Xylose 4.2% Galactose 44.6% Mannose 0.3% | - | 50 mg/kg;150 mg/kg | Reduced the COX-2 expression dose dependently | [112] | |
| Ecklonia cava | Fucose 77.9 mol% Rhamnose 2.3 mol% Galactose 10.1 mol% Glucose 2.2 mol% Xylose 7.5 mol% | - | 50–100 µg/mL | 39.1% | Reduced NO production and levels of TNF-α, IL-1β, and IL-6 | [158] |
| Fucus vesiculosus | Molar rate 1:0.03:0.02:0.04:0.2:1.2 for Fucose, Galactose, Mannose, Xylose, Uronic acid, and sulfate | - | 30–60 mg/kg | 27% | Inhibition of COX, hyaluronidase, and MAPK p38 enzymes. | [159] |
| Cladosiphon okamuranus | Fucose 30.9% Xylose 0.7% Glucose 2.2% Uronic acid 23.4% | - | 4.0 mg/kg | 15.1% | Inhibition of neutrophil extravasation into peritoneal cavity | [115] |
| Fucus vesiculosus | Fucoidan | - | 0–100 mg/mL | Inhibited the release of nitric oxide, IL-1b, TNF-a, prostaglandin E2 and monocyte chemoattractant protein-1 by inhibiting NF-κB, Akt and MAPK kinases activation | [160] | |
| Sargassum hemiphyllum | Fucose 210.99 mmol/g | - | 100 mg/mL | 38.99.4% | Inhibition of IL-1b, TNF-a, and reduction of IL-10, IFN-c in production LPS treated cells | [161] |
| Macrocystis pyrifera | Fucose 25.77% Galactose 3.93% Glucose 1.14% Mannose 1.12% Xylose 0.84% Uronic acid 5.54% | - | 5-100 μg/mL | 27.32% | Delayed the apoptosis and promote pro-inflammatory cytokine production in human neutrophils | [151] |
| Ascophyllum nodosum | Fucose 39.8% Galactose 3.37% Glucose 0.88% Mannose 0.72% Xylose 3.68% Uronic acid 1.72% | - | 50–100 μg/mL | 24.07% | Delayed the apoptosis and promote pro-inflammatory cytokine production in human neutrophils | [151] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wang, P.; Imre, B.; Wong, A.C.Y.; Hsieh, Y.S.Y.; Wang, D. Brown Algae Carbohydrates: Structures, Pharmaceutical Properties, and Research Challenges. Mar. Drugs 2021, 19, 620. https://doi.org/10.3390/md19110620
Li Y, Zheng Y, Zhang Y, Yang Y, Wang P, Imre B, Wong ACY, Hsieh YSY, Wang D. Brown Algae Carbohydrates: Structures, Pharmaceutical Properties, and Research Challenges. Marine Drugs. 2021; 19(11):620. https://doi.org/10.3390/md19110620
Chicago/Turabian StyleLi, Yanping, Yuting Zheng, Ye Zhang, Yuanyuan Yang, Peiyao Wang, Balázs Imre, Ann C. Y. Wong, Yves S. Y. Hsieh, and Damao Wang. 2021. "Brown Algae Carbohydrates: Structures, Pharmaceutical Properties, and Research Challenges" Marine Drugs 19, no. 11: 620. https://doi.org/10.3390/md19110620







